Vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation
A technology for trifluoromethanesulfonic acid and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations containing vilanterol trifluoromethanesulfonate, can solve problems such as long action time, improve health status, reduce the number of acute exacerbations, clinical efficacy and good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
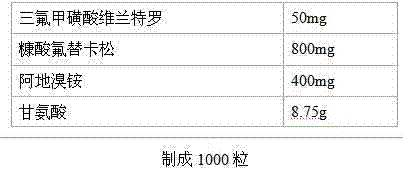
[0032] Vilanterol triflate / fluticasone furoate aclidinium bromide granules / tablets / capsules Prescription:
[0033]
[0034] Preparation steps: mix the main ingredient, lactose, and croscarmellose sodium uniformly in equal amounts, prepare a soft material with 3% PVP k30 as a binder, granulate with a 20-mesh sieve, dry at 60°C, and dry at 20-mesh The granules are obtained by sieving and granulating. Add the prescribed amount of magnesium stearate after granulation, mix uniformly and compress to obtain tablets or fill capsules to obtain capsules.
Embodiment 2
[0036] Vilanterol triflate / fluticasone furoate aclidinium bromide orally disintegrating tablets:
[0037]
[0038] Preparation steps: mix the main ingredient and auxiliary materials uniformly by the equal-volume incremental method, pass through a 100-mesh sieve, and directly compress into tablets.
Embodiment 3
[0040] Vilanterol triflate / fluticasone furoate aclidinium bromide extended-release tablets prescription:
[0041]
[0042] Preparation steps: mix the main ingredient, microcrystalline cellulose, and hypromellose evenly in equal amounts, use 0.5% hydroxypropyl cellulose as a binder to prepare a soft material, granulate with a 20-mesh sieve, and dry at 60°C , 20-mesh sieve, adding magnesium stearate in prescribed amount, mixing evenly, and compressing into tablets to obtain sustained-release tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com