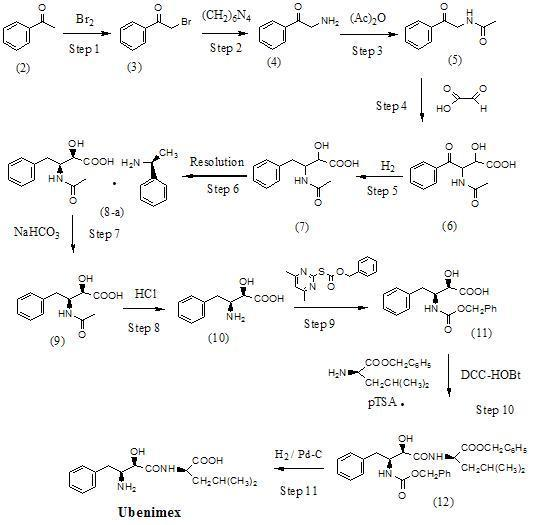
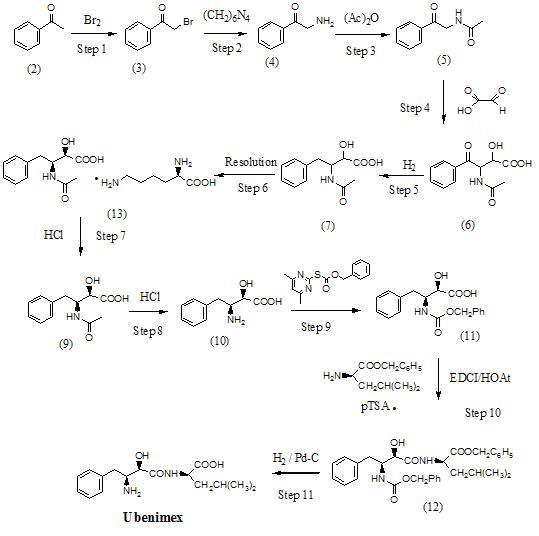
Preparation method for ubenimex
A technology of ubenimex and acetophenone, which is applied in the field of preparation of anti-tumor drugs, can solve the problems of no accurate method for content determination, large-scale production of expensive reagents, and reduction of the effectiveness of ubenimex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1 : Preparation of the L-lysine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutanoic acid (13) (Method A)
[0112]
[0113] The threo racemate of compound (7) (10.0 g, 42.1 mmol) and L-lysine (6.16 g, 42.1 mmol) were dissolved in a mixed solvent system of 95% ethanol (200 mL) and acetonitrile (50 mL) , heated to 60-65°C, stirred for 30 minutes, then cooled to 10-15°C, and the reaction mixture was stirred for 3 hours. The precipitated solid was collected by filtration, and the wet product was transferred to isopropyl acetate (50 mL) and stirred at room temperature for 24 hours. The white solid was collected by filtration, and then recrystallized twice from ethanol to obtain the L-lysine salt of (2S,3R)-3-acetylamino-2-hydroxy-4-phenylbutyric acid. 6.1 g of compound (13) was obtained by vacuum drying at 35 ~ 40 °C, with a yield of 37.7%. After detection, the obtained compound (13) was:
[0114] [α] = + 33.2°, m.p.: 130 ~ 130.5°C,
[0115] NMR (DMSO-d 6...
Embodiment 2
[0116] Example 2 : Preparation of the L-lysine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutanoic acid (13) (Method B)
[0117] The threo racemate (10.0 g, 42.1 mmol) of compound (7) was dissolved in isopropanol (250 mL), and after heating to 60-65 °C, L-lysine (2.46 g, 16.8 mmol) Dissolve in water (25 mL), add to the isopropanol solution of compound (7), heat to 60 ~ 65 ° C, stir for 30 minutes, then cool to 5 ~ 10 ° C, continue to stir the reaction mixture for 3 hours, pour The upper layer solution was removed, and the precipitated semi-solid was dispersed with acetone (50 mL). After stirring at room temperature for 48 hours, the crystals were collected by filtration and recrystallized twice with isopropanol. After vacuum drying at 35-40℃, 6.2 g of compound (13) was obtained with a yield of 38.4%. After detection, the obtained compound (13) was:
[0118] [α] = + 33.3°, m.p.: 130 ~ 130.5°C.
Embodiment 3
[0119] Example 3 : Preparation of the L-lysine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid (13) (Method C)
[0120] The threo racemate of compound (7) (10.0 g, 42.1 mmol) and L-lysine (6.16 g, 42.1 mmol) were dissolved in a mixed solvent system of 95% ethanol (200 mL) and acetonitrile (50 mL) , heated to 60-65°C, stirred for 30 minutes, then cooled to 10-15°C, and the reaction mixture was stirred for 3 hours. The precipitated solid was collected by filtration, and the wet product was transferred to isopropyl acetate (50 mL) and stirred at room temperature for 24 hours. The white solid was collected by filtration, and then recrystallized twice from ethanol to obtain the L-lysine salt of (2S,3R)-3-acetylamino-2-hydroxy-4-phenylbutyric acid. After vacuum drying at 35-40℃, 4.8 g of compound (13) was obtained with a yield of 29.7%. After detection, the obtained compound (13) was:
[0121] [α] = + 33.2°, m.p.: 130 ~ 130.5°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com