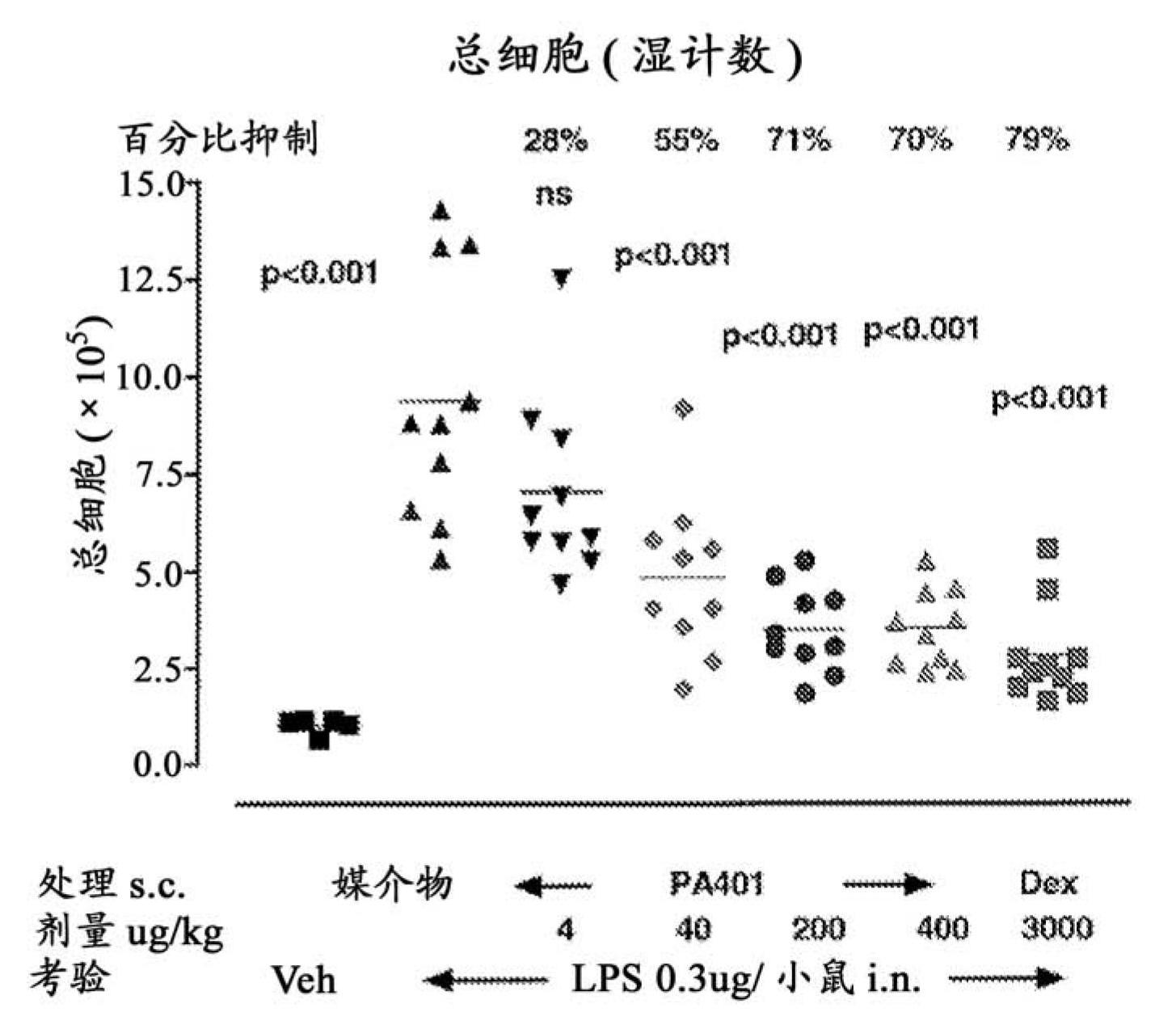
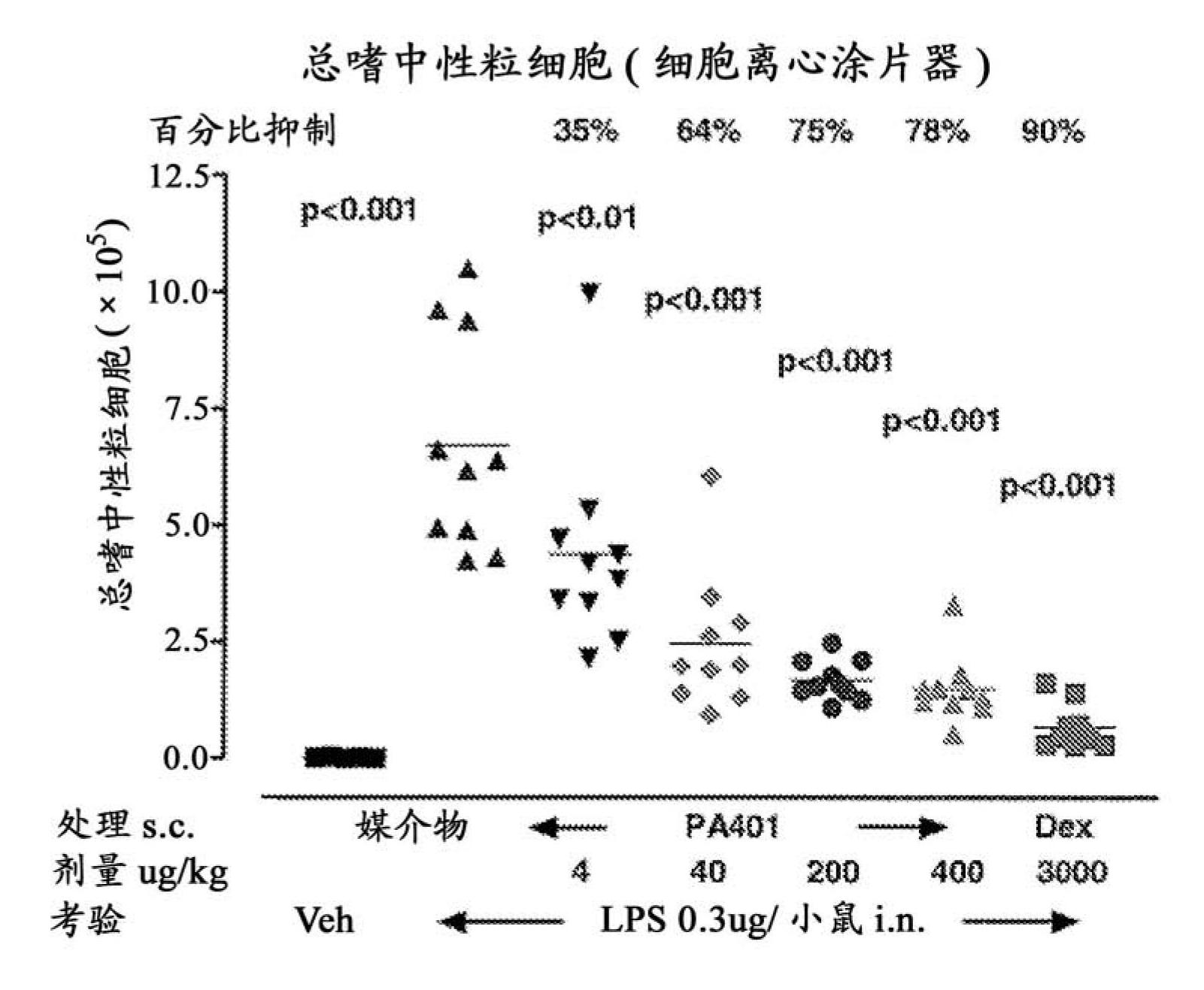
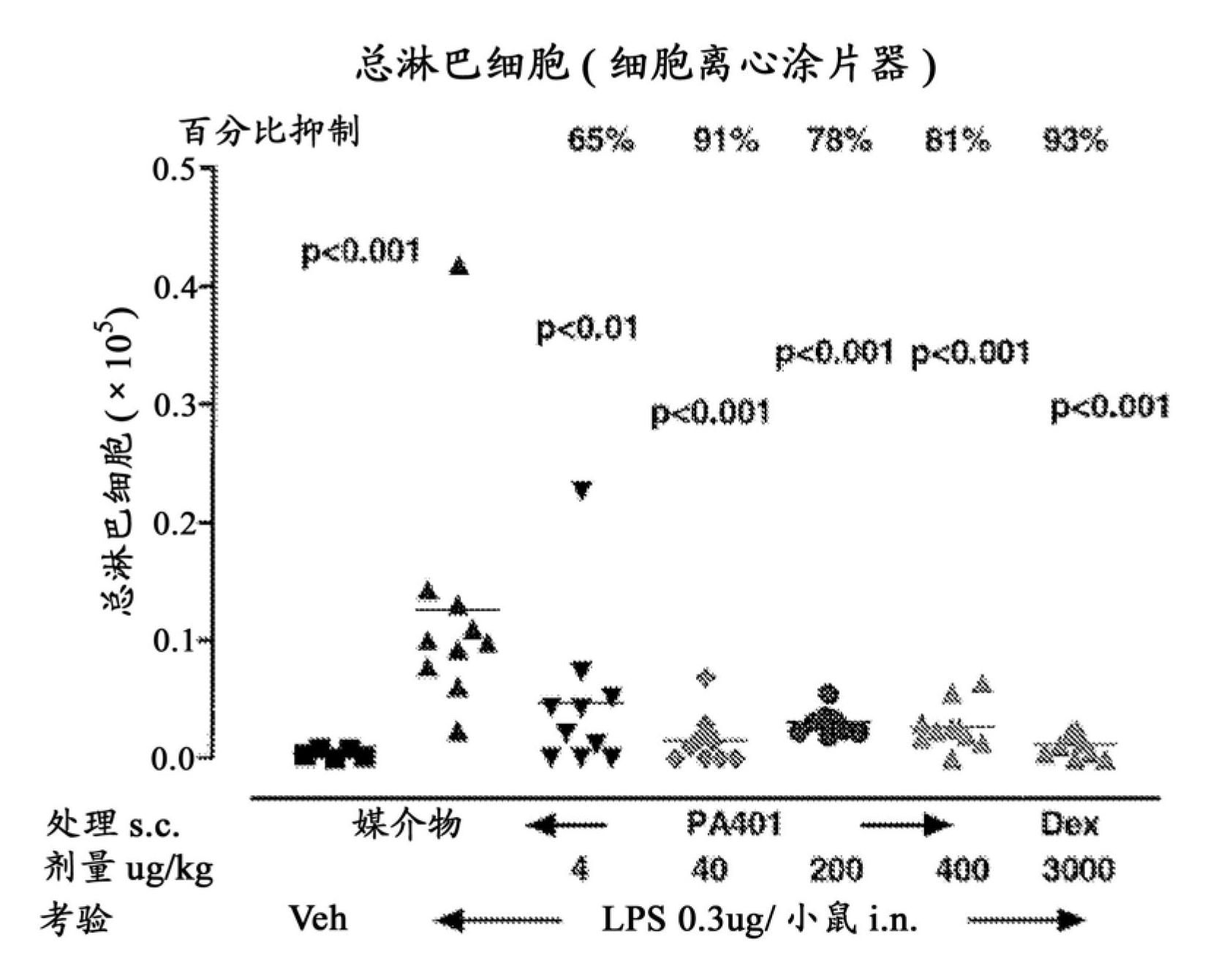
Patents
Literature
37 results about "Severe asthma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The symptoms of severe asthma are similar to the symptoms of mild to moderate asthma. But severe asthma symptoms tend to be more intense, potentially life-threatening, and are difficult to control with asthma treatments. Signs and symptoms of severe asthma may include: shortness of breath that continues to worsen. pain or tightness in your chest.
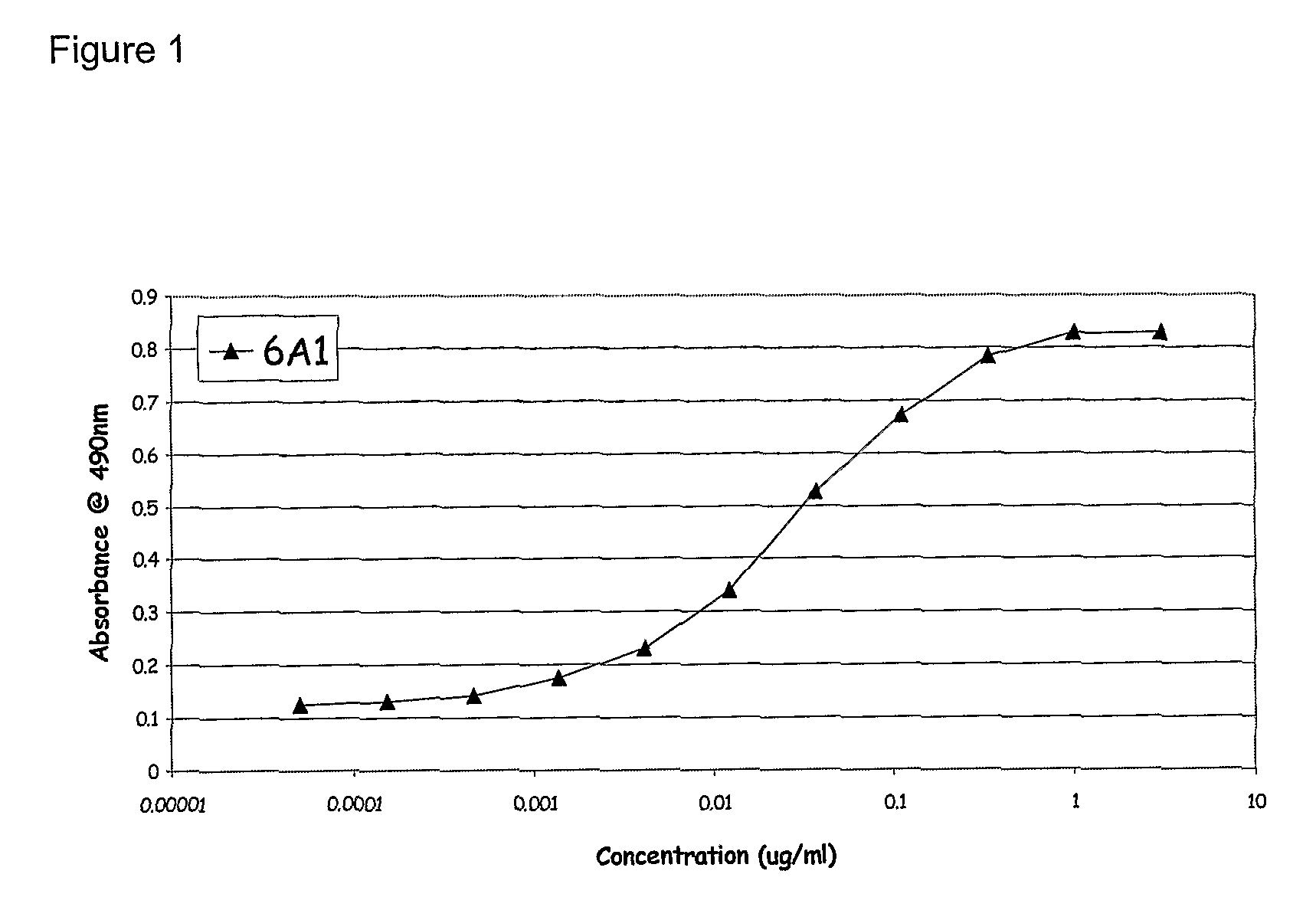
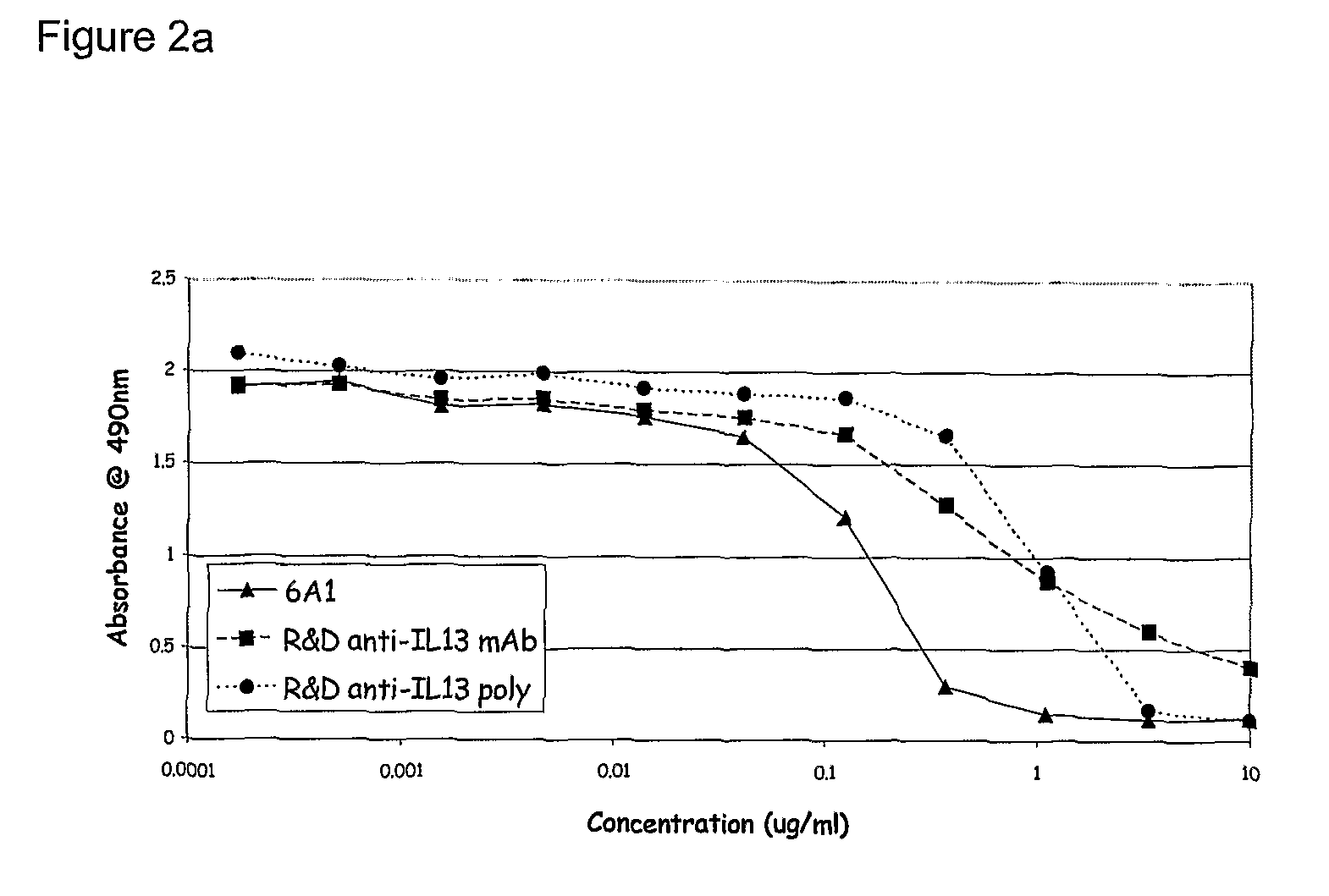
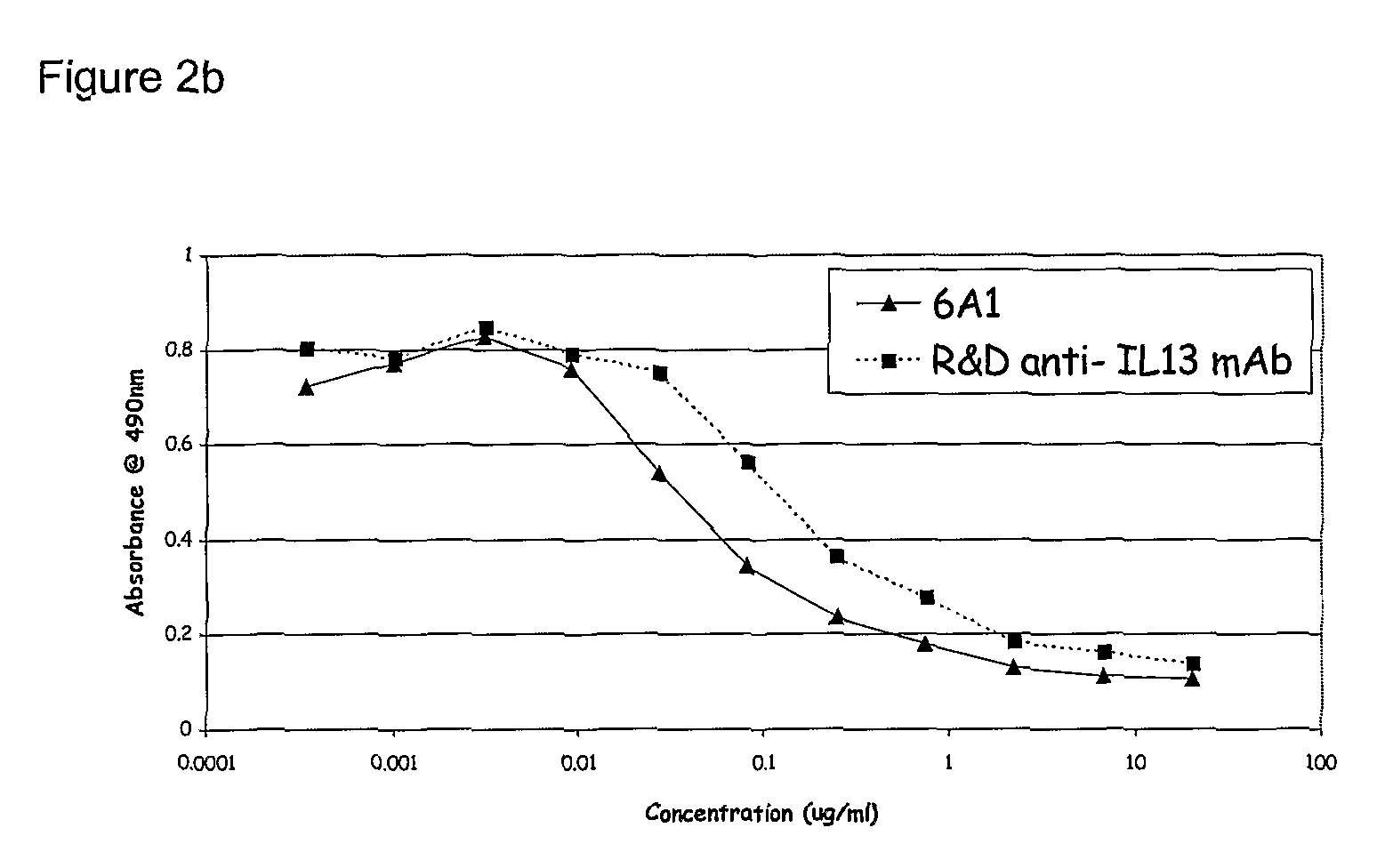
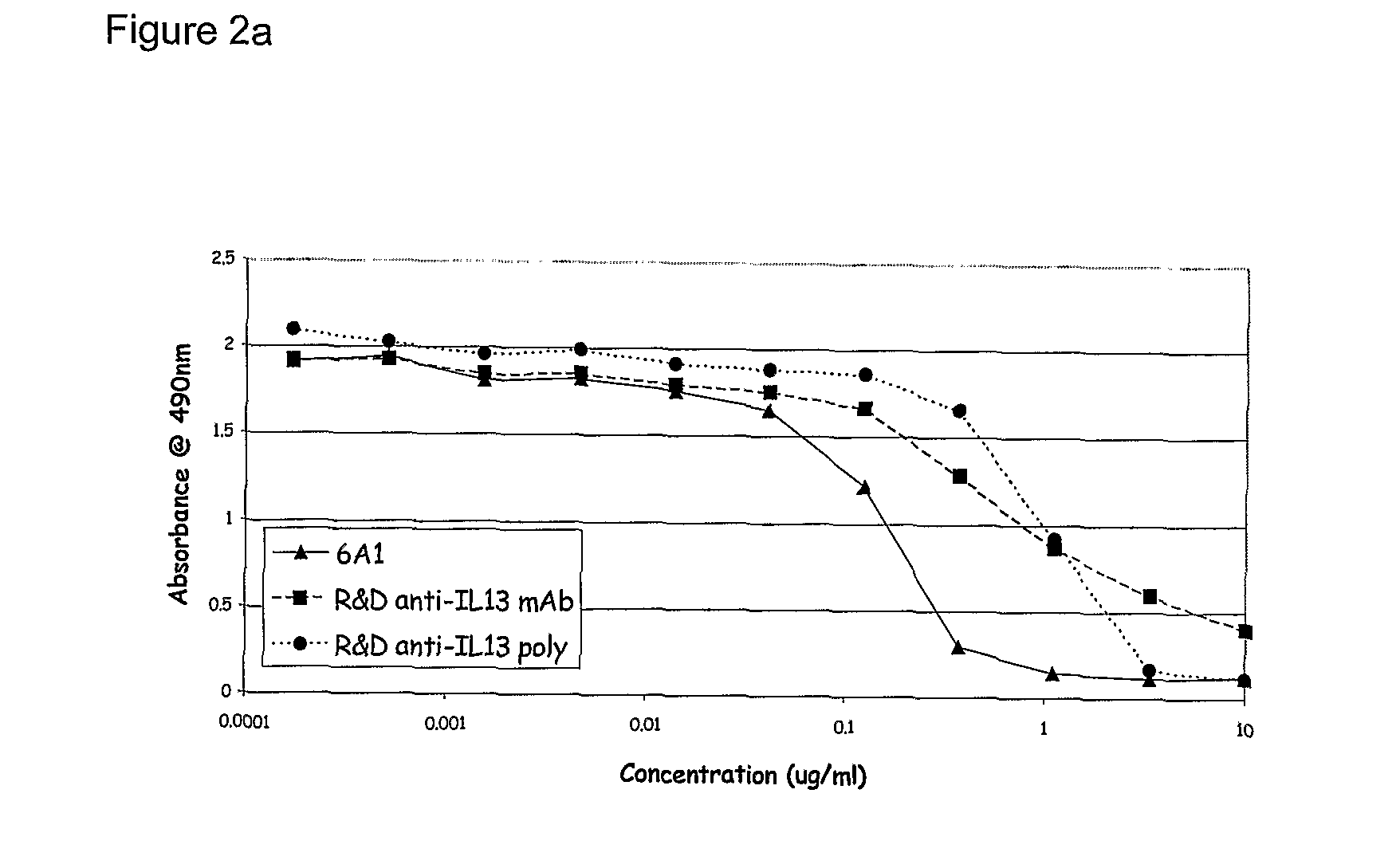
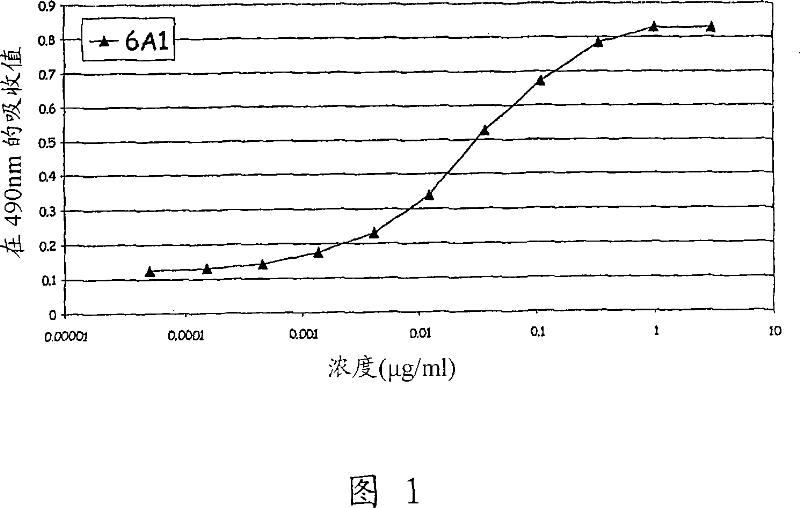
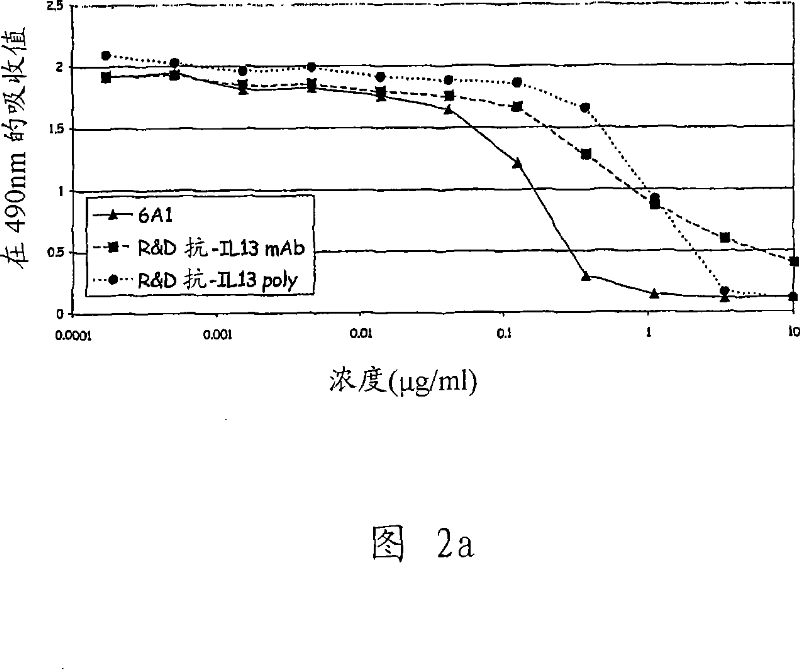
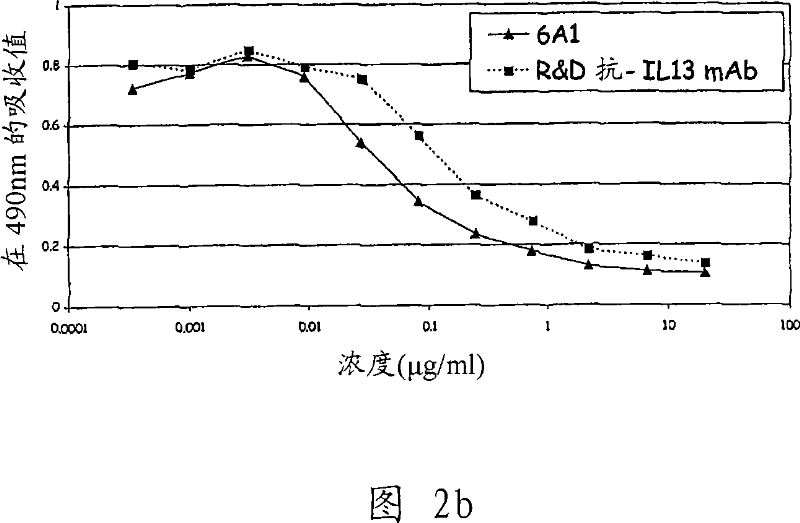
Chimeric and humanised monoclonal antibodies against Interleukin-13
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GRP LTD
Classification of medical diagnostic images
InactiveUS20110280457A1Image enhancementImage analysisParenchymal lung diseaseClassification methods
The invention provides methods for automated classification of a medical diagnostic image of a lung according to its deduced probability of relating to a lung of a patient who is suffering from a diffuse parenchymal lung disease such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, or severe asthma, or to a class of patients characterised by the severity of such a condition, or to a class of patients characterised by a prognostic likelihood of developing such a condition or severity of condition.
Owner:UNIVERSITY OF COPENHAGEN
Chimeric and Humanised Monoclonal Antibodies Against Interleukin-13
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GROUP LTD
Classification of medical diagnostic images
InactiveUS8811724B2Image enhancementImage analysisParenchymal lung diseaseObstructive Pulmonary Diseases
The invention provides methods for automated classification of a medical diagnostic image of a lung according to its deduced probability of relating to a lung of a patient who is suffering from a diffuse parenchymal lung disease such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, or severe asthma, or to a class of patients characterized by the severity of such a condition, or to a class of patients characterized by a prognostic likelihood of developing such a condition or severity of condition.
