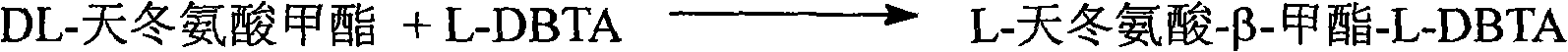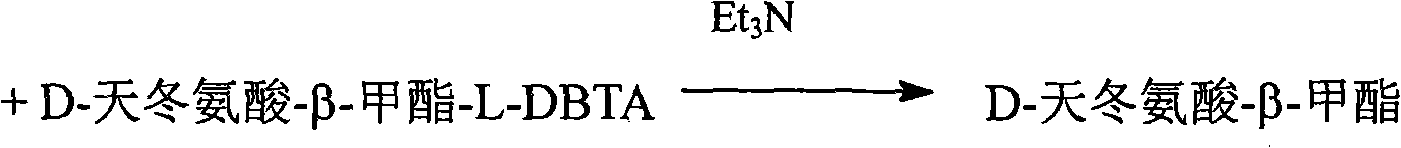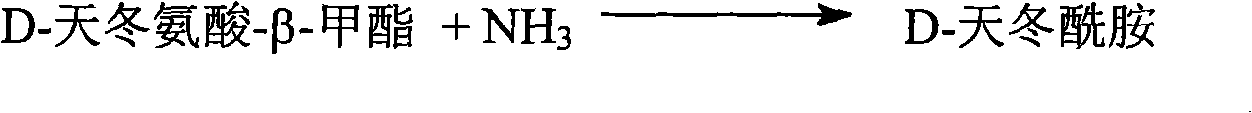Method for preparing D-asparagine and D-homoserine
A technology of homoserine and asparagine, which is applied in the preparation of cyanide reaction, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of high cost, long route, unsuitable for large-scale production, etc., and achieves environmental pollution. The effect of small size, simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Take 15g of DL-aspartic acid-β-methyl ester into a three-necked flask, add 50-100mL of water, raise the temperature to 40-95°C, and add L-dibenzoyl tartaric acid (L-DBTA, 18.8g) into it The methanol solution was cooled to room temperature after reacting for 1 hour, and filtered to obtain 21.7 g of D-aspartic acid-β-methyl ester·L-DBTA, with a yield of 86%.
Embodiment 2
[0047] Take 30g of D-aspartic acid-β-methyl L-DBTA, put it in a 250mL three-neck bottle, add 30-150mL of ethanol and 17mL of triethylamine, stir and react at room temperature for 2-4 hours, filter to obtain a white powder Solid D-aspartic acid methyl ester 6.58g, yield 74.8%.
Embodiment 3
[0049] Add 10g of D-aspartic acid-β-methyl ester and 50-300mL of methanol into the three-neck flask, stir well and then feed NH 3 Gas, the temperature is controlled at -5 ~ 15 ° C for 1-3 hours, after rising to room temperature, continue to feed NH 3 Gas for 2 to 3 hours, stand in a water bath at 30°C for 48 hours, then concentrate to dryness under reduced pressure; dissolve the residue in 30ml of distilled water, add 150ml of methanol to precipitate, freeze and crystallize, and filter to obtain 4.8g of D-asparagine . Dissolve 4.g of crude D-asparagine in hot distilled water, add a small amount of activated carbon for decolorization, add 150ml of methanol under stirring, and filter to obtain 4.1g of white orthorhombic crystalline D-asparagine, with a total yield of 49.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com