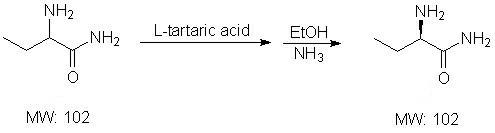
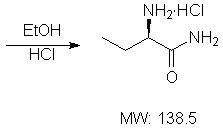
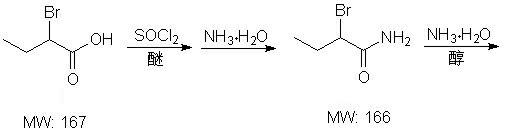Method for synthesizing intermediate L-2-aminobutyrylamide hydrochloride of chiral drug levetiracetam
A technology of aminobutanamide and chiral drugs, which is applied in the field of preparation of chiral drug intermediates, can solve the problems of safety, three-waste treatment and high environmental protection requirements, long reaction steps, influence on process safety and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 1000ml reaction flask, add 83.5g of 2-bromobutyric acid, 125g of 28% ammonia water and 500ml of diethyl ether, cool down to -5°C, slowly add 59.5g of thionyl chloride dropwise under stirring, keep stirring and react after dropping 10 hours. After the reaction, the layers were separated, the organic layer was washed with water, and concentrated to dryness to obtain 73 g of white solid 2-bromobutyramide with a yield of 88% and a purity of 98.5%.
Embodiment 2
[0027] In a 2000ml reaction flask, add 167g of 2-bromobutyric acid, 250g of 28% ammonia water and 1000ml of methyl tert-butyl ether, cool down to 0°C, slowly add 119g of thionyl chloride dropwise under stirring, and naturally The temperature was raised to 20°C, and the reaction was carried out under heat preservation and stirring for 2 hours. After the reaction was complete, the layers were separated, the organic layer was washed with water, and concentrated to dryness to obtain 143 g of white solid 2-bromobutyramide with a yield of 86% and a purity of 98.9%.
Embodiment 3
[0029] In a 2000ml reaction flask, add 167g of 2-bromobutyric acid, 250g of 28% ammonia water and 1000ml of methyl tert-butyl ether, cool down to 0°C, slowly add 119g of thionyl chloride dropwise under stirring, and naturally Raise the temperature to 10°C, heat and stir for 4 hours. After the reaction was complete, the layers were separated, the organic layer was washed with water, and concentrated to dryness to obtain 148 g of white solid 2-bromobutyramide, with a yield of 89.2% and a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com