Owner:UNIVERSITY OF COPENHAGEN
Composition for treatment of CXCL8-mediated lung inflammation
InactiveCN102596227AHigh binding affinityElevated GPCR activityPeptide/protein ingredientsAntipyreticObstructive Pulmonary DiseasesARDs - Acute respiratory distress syndrome
Owner:PROTAFFIN BIOTECHNOLOGIE AG
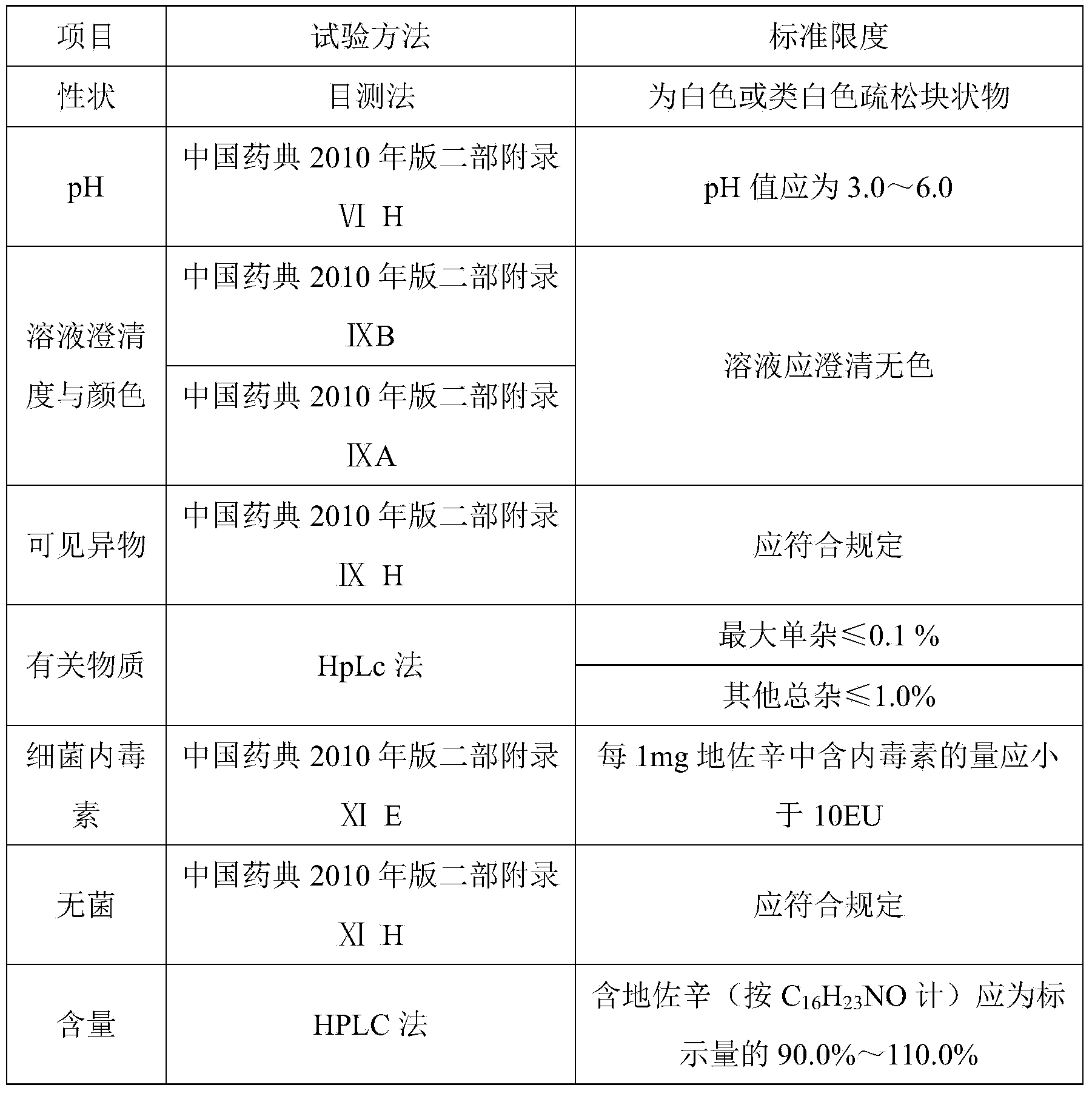
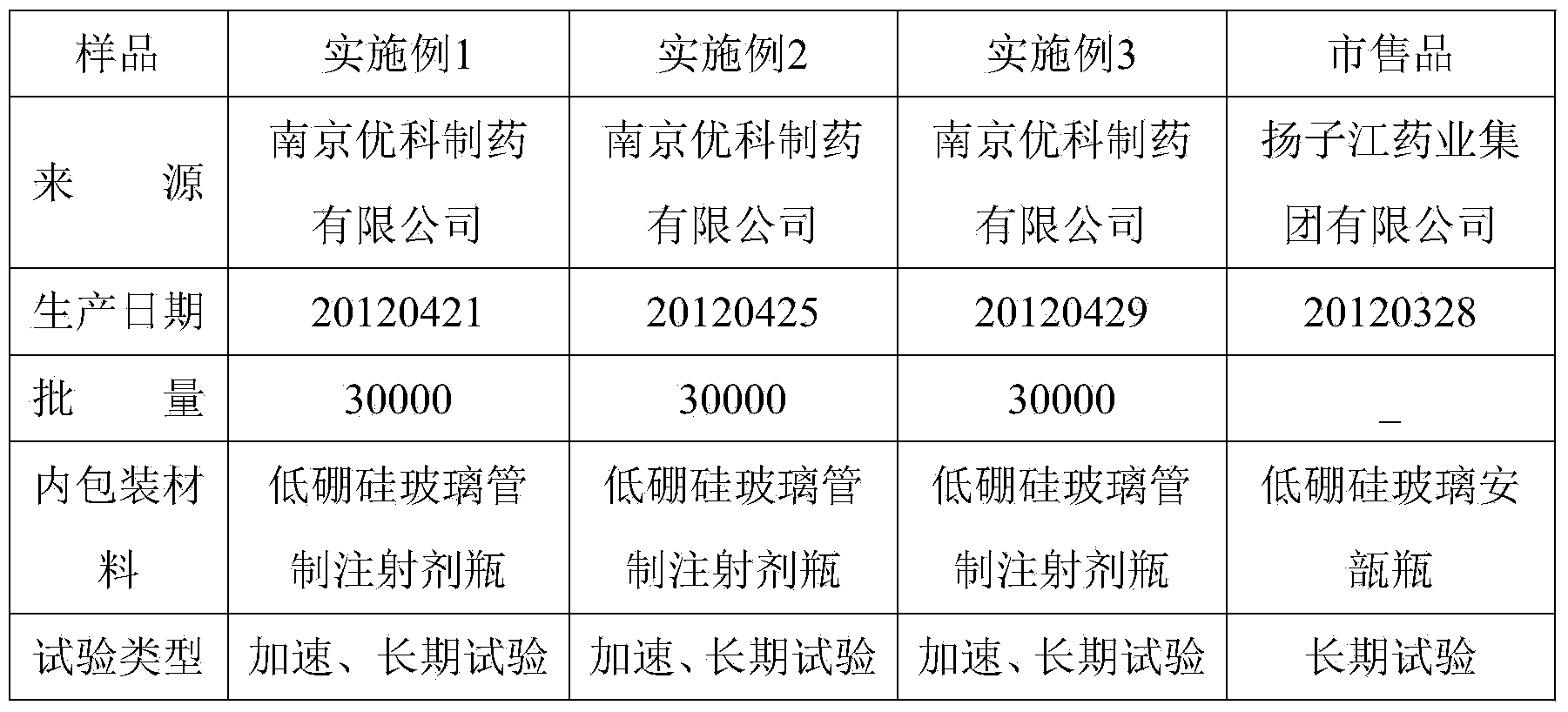
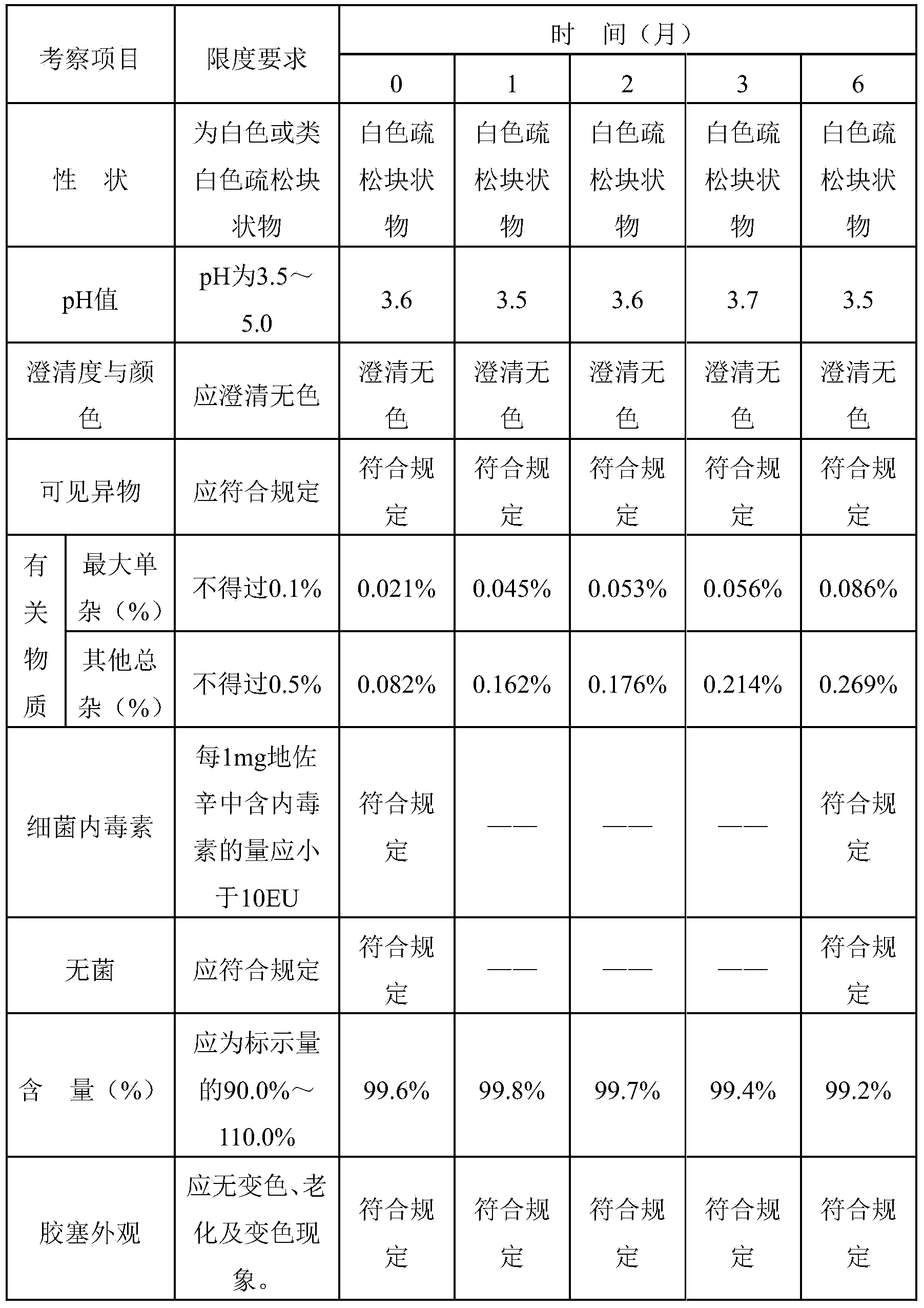
Dezocine freeze-dried powder injection and preparation method thereof
InactiveCN104224734AAvoid safety hazardsSubstance lessOrganic active ingredientsPowder deliveryAntioxidantFreeze-drying
The invention discloses dezocine freeze-dried powder injection. The dezocine freeze-dried powder injection consists of the following components by dosage: 0.5-50mg of dezocine / per injection, 1-25% of a freeze-dried excipient and 0.5-50mg of an antioxidant / per injection, wherein the pH value of the freeze-dried solution is regulated to 3.0-6.0 by use of a pH regulator. Compared with the commercially available preparation, the dezocine freeze-dried powder injection has the advantage that the auxiliary material components are not added with sodium pyrosulfite which is taken as the antioxidant, and the sulfate possibly causes fateful anaphylactic reaction and severe asthma for a susceptible person, so that certain potential safety hazards caused by the sulfate are avoided. The commercially available preparation is added with 30% of propylene glycol as an anti-freezing agent, and made into freeze-dried powder injection in which a special solvent is not needed for separation under a low temperature condition. In a preparation process of the dezocine injection, high-temperature sterilization is needed, and associated substances in the sterilization process are suddenly increased. Compared with the injection, the dezocine freeze-dried powder injection contains less associated substances without being sterilized at the high temperature; and meanwhile, the injection solids are easier to store than the liquid, so that the increase of the associated substances is less, and thus, additional risks are reduced for clinical application.
Owner:NANJING YOKO PHARMA GRP CO LTD
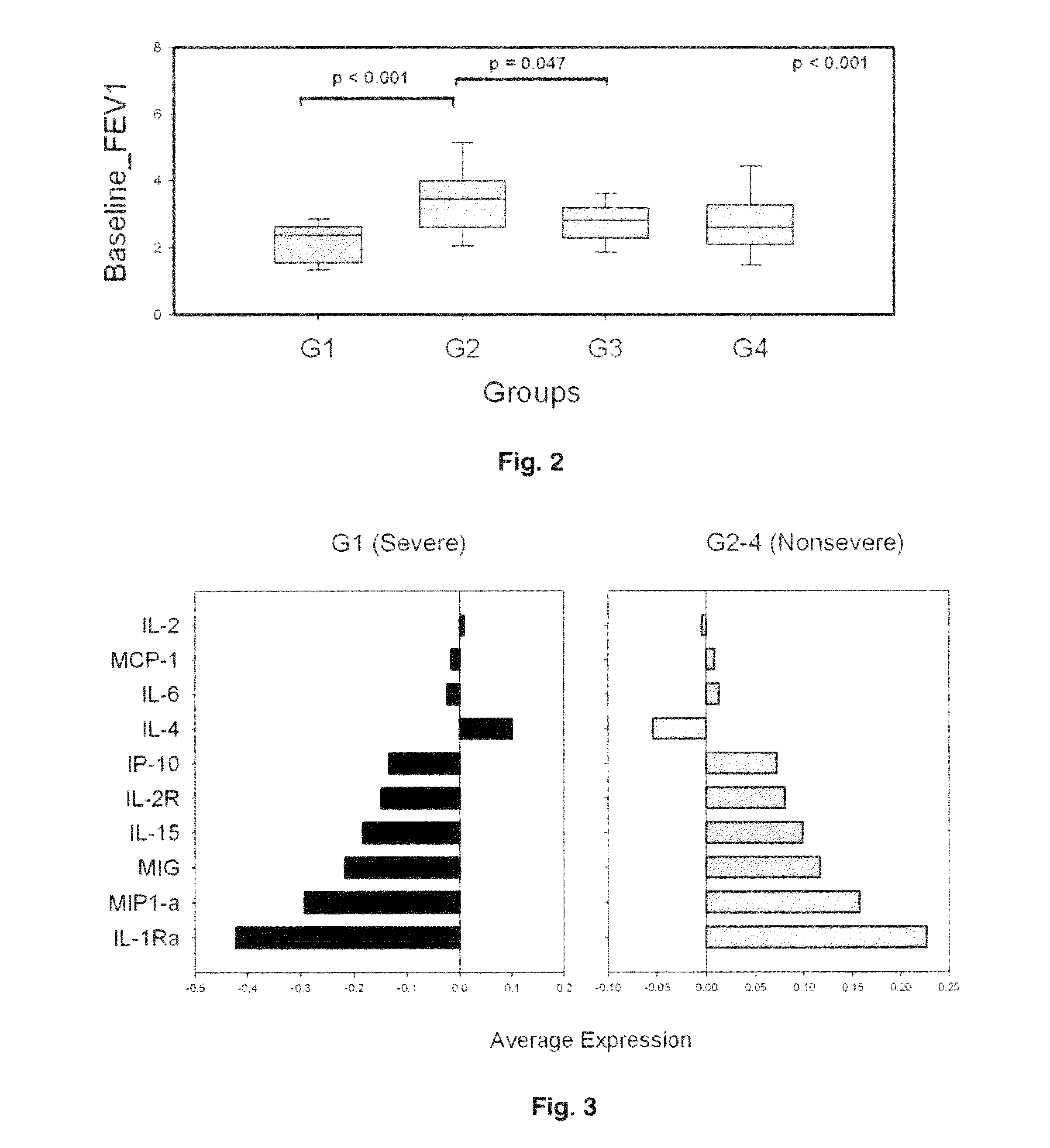
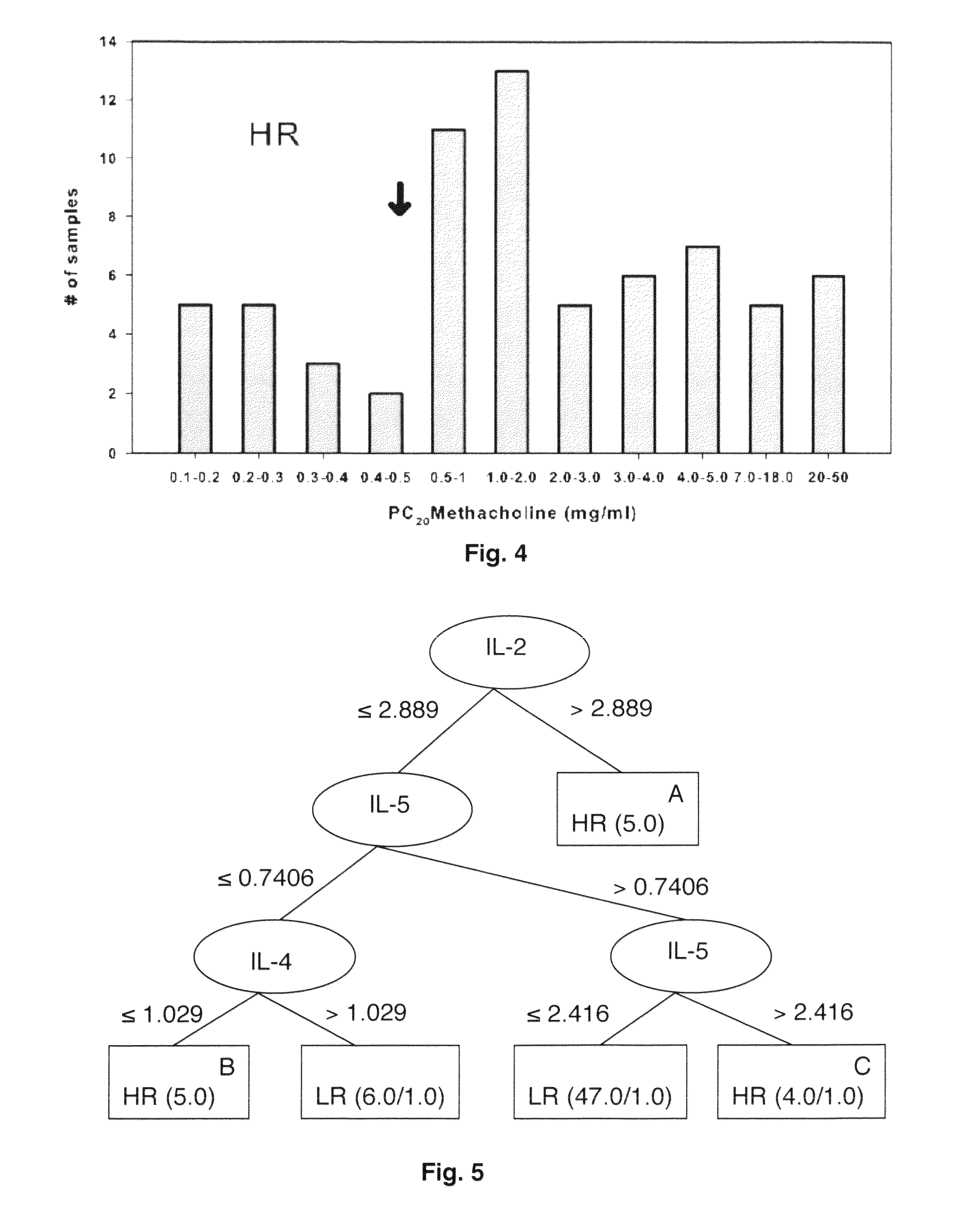
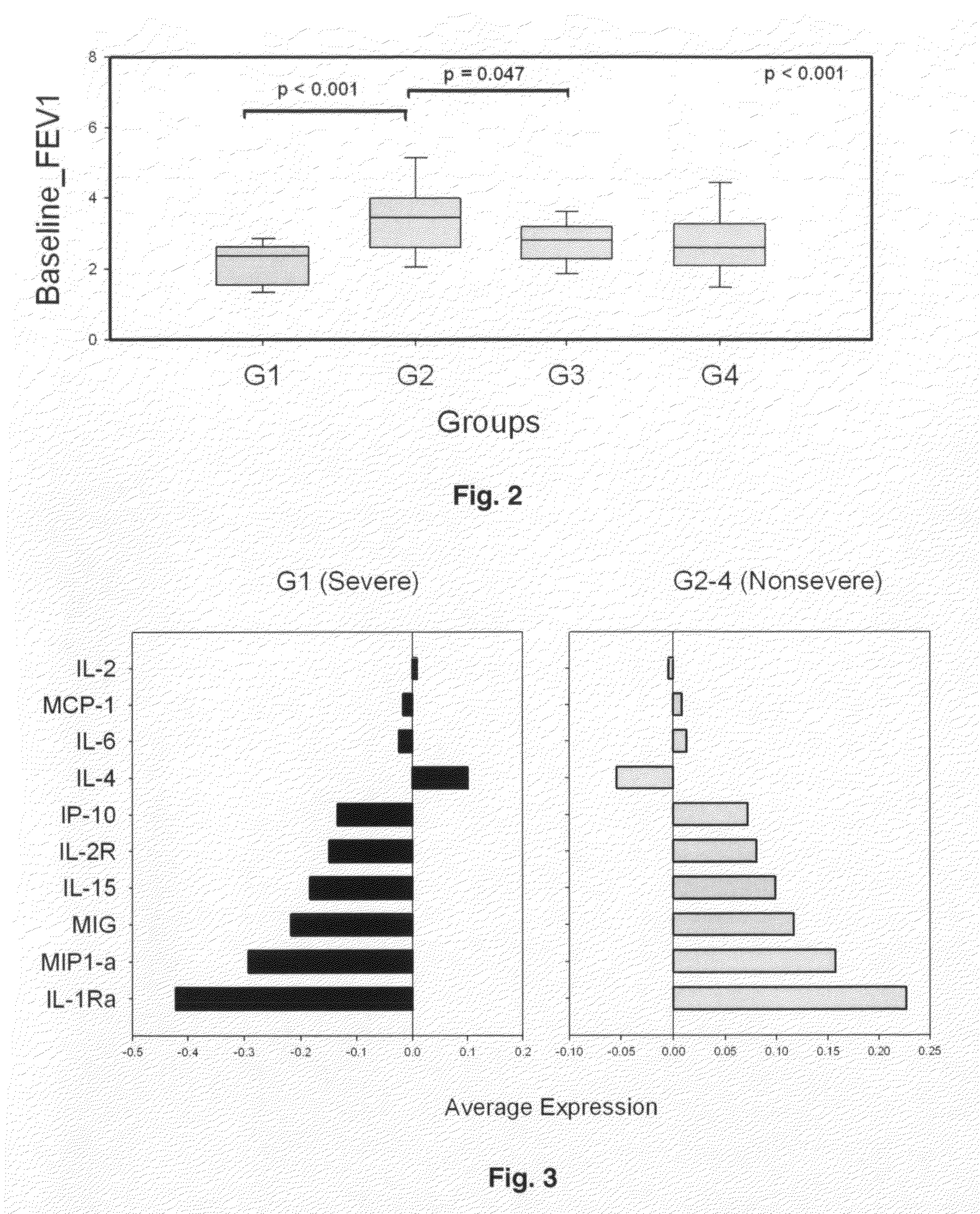
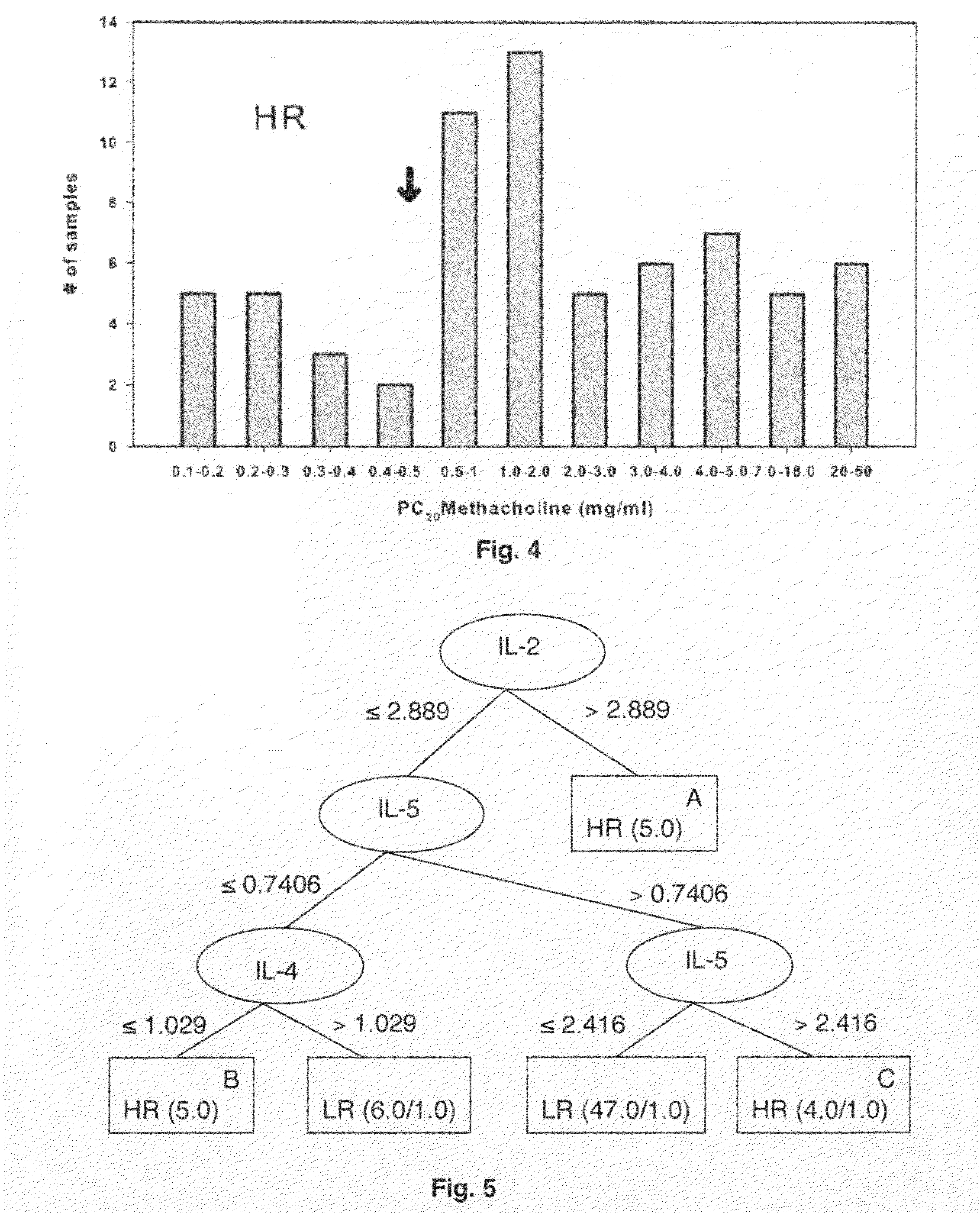
Molecular phenotyping of severe asthma
ActiveUS8053199B2Convenient investigationMicrobiological testing/measurementDisease diagnosisIntensive treatmentMedicine
The present invention discloses a method for classifying individuals into those who have airway hyperreactvitiy and those with asthma based on cytokine expression patterns. It is contemplated that such a method will enable rapid identification of individuals requiring intensive treatment for asthma, thereby reducing morbidity and improving quality of life for those affected.
Owner:BRASIER ALLAN R +2
Asthma resuscitation system and method
