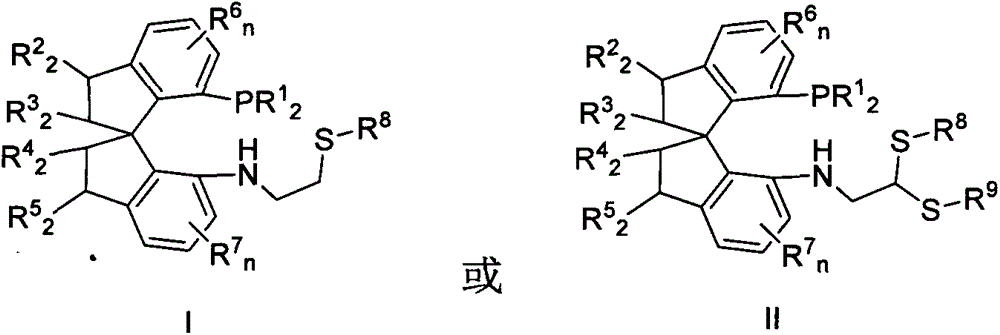
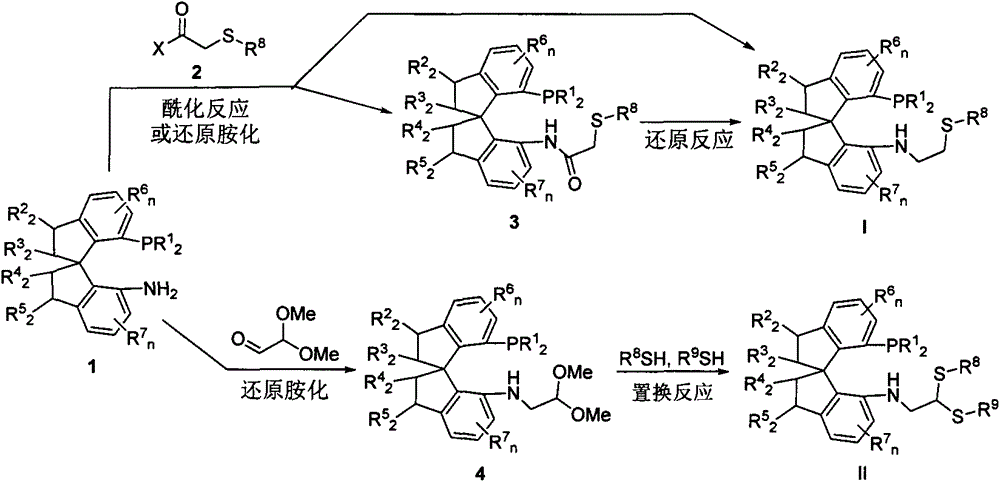
Chiral spiro phosphine-nitrogen-sulfur tridentate ligand and preparation method and application thereof
一种螺环、手性的技术,应用在羧酸酰胺旋光异构体制备、羧酸酯制备、羧酸酰胺制备等方向,达到活性高、反应条件温和、效率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
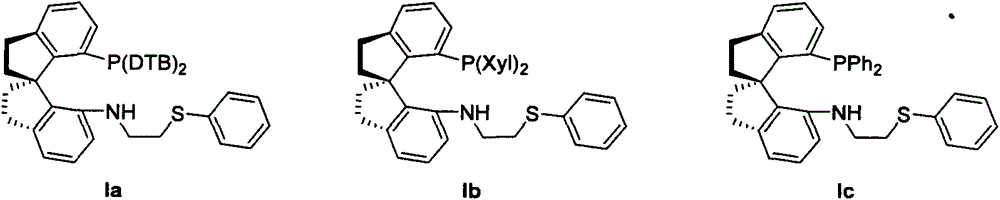
[0032] (R)-N-(2-(phenylthio)ethyl)-7-bis-(3,5-di-tert-butylphenyl)phosphino-7'-amino-1,1'-spirodihydro Synthesis of Indene(Ia)
[0033]
[0034] In a nitrogen atmosphere, weigh (R)-7'-bis-(3,5-di-tert-butylphenyl)phosphino-7'-amino-1,1'-spirodihydroindene (193mg, 0.3mmol ), pyridine (119 mg, 1.5 mmol) and 2 mL of dichloromethane in a 15 mL dry Schlenk tube. After stirring at room temperature to dissolve the solid, a dichloromethane solution (2 mL) of thiophenylacetyl chloride (84 mg, 0.45 mmol) was added dropwise to the system under cooling in an ice-water bath. After the drop was completed, after stirring at room temperature for 2 hours, TLC detected that the reaction was complete (petroleum ether: ethyl acetate = 10:1). The organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, and allowed to stand. The desiccant was removed by suction filtration, and the filtrate was desolvated by a rotary evaporator, and the crude product obtained was ...
