Pulse-controlled release tablet containing fluticasone furoate and vilanterol and preparation method thereof
A technology of fluticasone furoate and controlled-release tablets, which is applied in the field of pulse controlled-release tablets containing fluticasone furoate and vilanterol and its preparation, which can solve problems such as complicated operation steps, toxic and side effects of preparations, and difficulty in achieving inhalation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
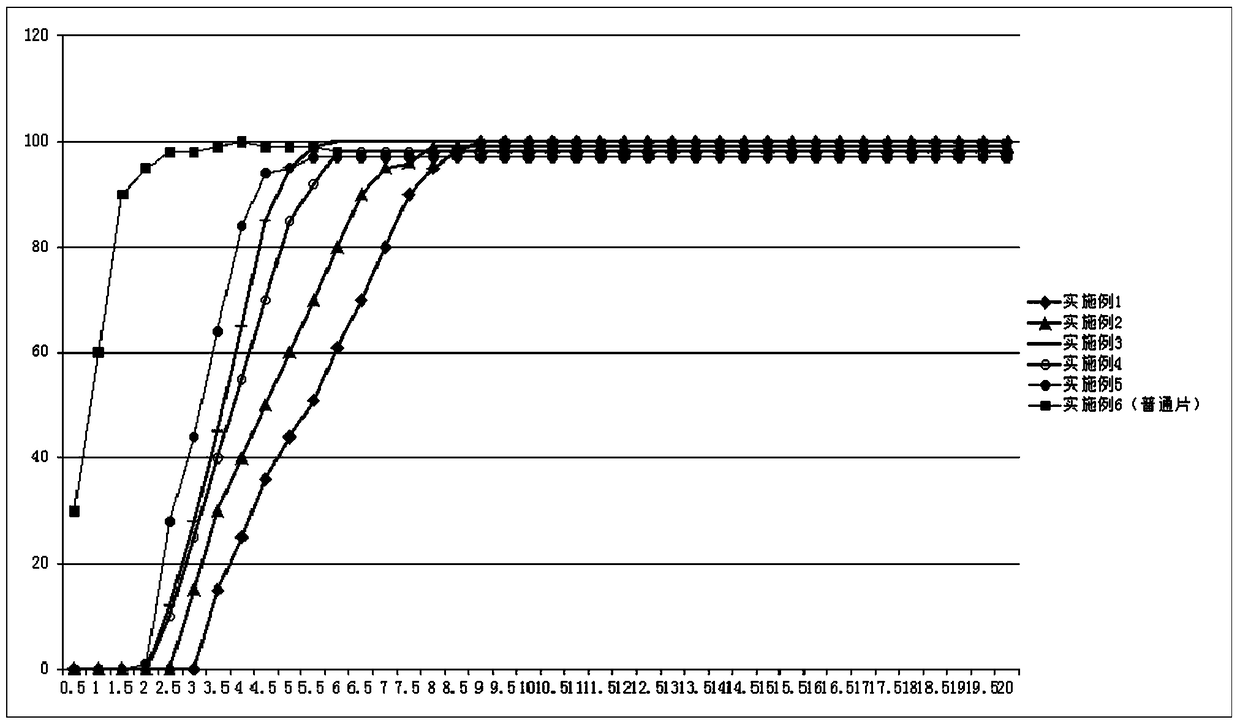
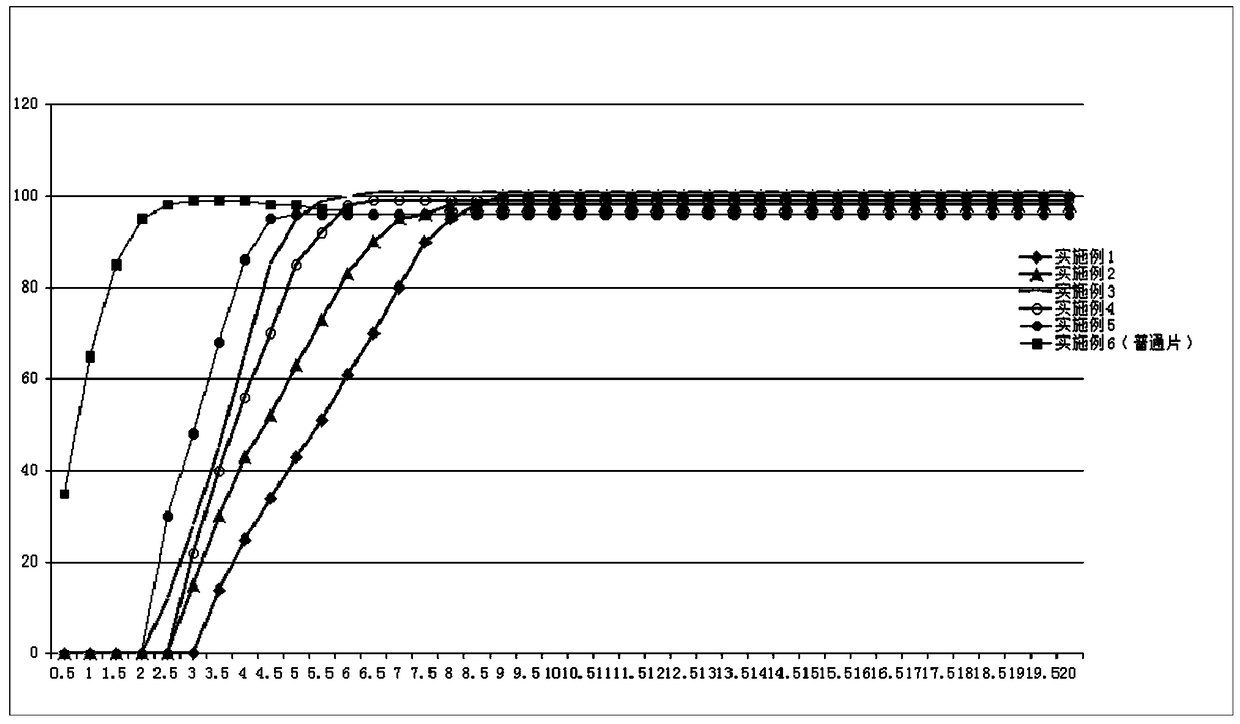
Image
Examples
Embodiment 1
[0034] Example 1. Formulation and preparation of pulse controlled-release tablets of fluticasone furoate and vilanterol
[0035] 1. Tablet core prescription (20000 tablets)
[0036] a. Prescription for immediate release: 10.0g fluticasone furoate, 2.0g vilanterol, 20.0g low-substituted hydroxypropyl cellulose, 200.0g lactose, 2.0g povidone K30.
[0037] b. Prescription for controlled release: 20.0g fluticasone furoate, 10.0g vilanterol, 30.0g low-substituted hydroxypropyl cellulose, 250.0g lactose; 40.0g Eudragit L100.
[0038] C. Lubricant: 3.0g magnesium stearate
[0039] 2. Sponge prescription
[0040] 800.0 g hydroxypropyl methylcellulose (HPMC), 2200.0 g lauryl alcohol, 20.0 g magnesium stearate.
[0041] 3. Preparation method:
[0042] (1) Preparation of tablet core
[0043] a. Preparation of immediate-release granules: mix fluticasone furoate, vilanterol, low-substituted hydroxypropyl cellulose, and lactose evenly, spray 2% povidone k30 aqueous solution on the powd...
Embodiment 2
[0050] Example 2. Formulation and preparation of pulse controlled-release tablets of fluticasone furoate and vilanterol
[0051] 1. Tablet core prescription (20000 tablets)