A resuscitation system for the administration of cardiopulmonary resuscitation of asthma patients, and for teaching the cardiopulmonary resuscitation of asthma patients. The invention includes a deflatable bag and a gas flow channel connected with said bag, for connection with an indwelling endotracheal tube so that gas can flow from the bag into the patient and from said patient through said flow channel, an exhalation port in flow connection with said flow channel and an indicator mounted adjacent said system for detecting expiration flow and / or pressure within at least one of said flow channel and exhalation port to detect inadequacy in the expiratory component of ventilation during CPR and to train healthcare workers in the emergency ventilation of severe asthmatic patients in the field and emergency room.
Owner:LYNN LAWRENCE A
Molecular phenotyping of severe asthma
ActiveUS20090068689A1Facilitate clinical investigationConvenient investigationMicrobiological testing/measurementDisease diagnosisIntensive treatmentMedicine
The present invention discloses a method for classifying individuals into those who have airway hyperreactvitiy and those with asthma based on cytokine expression patterns. It is contemplated that such a method will enable rapid identification of individuals requiring intensive treatment for asthma, thereby reducing morbidity and improving quality of life for those affected.
Owner:BRASIER ALLAN R +2
Chimeric and humanised monoclonal antibodies against interleukin-13
InactiveCN101039960AImmunoglobulins against cytokines/lymphokines/interferonsImmunological disordersDiseaseCOPD
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GROUP LTD
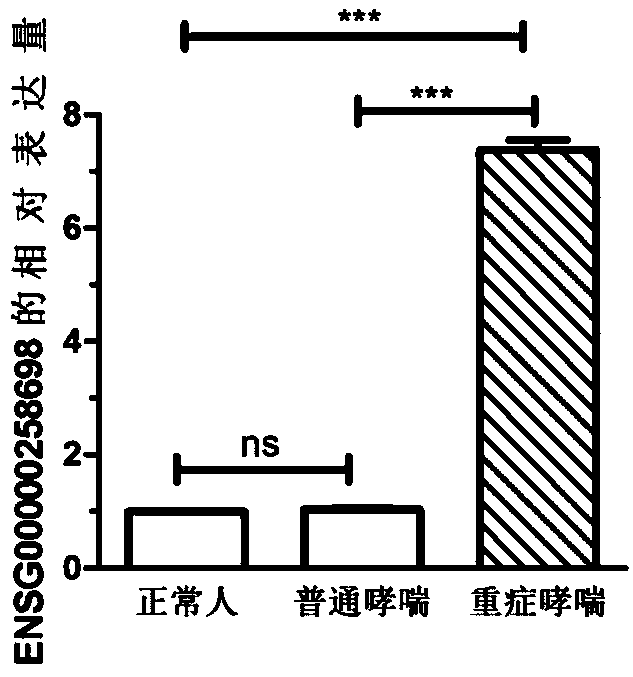
Molecular marker for diagnosing severe asthma
InactiveCN109439744AAchieve early diagnosisImprove the quality of lifeMicrobiological testing/measurementRespiratory disorderSevere asthmaMolecular diagnostics
The invention discloses a molecular marker for diagnosing severe asthma. The molecular marker is ENSG00000258698. The invention discloses that the expression of the ENSG00000258698 is up-regulated insevere asthma patients; the expression level of the ENSG00000258698 is detected to judge whether subjects have the severe asthma or not, so that early-period molecular diagnosis of the severe asthma is realized.
Owner:CHANGZHOU NO 2 PEOPLES HOSPITAL
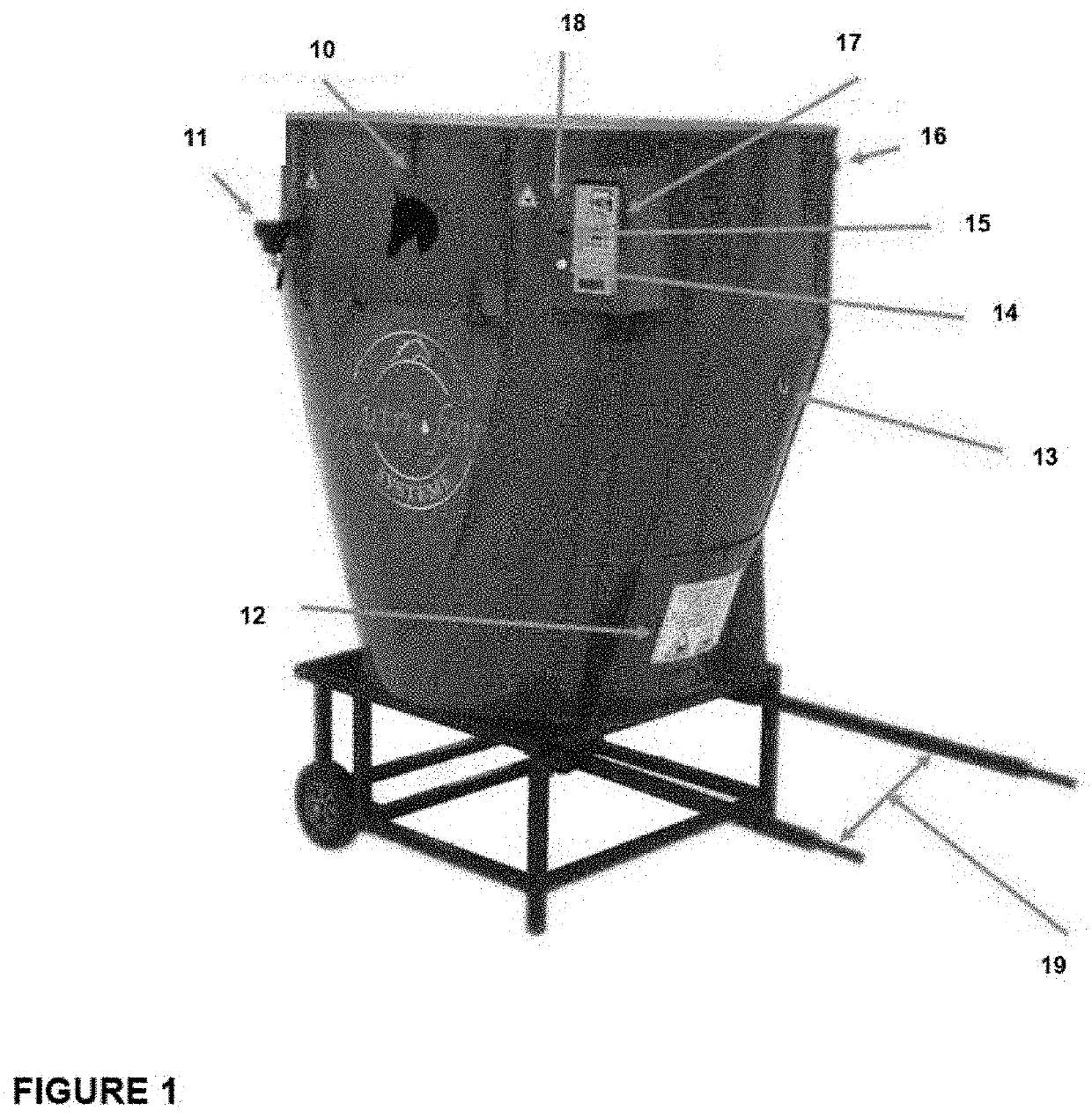
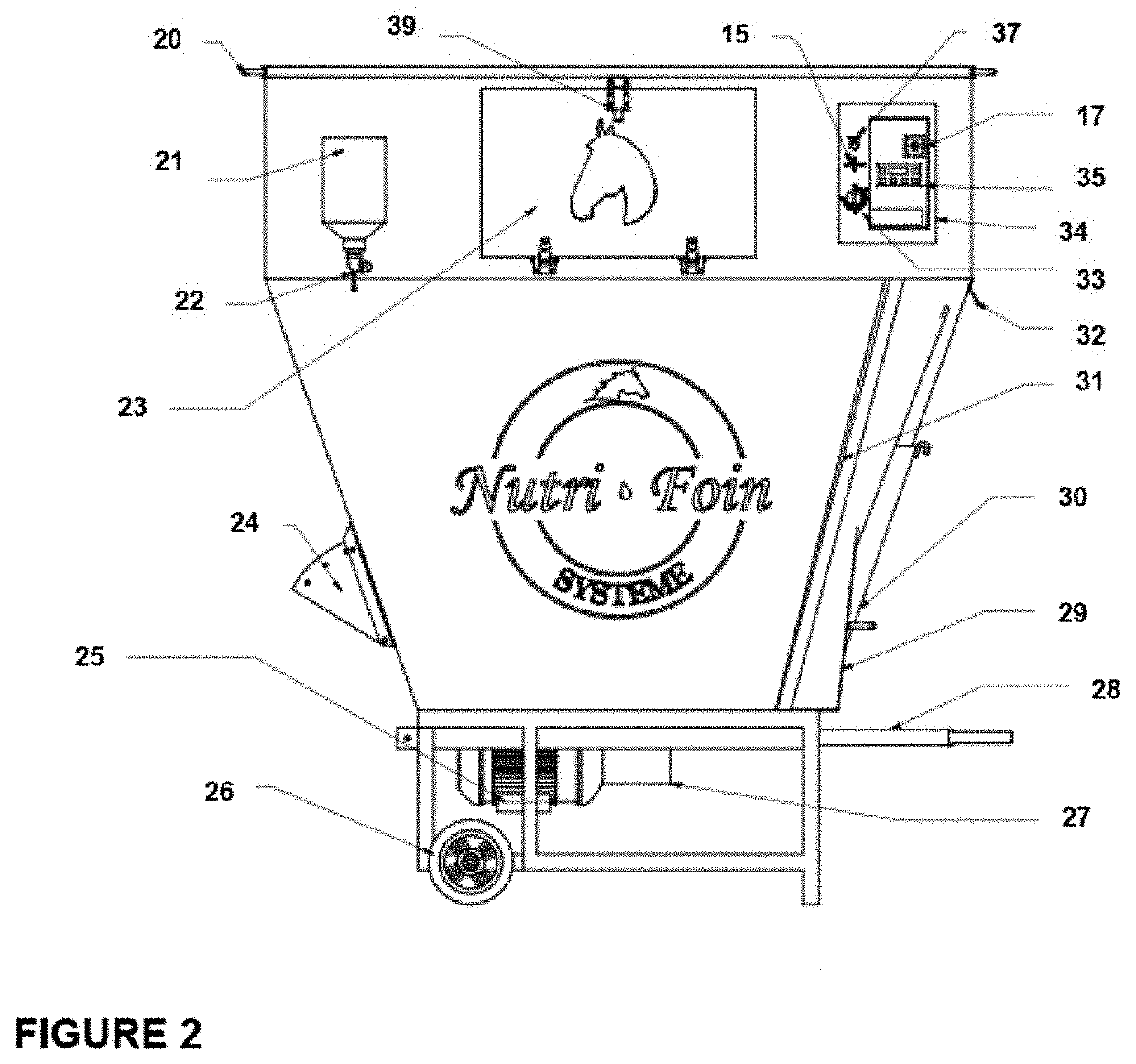
Hay-based material free of respirable dust emission: process for its preparation, use and associated machine
A process for preparing a hay-based material for feeding a horse is provided. The process comprises chopping / blending together hay and a binding agent or dust-trapping agent. A machine for conducting the process of the invention is also provided. The hay-based material of the invention may be used to feed horses to improve their general health, particularly horses with severe asthma.