Embodiment 2
[0039] (R)-N-(2-(phenylthio)ethyl)-7'-bis-(3,5-dimethylphenyl)phosphino-7'-amino-1,1'-spirodihydro Synthesis of Indene(Ib)
[0040]
[0041] Refer to Example 1 for specific operation, white solid, yield: 65%.
[0042] Mp 58-60°C, 277.0 (c 0.5, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ7.28-7.08(m, 8H), 7.03(t, J=7.6Hz, 1H), 6.84(s, 1H), 6.81(s, 1H), 6.72-6.63(m, 3H), 6.58(d , J=7.1Hz, 2H), 5.92(d, J=7.9Hz, 1H), 3.17-3.11(m, 1H), 3.08-2.96(m, 4H), 2.78(td, J=12.9, 6.5Hz, 1H), 2.68-2.53(m, 2H), 2.53-2.43(m, 1H), 2.43-2.33(m, 2H), 2.33-2.23(m, 1H), 2.17(s, 6H), 2.16(s, 6H); 31 P NMR (162MHz, CDCl 3 )δ-22.6(s); 13 C NMR (101MHz, CDCl 3)δ153.1 (d, J=25.3Hz), 144.6 (d, J=2.8Hz), 144.1 (d, J=7.7Hz), 143.5 (d, J=1.8Hz), 139.5 (d, J=13.0 Hz), 137.3(d, J=6.0Hz), 137.1(d, J=8.1Hz), 136.2(d, J=13.3Hz), 135.8, 135.0, 134.8, 134.4(d, J=2.9Hz), 133.4 (d, J=3.4Hz), 132.5, 132.2, 130.9, 130.7, 130.4, 130.1, 129.5, 129.0, 128.1, 127.5, 126.4, 126.0, 113.7, 107.9, 61.7 (d, J=3.2Hz), 41...
Embodiment 3
[0044] Synthesis of (R)-N-(2-(phenylthio)ethyl)-7'-diphenylphosphino-7'-amino-1,1'-spiroindene (Ic)
[0045]
[0046] See Example 1 for specific operation, white solid, yield: 51%.
[0047] Mp 55-58°C, 245.5 (c 0.5, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ7.31-6.91(m, 19H), 6.65(d, J=7.3Hz, 1H), 5.93(d, J=7.9Hz, 1H), 3.27-3.18(m, 1H), 3.11-2.93(m , 4H), 2.93-2.81(m, 1H), 2.68(t, J=6.5Hz, 2H), 2.53-2.33(m, 3H), 2.32-2.23(m, 1H), 2.18-2.08(m, 1H ); 31 P NMR (162MHz, CDCl 3 )δ-22.3(s); 13 C NMR (101MHz, CDCl 3 )δ153.3 (d, J=25.3Hz), 144.6 (d, J=3.1Hz), 144.3 (d, J=7.8Hz), 143.4, 139.7 (d, J=13.9Hz), 136.8 (d, J =14.0Hz), 135.6, 134.5(d, J=2.5Hz), 134.34, 134.25, 134.1, 134.0, 133.3, 133.2(d, J=3.5Hz), 133.1, 130.0, 129.0, 128.4(d, J=3.6 Hz), 128.1(d, J=5.5Hz), 128.0(d, J=7.3Hz), 127.7(d, J=9.9Hz), 126.4, 126.3, 113.8, 108.0, 61.7(d, J=3.2Hz) , 41.3, 40.0 (d, J=5.3Hz), 36.2, 33.9, 31.5, 31.1. HRMS (MALDI) Calcd for C 37 h 35 NPS[M+H] + : 556.2222; Found: 556.2216.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com