[0052] a. Prescription for immediate release: 20.0g fluticasone furoate, 4.0g vilanterol, 20.0g croscarmellose sodium, 100.0g mannitol, 100.0g microcrystalline cellulose, 3.0g povidone k30.
[0053] b. Prescription for controlled release: 30.0g fluticasone furoate, 16.0g vilanterol, 40.0g croscarmellose sodium, 100.0g mannitol, 200.0g microcrystalline cellulose, 45.0g Kollicoat MAE30DP.
[0054]c. Lubricant: 4.0g magnesium stearate sodium fumarate
[0055] 2. Sponge prescription
[0056] 1000.0g Sodium Alginate, 3200.0g Myristyl Alcohol, 24.0g Magnesium Stearate Sodium Fumarate.
[0057] 3. Preparation
[0058] (1) Preparation of tablet core
[0059] a. Preparation of immediate-release granules: mix fluticasone furoate, vilanterol, croscarmellose sodium, mannitol, and microcrystalline cellulose, and ...
Embodiment 3
[0066] Example 3. Formulation and preparation of pulse-controlled release tablets of fluticasone furoate and vilanterol
[0067] 1. Tablet core prescription (20000 tablets)
[0068] a. Prescription for immediate release: 30.0g fluticasone furoate, 10.0g vilanterol, 10.0g sodium starch glycolate, 10.0g low-substituted hydroxypropyl cellulose, 150.0g mannitol, 150.0g lactose, 4.0g hydroxy Propyl methylcellulose.
[0069] b. Prescription for controlled release: 40.0g fluticasone furoate, 20.0g vilanterol, 44.0g sodium starch glycolate, 200g mannitol, 100g microcrystalline cellulose, 100g lactose, 50.0g KollicoatMAE100P.
[0070] c. Lubricant: 4.0g magnesium stearate, 2.0g talc.
[0071] 2. Sponge prescription
[0072] 1000.0g sodium alginate, 200.0g hydroxyethyl cellulose, 5600.0g cetyl alcohol, 30.0g magnesium stearate.
[0073] 3. Preparation
[0074] (1) Preparation of tablet core
[0075] a. Preparation of immediate-release granules: mix fluticasone furoate and vilanter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com