Owner:NUTRI FOIN SYST INC
Method for preparing nanometer traditional Chinese medicine for treating severe asthma and production method thereof
InactiveCN102100814AHighlight substantive featuresSignificant progressAnthropod material medical ingredientsPharmaceutical delivery mechanismImmunityPharmaceutical drug
The invention relates to a method for preparing a nanometer traditional Chinese medicine for treating severe asthma and a production method thereof. The nanometer traditional Chinese medicine comprises the following raw materials in part by weight: 10 to 20 parts of radish seed, 10 to 20 parts of shizandra berry, 15 to 30 parts of malaytea scurfpea, 6 to 12 parts of common threewingnut root and the like. The production process comprises the following steps of: ultrasonic extracting, microwave extracting, supersonic crushing, nanometer grinding, mixing, filling and the like. In the method, the nanometer traditional Chinese medicine has the synergetic and multi-targeting effects of nanometer medicaments. Products have the effects of concurrent treatment of lung, spleen, kidney and liver, tonification and purgation in combination and the like by treatment methods of warming yang, keeping strong and healthy, replenishing qi to invigorate spleen, warming lung to eliminate cold, resolving sputum, relieving asthma, dispelling dampness, resolving sputum, tonifying kidney, holding qi and the like. The nanometer traditional Chinese medicine has good effect of treating the severe asthma, can enhance the immunity in human bodies and does not have toxic or side effects.
Owner:SUZHOU ZHIWEITANG BIOLOGICAL TECH +1
Composition for treatment of cxcl8-mediated lung inflammation
InactiveUS20120288474A1Increased GAG binding affinityInhibitory activityPeptide/protein ingredientsAntipyreticInterleukin 8ALI - Acute lung injury
The present invention provides a composition comprising a modified interleukin 8 (IL-8) having increased GAG binding affinity and further inhibited or down-regulated GPCR activity compared to the respective wild type IL-8 for use in preventing or treating lung inflammation with neutrophilic infiltration, for example for the prevention or treatment of chronic obstructive pulmonary disease, cystic fibrosis, severe asthma, bronchitis, broncheolitis, acute lung injury and acute respiratory distress syndrome.
Owner:PROTAFFIN BIOTECHNOLOGIE AG
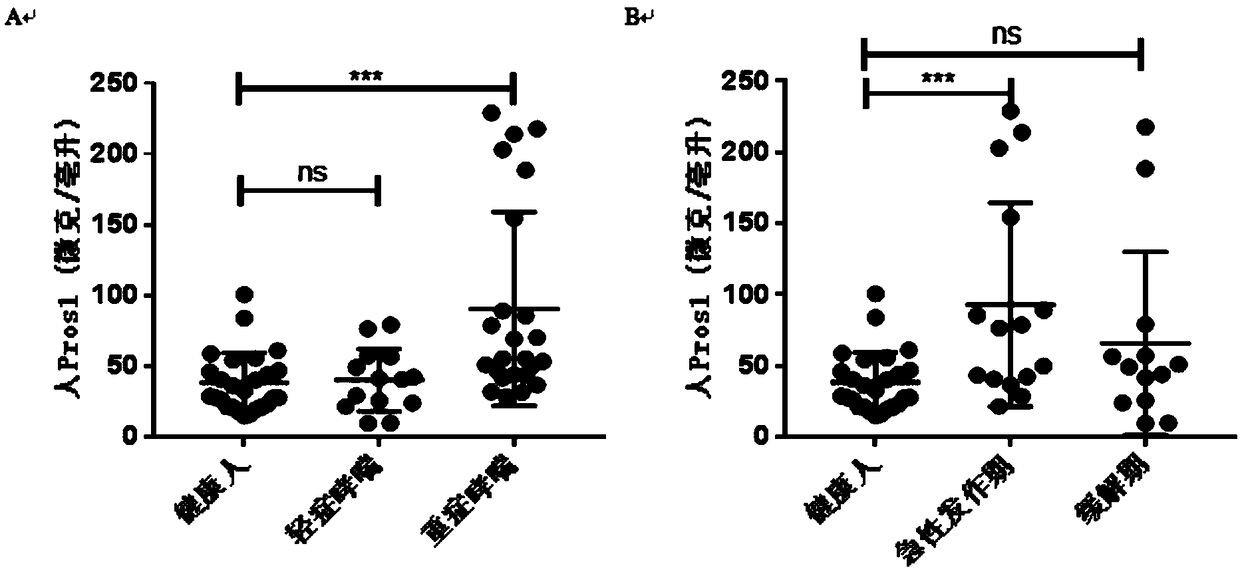
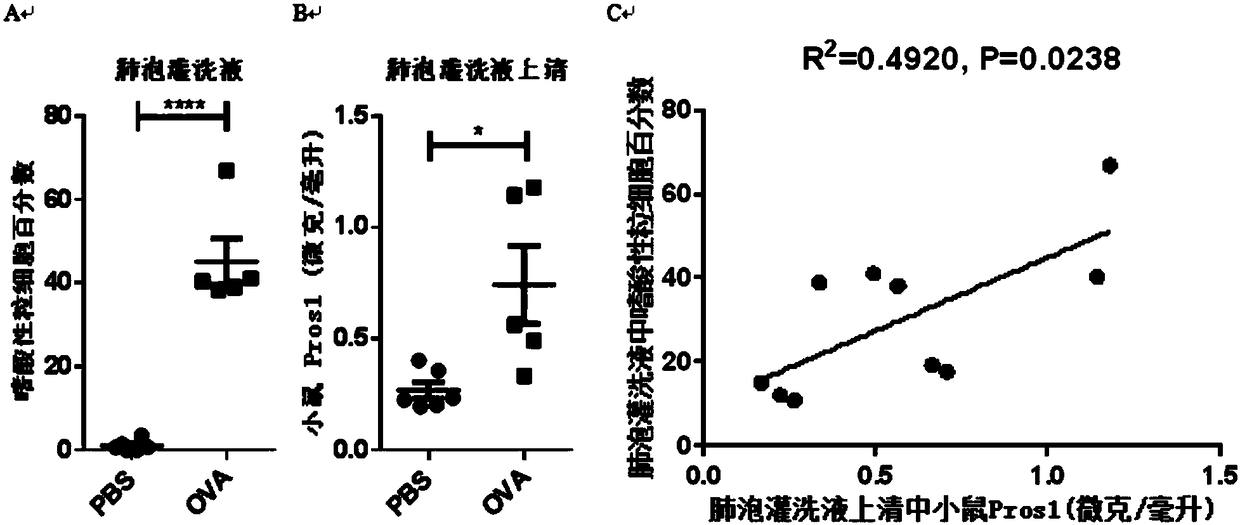
Application of protein S as biological marker in preparation of reagent for diagnosis of asthma and evaluation of prognosis effect
InactiveCN108546751AGood correlationMicrobiological testing/measurementDisease diagnosisDiseaseProtein S
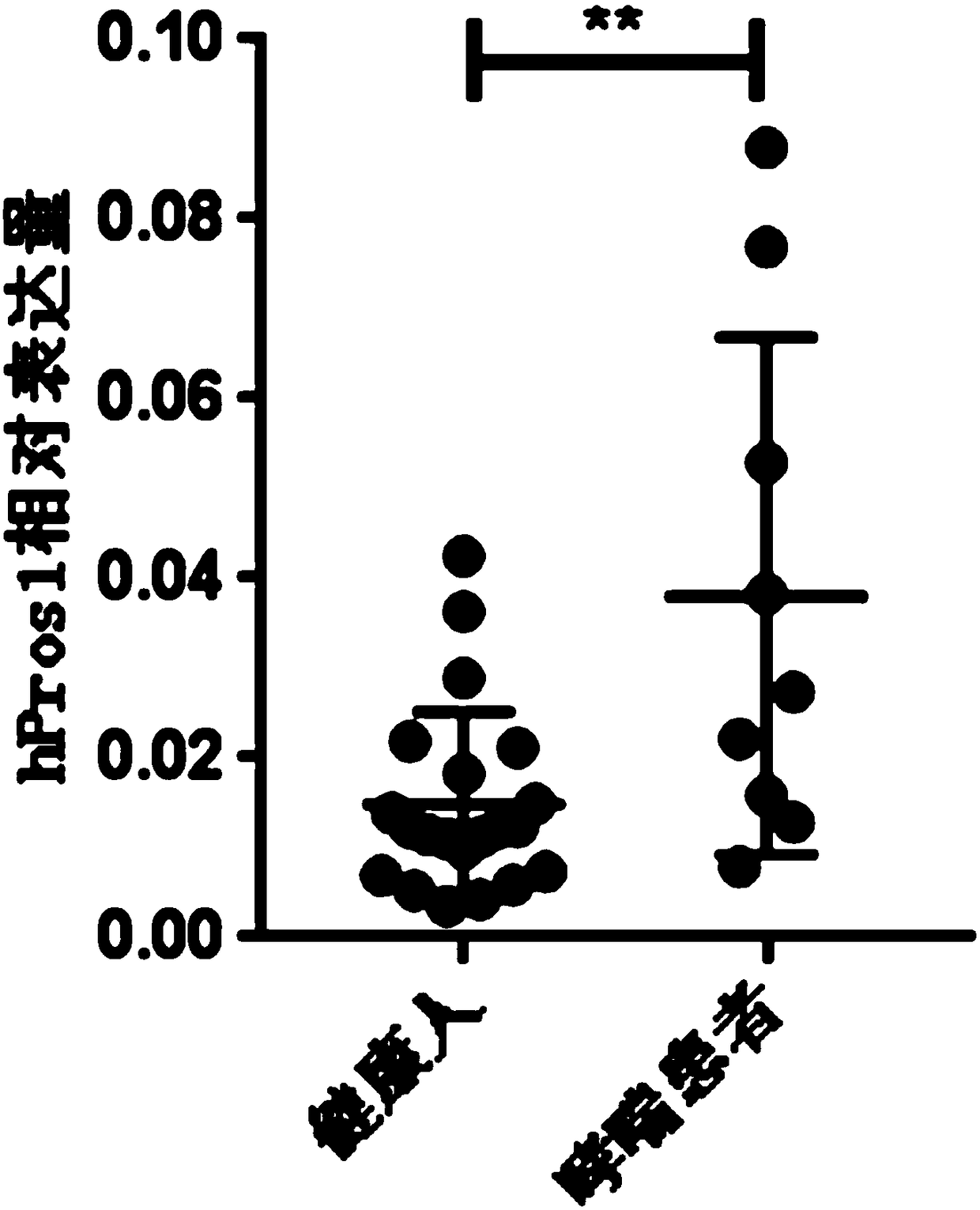
The invention relates to the field of biological medicines and discloses application of protein S (Pros1) as a biological marker in preparation of a reagent for diagnosis of asthma and evaluation of the prognosis effect. The inventors find that the RNA level of Pros1 in peripheral blood of patients suffering from asthma is obviously higher than that of healthy people; further, the ELISA detectionis further performed on Pros1 in peripheral blood of patients suffering from mild and severe asthma and healthy people and finds that the protein level of Pros1 in peripheral blood of patients suffering from severe asthma is obviously higher than that of healthy people; and furthermore, for patients suffering from asthma, the protein level of Pros1 in peripheral blood of patients in the acute stage is higher than that of patients in the remission stage, so that the protein S can serve as the biological marker for diagnosis of asthma and evaluation of the prognosis effect.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
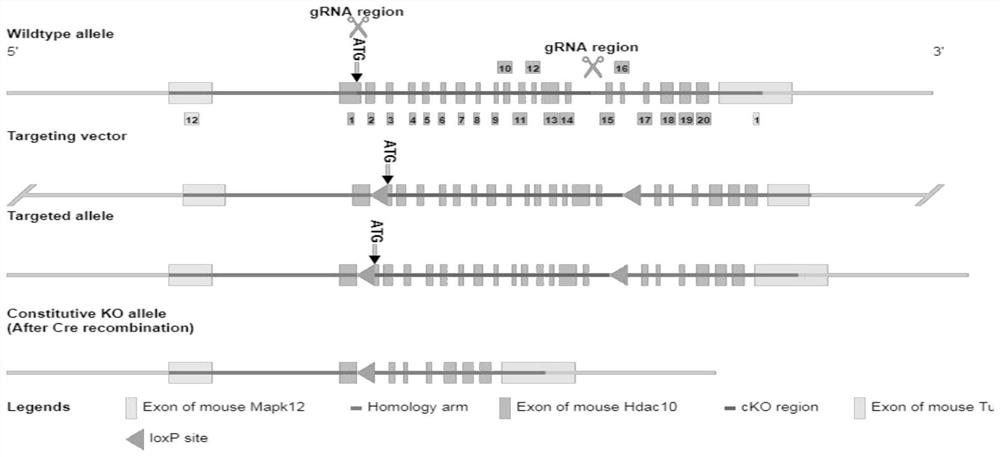
Medicine for relieving severe asthma, application and animal model construction method
PendingCN113018439AIncrease transcriptional activityImprove airway inflammationRespiratory disorderPharmaceutical active ingredientsIntraperitoneal routeAirway hyperreactivity
The invention belongs to the technical field of severe asthma action mechanisms, and discloses a medicine for relieving severe asthma, application and an animal model construction method, the medicine is an HDAC10 inhibitor, and the action mechanism of HDAC10 for regulating and controlling Th17 / IL-17A expression in severe asthma is clarified by using the construction method; the interaction mode of HDAC10 and STAT3 is defined; it is proved that HDAC10 deacetylated STAT3 regulates and controls the expression of Th17 / IL-17A in severe asthma. it is determined that the HDAC10 inhibitor can relieve severe asthma, and an experimental basis is provided for HDAC10 targeted therapy of severe asthma. it is determined that the HDAC10 inhibitor can relieve severe asthma, and an experimental basis is provided for HDAC10 targeted therapy of severe asthma. Compared with a control group, the intraperitoneal injection of the HDAC10 inhibitor can improve airway inflammation, airway hyperreactivity and airway mucus secretion of a severe asthma mouse model mainly comprising neutrophile granulocytes.
Owner:AFFILIATED HOSPITAL OF GUANGDONG MEDICAL UNIV
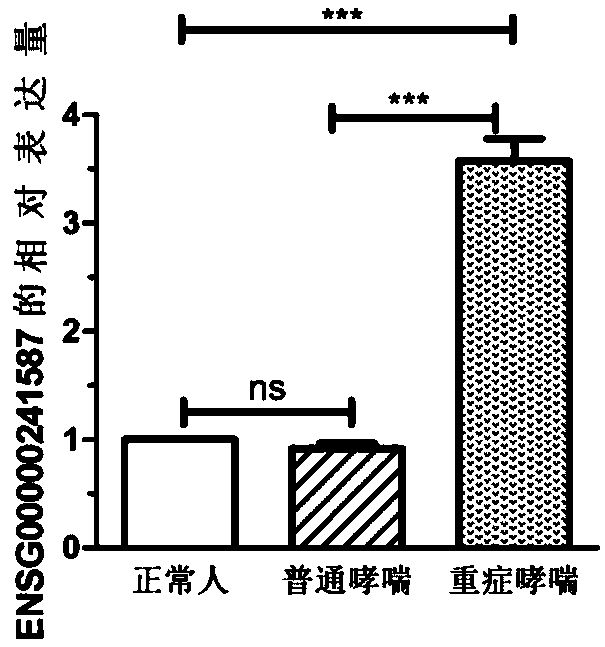
Biomarker for severe asthma and application thereof
InactiveCN109439743AAchieve early diagnosisReduce mortalityMicrobiological testing/measurementNormal peopleBiomarker (petroleum)
The invention discloses a biomarker for severe asthma and application thereof. The biomarker is ENSG00000241587. The expression level of lncRNA (long non-coding Ribonucleic Acid) in blood samples of normal people, common asthma patients and severe asthma patients is detected, finding that the ENSG00000241587 has remarkable difference in the severe asthma for the first time, which shows that the ENSG00000241587 can be used as a detection target to be applied to auxiliary diagnosis of the severe asthma.
Owner:CHANGZHOU NO 2 PEOPLES HOSPITAL
Treatment of type 1 immune response-mediated inflammatory lung disease by modulation of ifn-gamma activity
InactiveUS20070031377A1Reduce needPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsObstructive Pulmonary DiseasesIn vivo
The present invention relates to preventing, or treating and / or reducing the severity or progression of Type 1 immune response-mediated inflammatory lung disease. More particularly, the present invention provides a method for preventing or treating chronic obstructive pulmonary disease (COPD), severe asthma, sarcoidosis, berylliosis or cystic fibrosis by neutralizing or reducing IFNγ bioactivity which can be achieved either by in vivo administration of IFNγ neutralizing molecules or by in vivo immunization with pharmaceutical compositions comprising immunogenic IFNγ proteins or IFNγ-derived (poly)peptides or their corresponding nucleic acid sequences.
