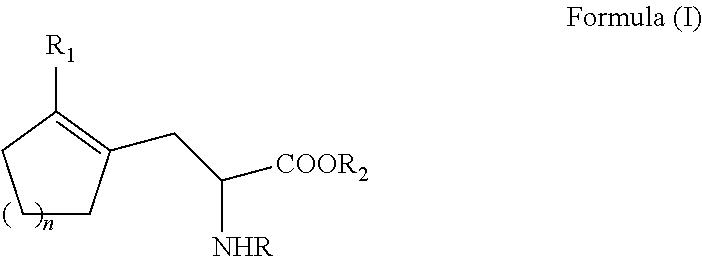
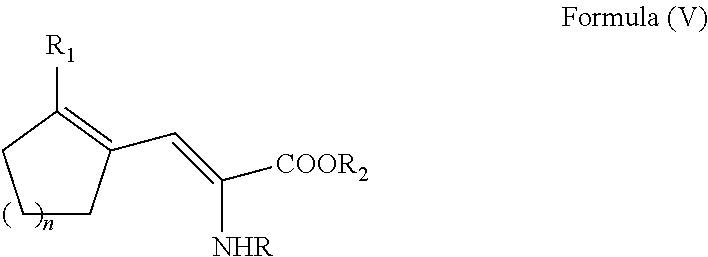
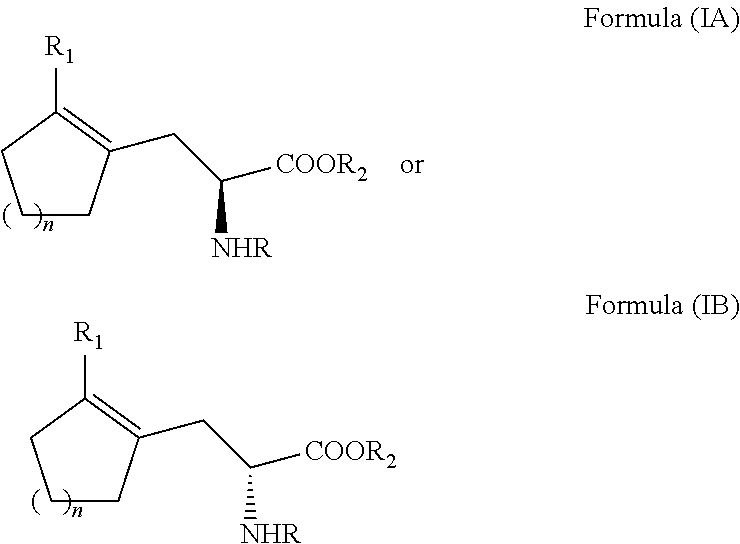
Enantioselective process for cycloalkenyl ?-substituted alanines
a technology of cycloalkenyl ?substituted alanines and selective process, which is applied in the preparation of carbonyl compounds, organic chemistry, carboxylic acid amides, etc., can solve the problems of waste of about half of the quantity of precursor materials and produce undesired isomers, and achieves easy conversion and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-chloro-1-formyl-1-cyclopentene (VIA)
[0056]
[0057]To a three-necked flask fitted with a stirrer, thermometer, reflux condenser, additional funnel, nitrogen inlet, and calcium chloride drying tube were added dimethylformamide (71.8 g, 0.9 mol) and 1,2-dichloroethane (150 mL). The resulting mixture was stirred under nitrogen and cooled to 5° C. with an external ice bath. Phosphorus oxychloride was added during approximately 1 hour through an additional funnel while the temperature of the stirred reaction mixture being maintained below 10° C. The mixture was then allowed to warm to room temperature. A solution of cyclopentanone (55.5 g, 0.66 mol) in 1,2-dichloroethane (100 mL) was added at such a rate that the temperature did not rise above 35° C. When the addition was completed, the mixture was heated at 55-60° C. for 3 hours. The reaction mixture was then cooled to below 35° C., and a solution of sodium acetate (240 g) in water (560 mL) was cautiously added through an ...
example 2
Preparation of 2-phenoxy-1-formyl-1-cyclopentene (VIB)
[0059]
[0060]To a solution of residue (100 g, 0.77 mol) obtained in example 1 in acetone (400 mL), phenol (79.3 g, 0.84 mol) and K2CO3 (126.8 g, 0.92 mol) were added. The mixture was stirred under nitrogen for 12 hours at room temperature and monitored by TLC (petroleum ether / ethyl acetate=10:1). After the reaction completed, the solvent was removed on a rotary evaporator, and water (300 mL) was added. The mixture was extracted with EtOAc (2×100 mL). The organic layer was washed with aqueous sodium carbonate (2×50 mL) and water (50 mL). After dried over sodium sulfate, the solvent was removed and 2-phenoxy-1-formyl-1-cyclopentene was obtained (155 g, contains some phenol), which was used directly in the next step.
example 3
Preparation of 4-((2-phenoxycyclopent-1-enyl)methylene-2-phenyloxzol-5(4H)-one (VIII)
[0061]
[0062]A mixture of 2-phenoxy-1-formyl-1-cyclopentene (150 g, obtained in example 2), acetic anhydride (196 g, 1.92 mol), sodium acetate (40 g, 0.48 mol) and benzoylglycine (125.7 g, 0.7 mol) was heated to 105-110° C. for about 1.5 h under nitrogen. The reaction was monitored by TLC (petroleum ether / ethyl acetate=15:1). After the reaction was completed, the mixture was cooled to 0° C., and then filtered. The cake was washed with methanol (2×150 mL) at room temperature. A brown to orange solid was obtained and dried in vacuum to afford 137 g product in 64.7% yield.
[0063]1H NMR (CDCl3, δ): 8.10-8.06 (m, 2H), 7.73-7.46 (m, 4H), 7.38-7.34 (m, 2H), 7.19-7.16 (m, 1H), 7.04-7.02 (m, 2H), 3.13 (t, 2H, J=7.2), 2.46 (t, 2H, J=7.6), 2.10-2.00 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| hydrogen pressure | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com