Owner:INNOGENETICS NV
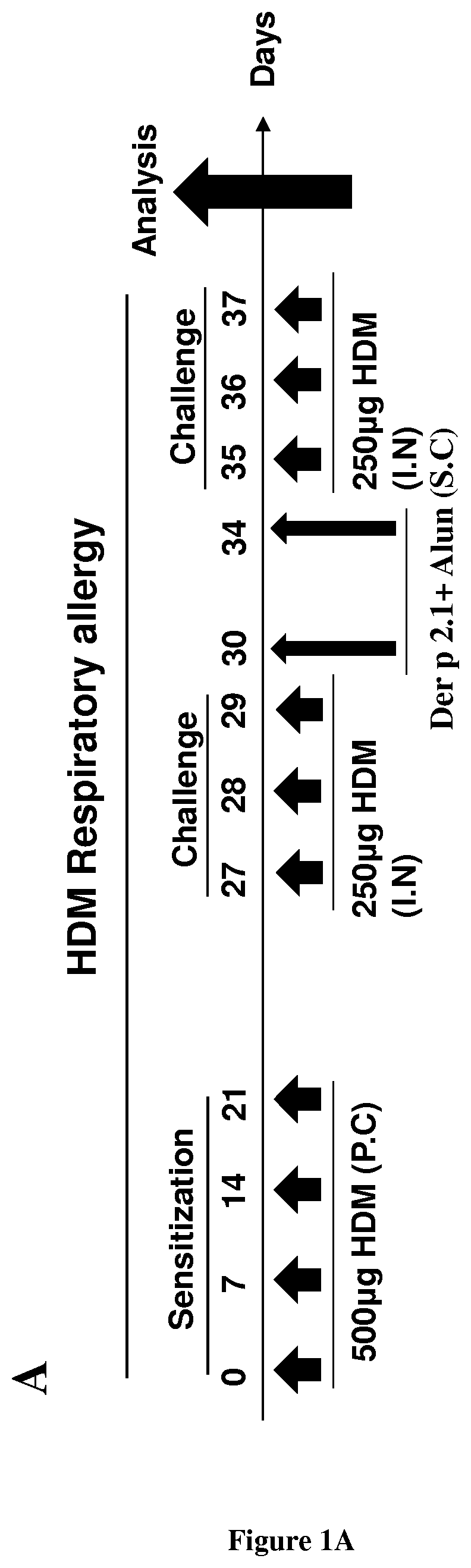
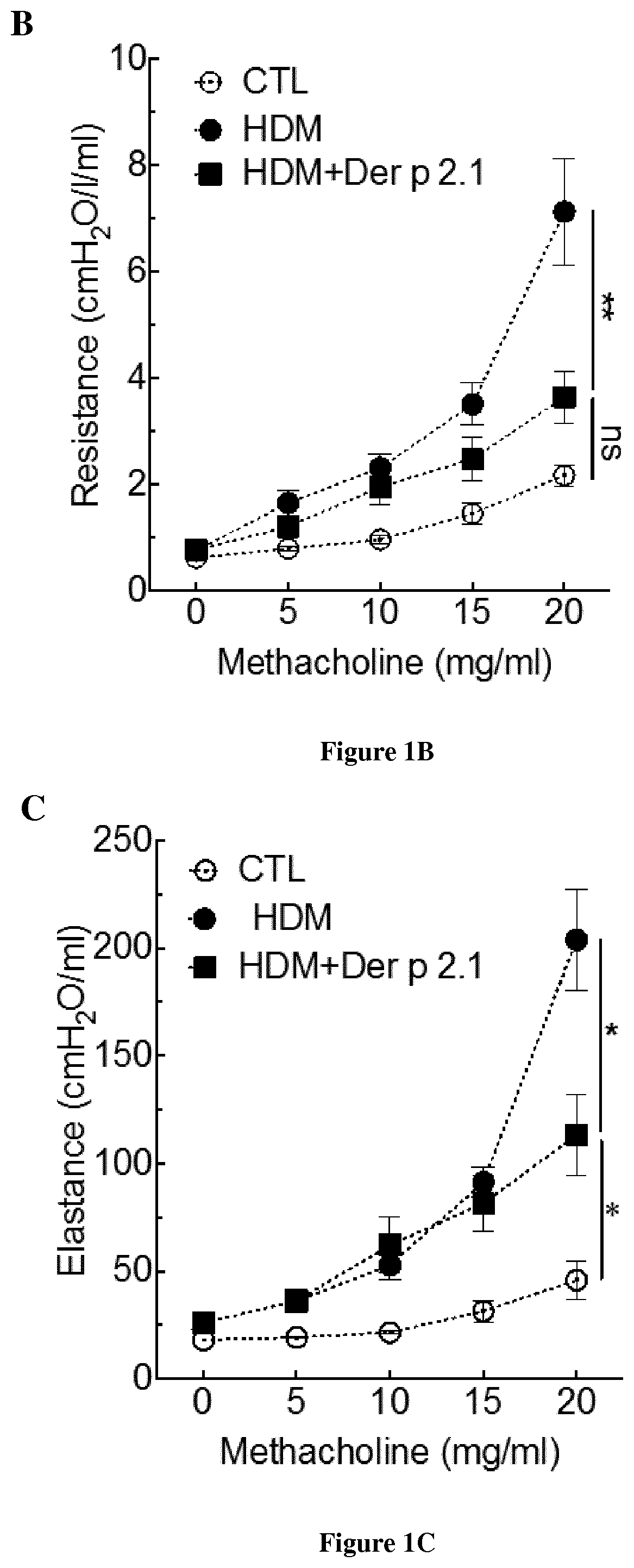
Methods and composition for the treatment of allergic asthma
PendingUS20210000917A1Improve permeabilityEase with which bacteria may be manipulated and grownPeptide/protein ingredientsPharmaceutical delivery mechanismDermatophagoides pteronyssimusBronchial epithelium
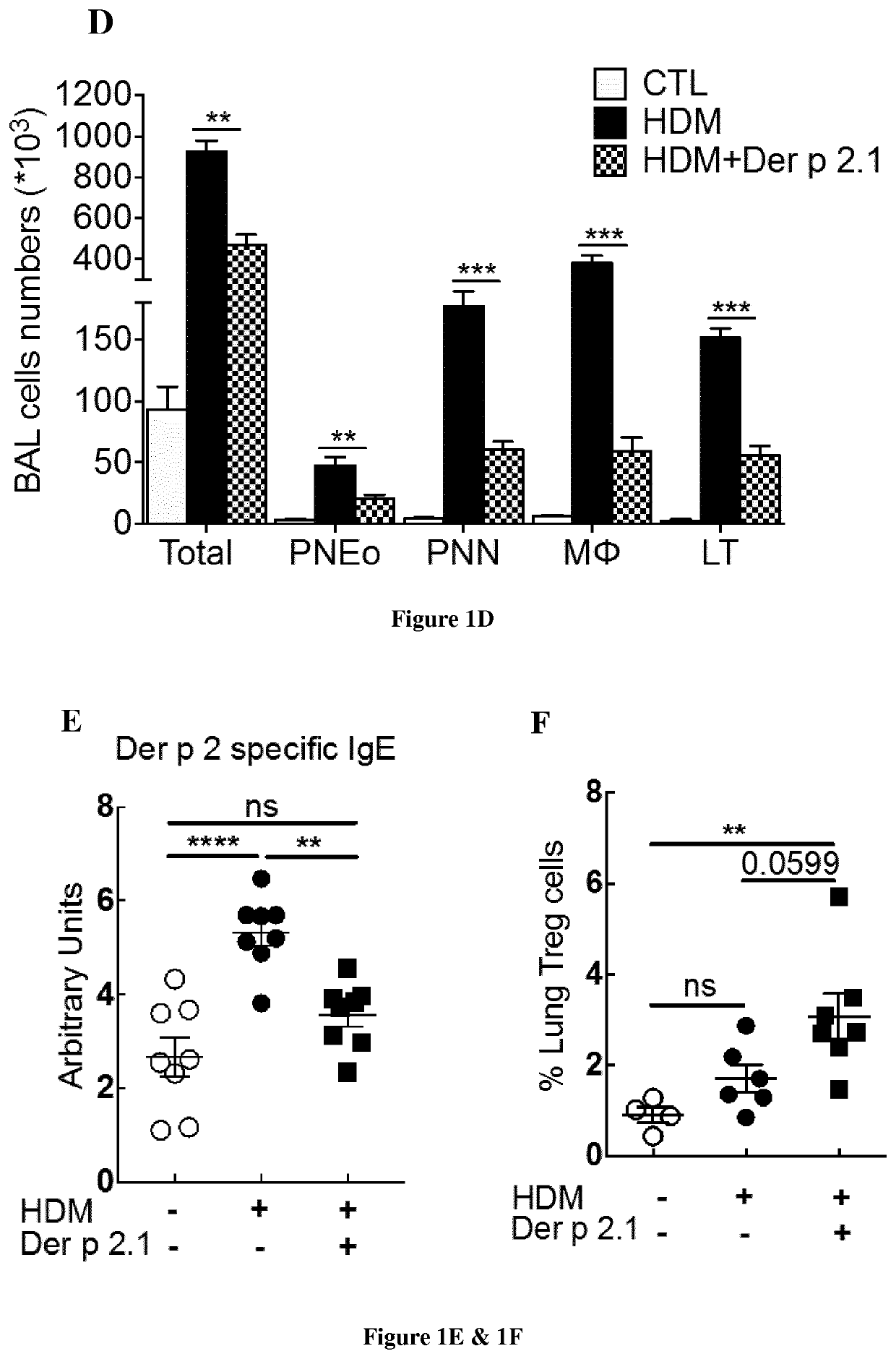
The present invention relates to a method of treating allergic asthma in a subject in need thereof comprising a step of administering to said subject a therapeutically effective amount of a polypeptide comprising or consisting of a biologically active fragment of the house-dust mite Dermatophagoides pteronyssinus Der p 2.1. Inventors have used a model allergy in a severe asthma allergic (mouse Balbc). They have surprisingly found that when they injected twice the polypeptide derp2.1 in the mouse model after a third asthma attack, the mouse presents a respiratory improvement, reduction of neutrophils and eosinophils in the broncho-alveolar lavage (BAL), an increase of regulators lymphocytes T and reduction of natural killer cells in the BAL. Thus, the polypeptide derp2.1 is a new tool to treat the allergic asthma by a restoration of lung function and reduction of inflammatory environment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Medicine preparation for treating severe myasthenia
InactiveCN100571710CQuick resultsRemissionPowder deliveryInorganic non-active ingredientsMyasthenia gravisCurative effect
The invention belongs to the field of medicines and relates to a medicine preparation for treating myasthenia gravis. The pharmaceutical preparation of the present invention comprises medicinal sodium thiosulfate of effective therapeutic dose, and pharmaceutically acceptable pharmaceutical auxiliary materials. The pharmaceutical preparation is injection solution or powder injection for acupoint injection. The pharmaceutical preparation of the present invention can cure patients with myasthenia gravis under the guidance of the treatment concept of integrated traditional Chinese and western medicine, and has quick effect, good curative effect, safety, short course of treatment, no recurrence after healing, and is easy to produce industrially, and is suitable for clinical application .
Owner:张国新
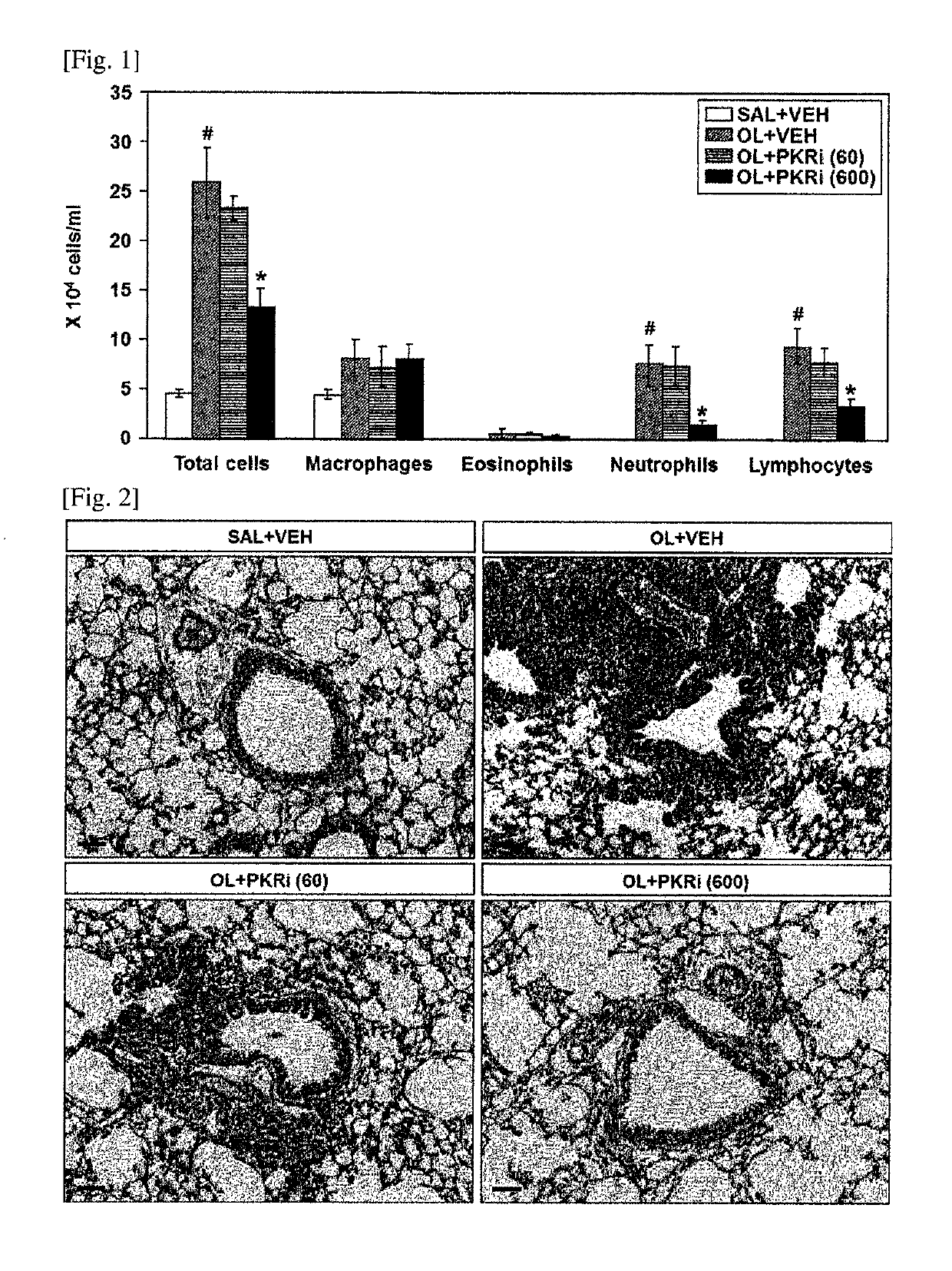
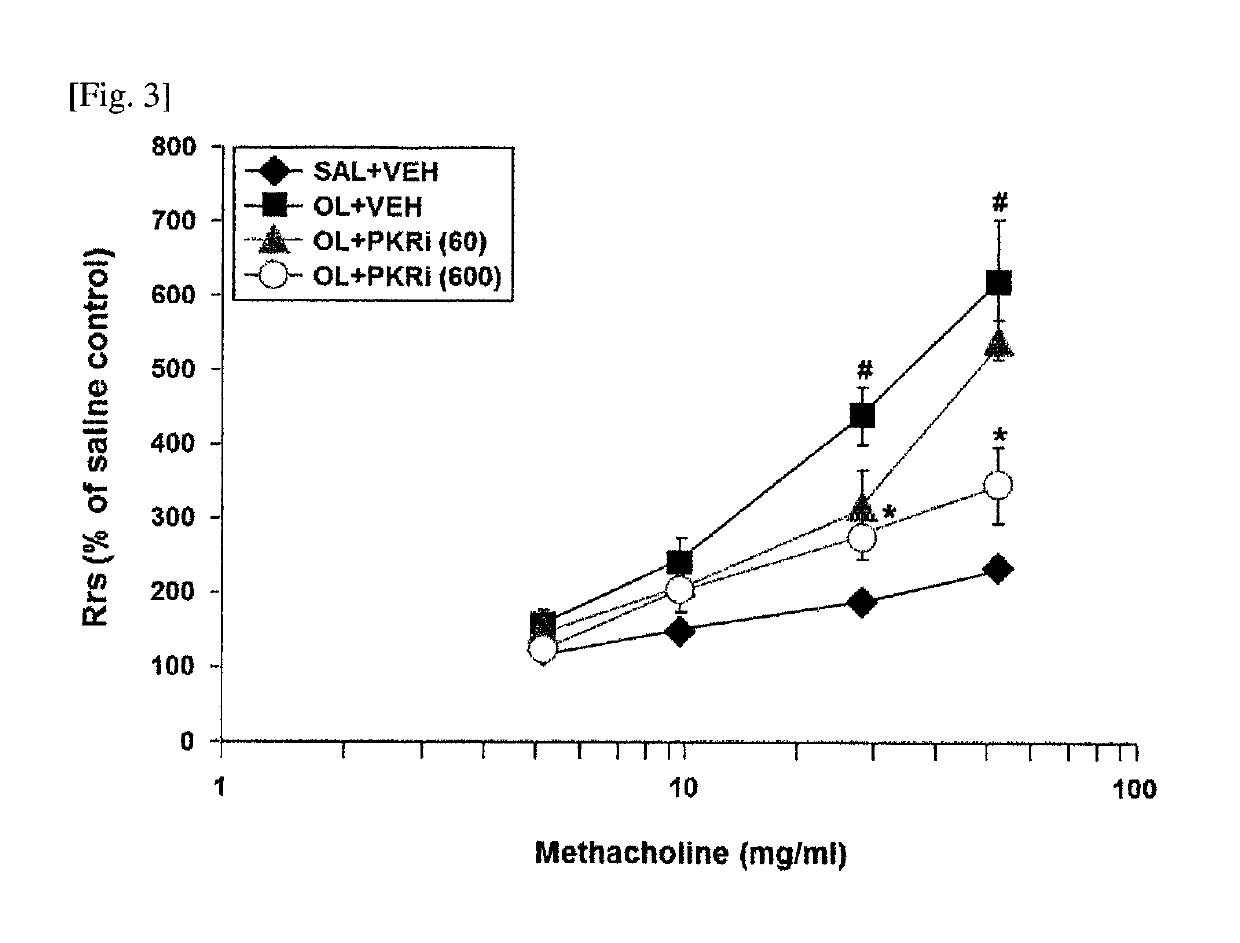
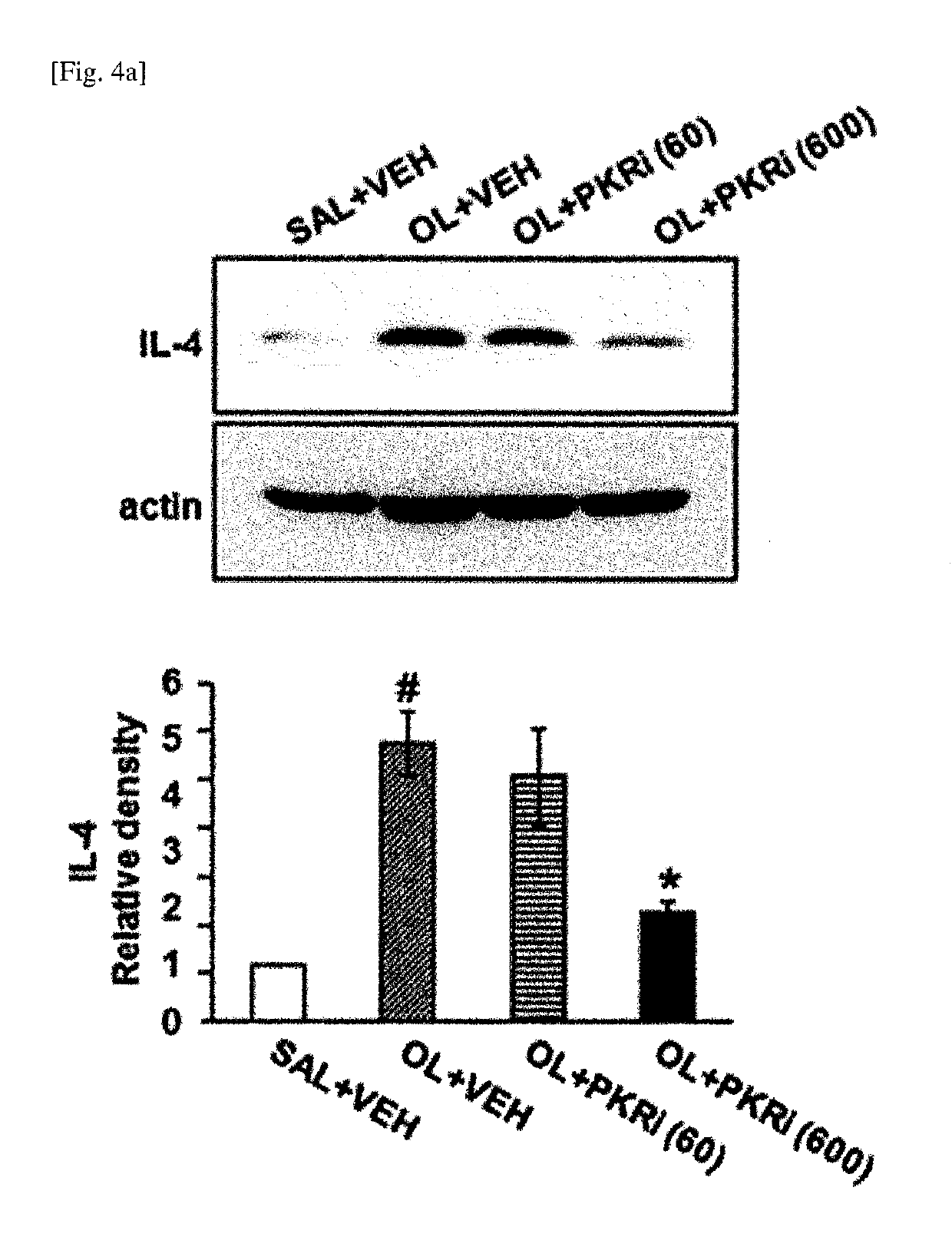
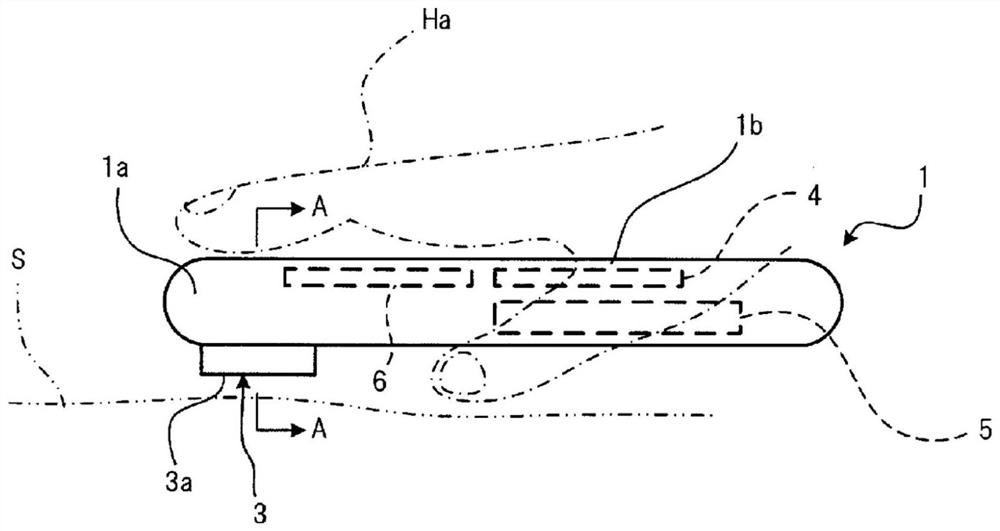
Composition for prevention or treatment of bronchial asthma comprising pkr inhibitor as active ingredient
ActiveUS20180000794A1Reducing airway inflammationReduce inflammationOrganic active ingredientsRespiratory disorderAdditive ingredientNeutrophil granulocyte
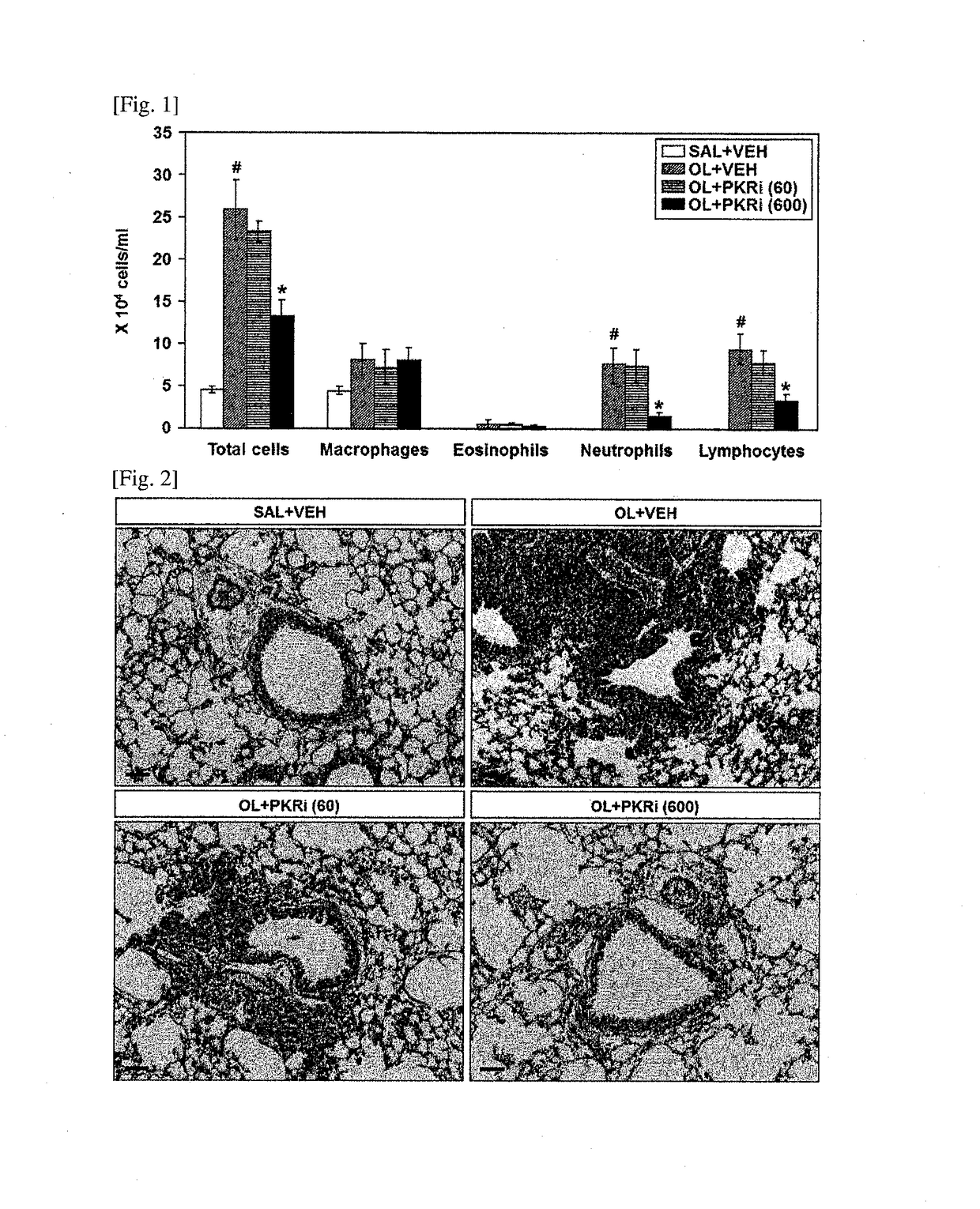
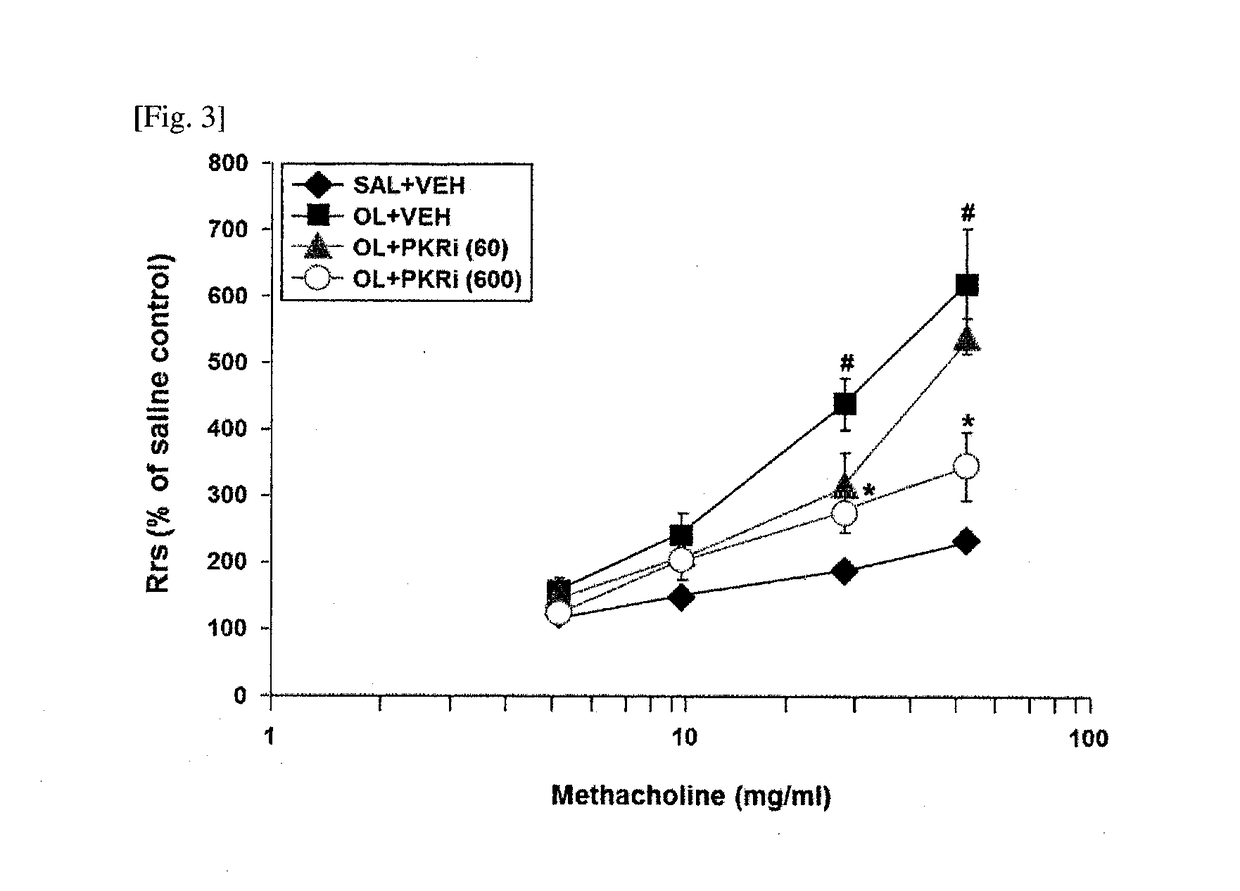
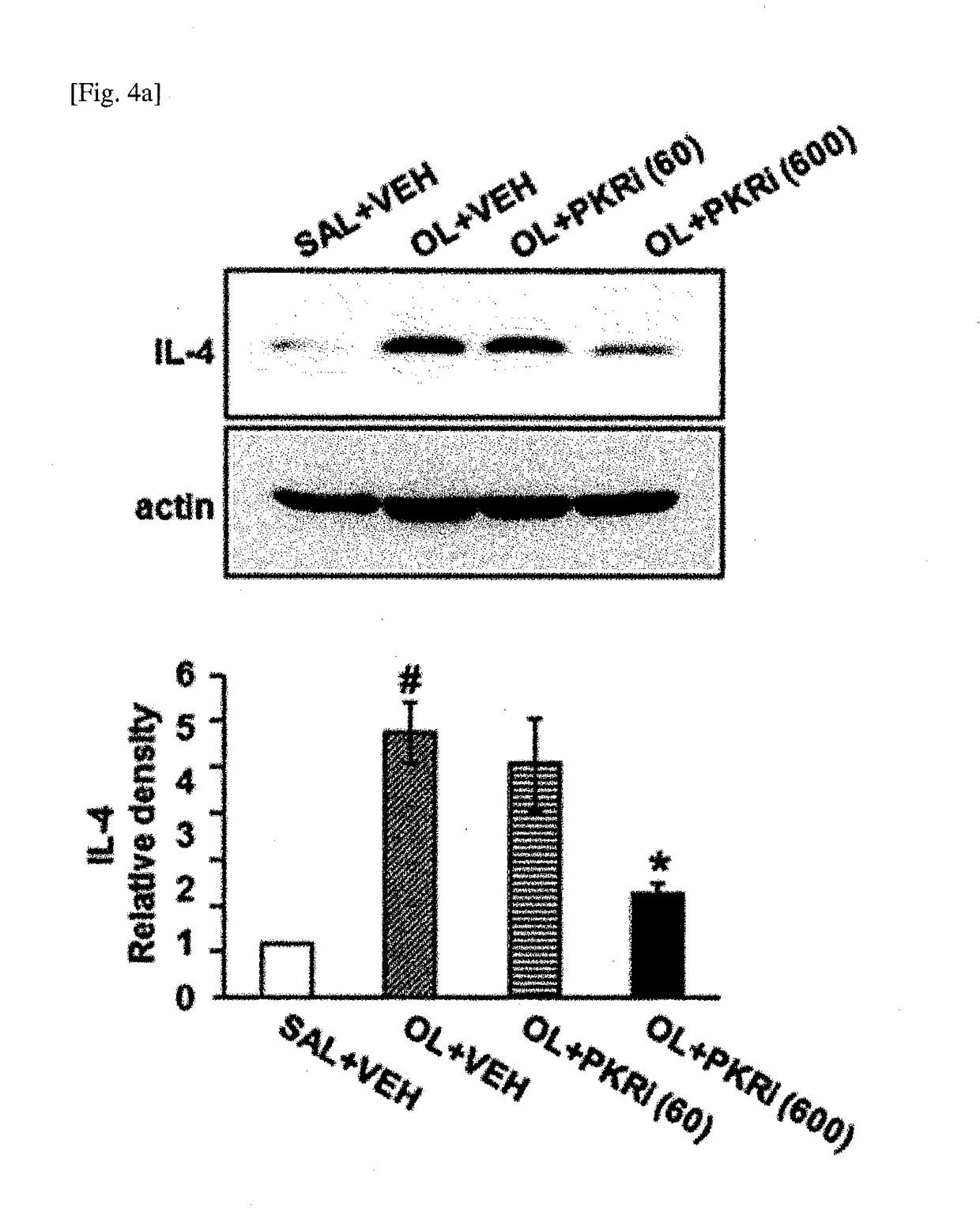
The present invention relates to a composition for prevention or treatment of bronchial asthma comprising a PKR inhibitor as an active ingredient. The PKR inhibitor and derivatives thereof according to the present invention can be used as a pharmaceutical for prevention, amelioration or treatment of bronchial asthma and as a supplementary health food because the PKR inhibitor and derivatives thereof reduce the total counts of inflammatory cells, eosinophils, neutrophils and lymphocytes in bronchoalveolar lavage fluid of neutrophilic severe asthma-induced mice, reduce airway inflammation and airway hyper-responsiveness, and reduce inflammatory mediators.
Owner:IND COOP FOUND CHONBUK NAT UNIV +1
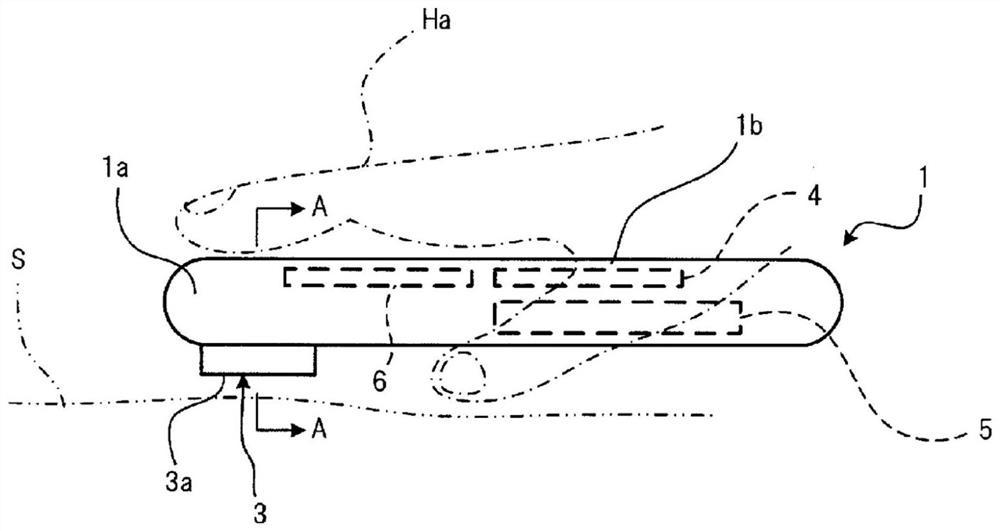
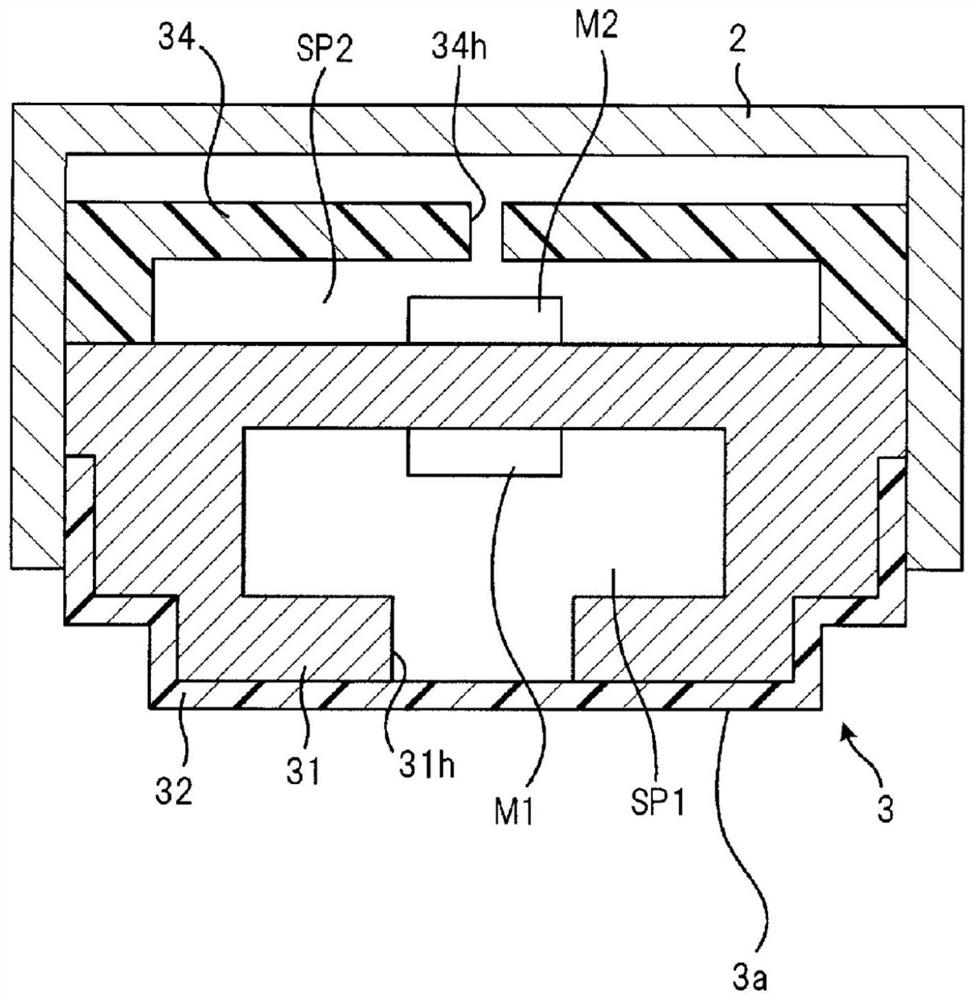
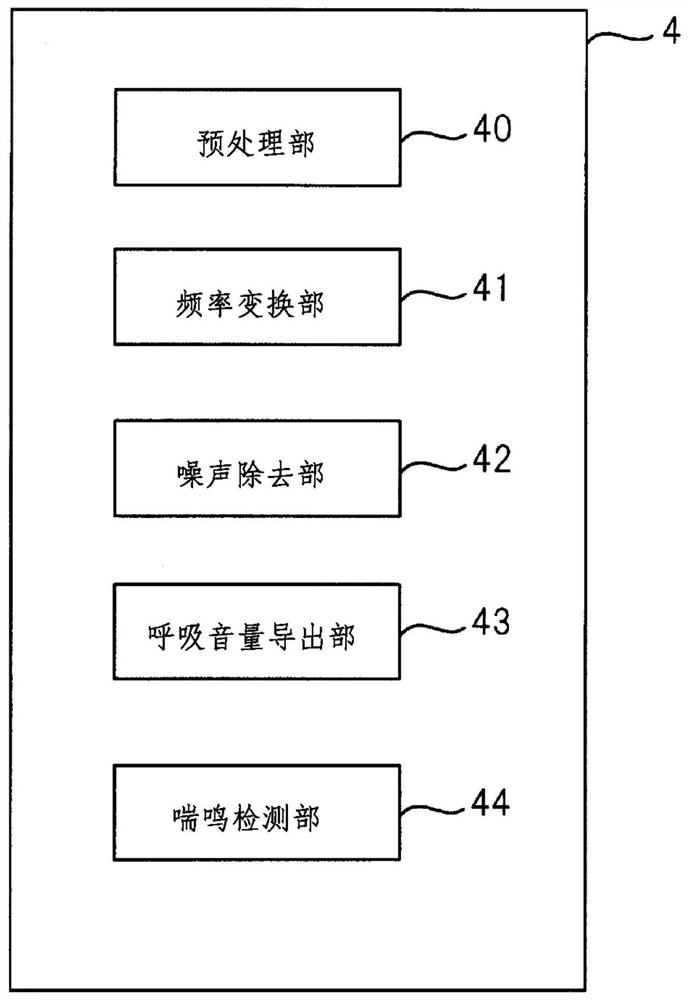
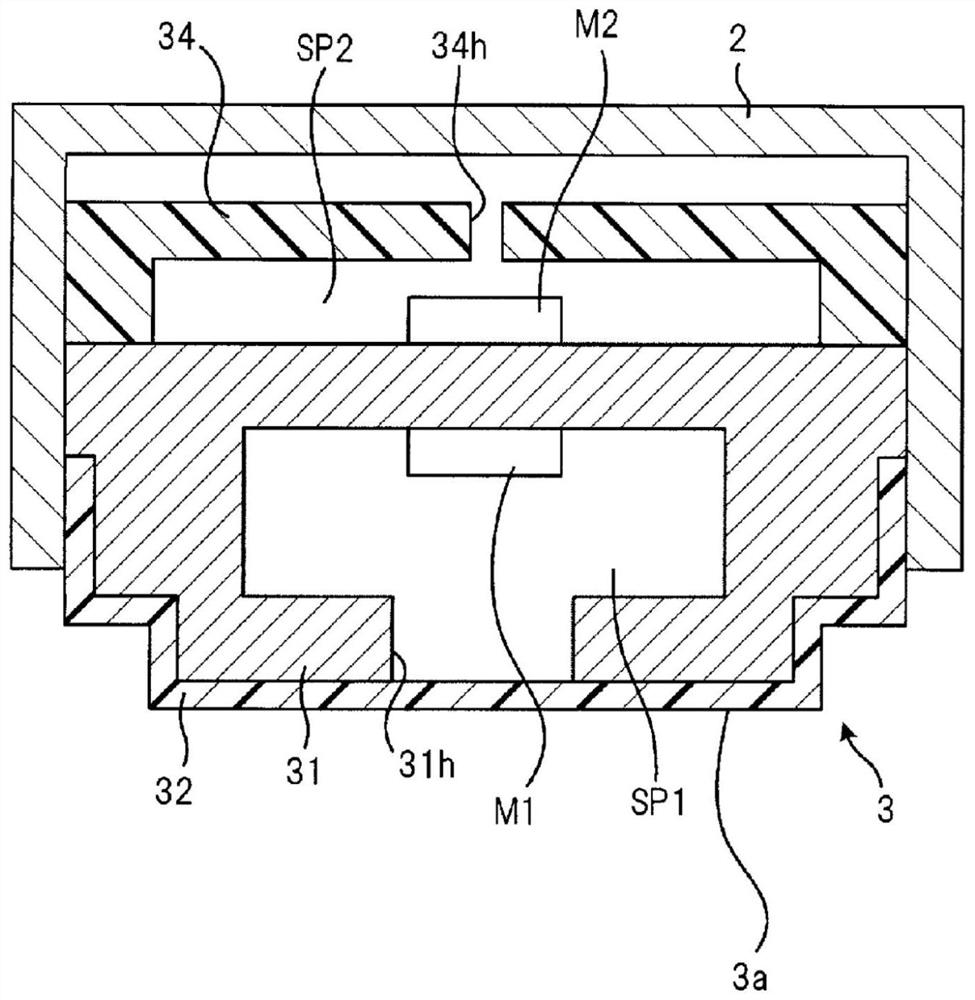
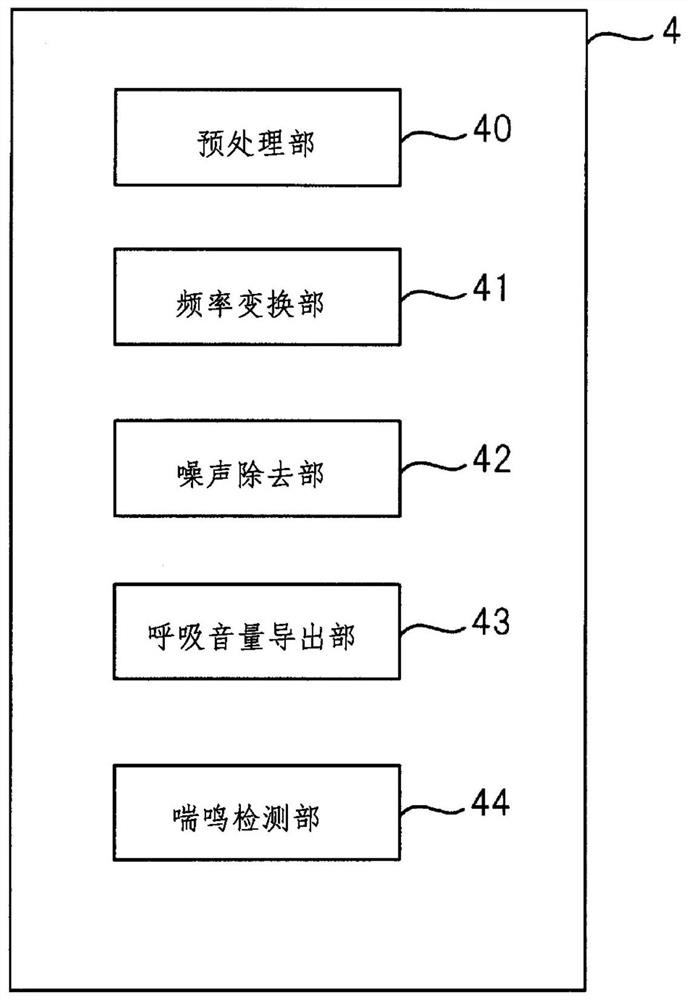
Wheeze detection apparatus, wheeze detection method, and wheeze detection program
Provided is a wheeze detection apparatus, a wheeze detection method, and a wheeze detection program which facilitate detection of wheezing during a severe asthma attack or wheezing of a heavy person or a person with a high BMI. The wheeze detection apparatus (1) includes a breathing sound volume deriving unit (43) which derives the breathing sound volume of a subject on the basis of sound measured by a first sound measuring device (M1) for measuring lung sounds from the subject, and a wheezing detection unit (44) which extracts a maximum point from the intensity distribution per frequency of said sound and detects wheezing on the basis of information on the maximum point. The wheezing detection unit (44) sets the detection sensitivity for wheezing to a higher value when the breathing sound volume is outside a specific range than when the breathing sound volume is within the specific range.
Owner:OMRON HEALTHCARE CO LTD
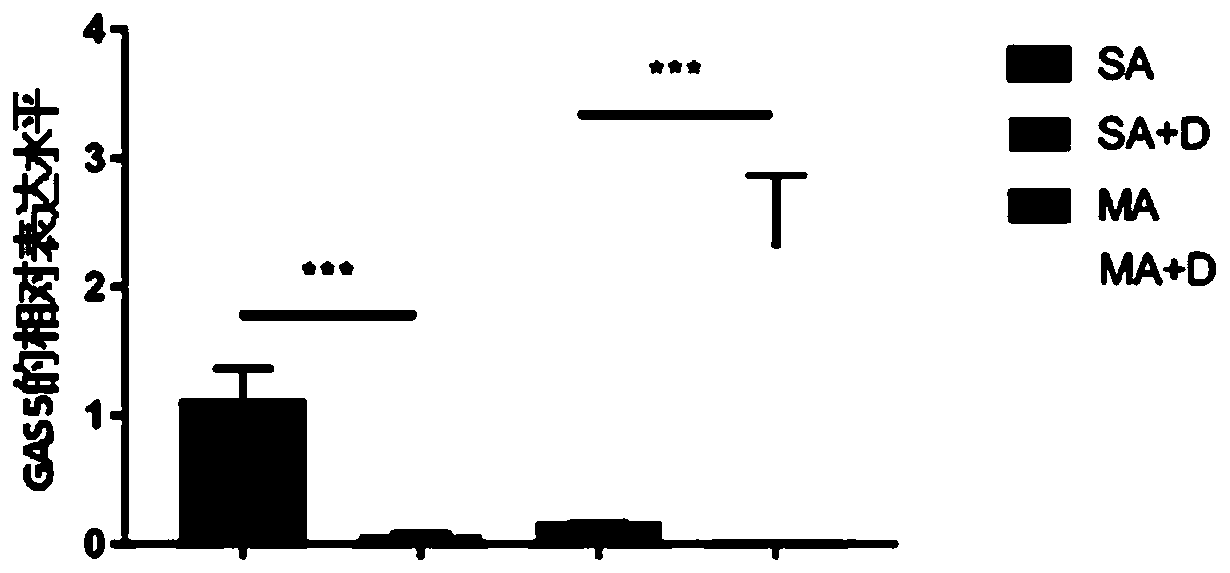
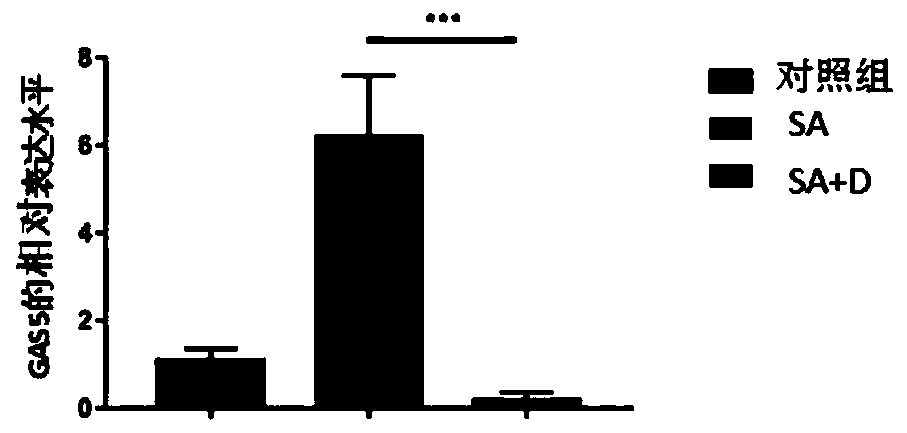
Application of GAS5 (growth arrest specific 5) in diagnosis of severe asthma
PendingCN110205382ASolve problems caused by individual differencesMicrobiological testing/measurementGlucocorticoidSevere asthma
The invention discloses application of GAS5 (growth arrest specific 5) in the diagnosis of severe asthma. It is discovered for the first time that GAS5 has a significant upregulation in severe asthma;whether a testee has severe asthma can be judged by detecting the expression level of GAS5. The invention also discloses a significant regulation of the expression level of GAS5 after glucocorticoiddrugs are applied to a severe asthma sample; whether the testee has severe asthma can be judged by detecting the expression level of GAS5 before and after the application of the glucocorticoid drugs;the problem of individual differences can be solved.
Owner:CHANGZHOU NO 2 PEOPLES HOSPITAL
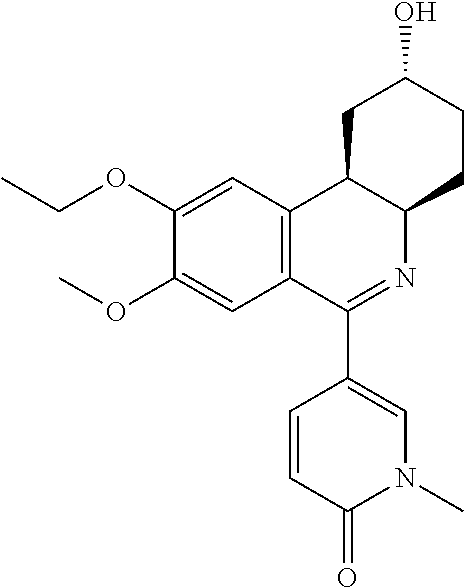
Treatment of Partly Controlled or Uncontrolled Severe Asthma
InactiveUS20160339010A1Respiratory disorderHeterocyclic compound active ingredientsSevere asthmaPhosphodiesterase-4
Method for the treatment of partly controlled or uncontrolled severe asthma comprising administering either a) a phosphodiesterase 4 (PDE4) inhibitor or b) a phosphodiesterase 4 (PDE4) inhibitor and a leukotriene modifier as add-on to standard of care in severe asthma.
Owner:TAKEDA GMBH
Composition for prevention or treatment of bronchial asthma comprising PKR inhibitor as active ingredient
ActiveUS10292969B2Reduce inflammationReduces inflammatory mediatorsOrganic active ingredientsRespiratory disorderNeutrophil granulocyteBULK ACTIVE INGREDIENT
The present invention relates to a composition for prevention or treatment of bronchial asthma comprising a PKR inhibitor as an active ingredient. The PKR inhibitor and derivatives thereof according to the present invention can be used as a pharmaceutical for prevention, amelioration or treatment of bronchial asthma and as a supplementary health food because the PKR inhibitor and derivatives thereof reduce the total counts of inflammatory cells, eosinophils, neutrophils and lymphocytes in bronchoalveolar lavage fluid of neutrophilic severe asthma-induced mice, reduce airway inflammation and airway hyper-responsiveness, and reduce inflammatory mediators.
Owner:IND COOP FOUND CHONBUK NAT UNIV +1
Stridor detection device and storage medium
The present invention provides a stridor detection device, a stridor detection method, and a storage medium capable of easily detecting wheezing during severe asthma attacks, and wheezing in persons with a large body weight or BMI. A stridor detection device (1) includes a breathing volume deriving unit (43) for deriving the breathing volume of the subject based on the sound measured by the first sound measuring device (M1) for measuring the lung sound of the subject and a stridor detection unit (44) that extracts a maximum point from the intensity distribution of each frequency of the sound, and detects stridor based on the information of the maximum point. When the breathing volume is outside the specific range, the stridor detection unit (44) sets the detection sensitivity of stridor to a higher value than when the breathing volume is within the specific range.
Owner:OMRON HEALTHCARE CO LTD
Methods of selectively treating asthma using IL-17 antagonists
ActiveUS10676522B2Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsSerum concentrationEosinophil
Owner:NOVARTIS AG
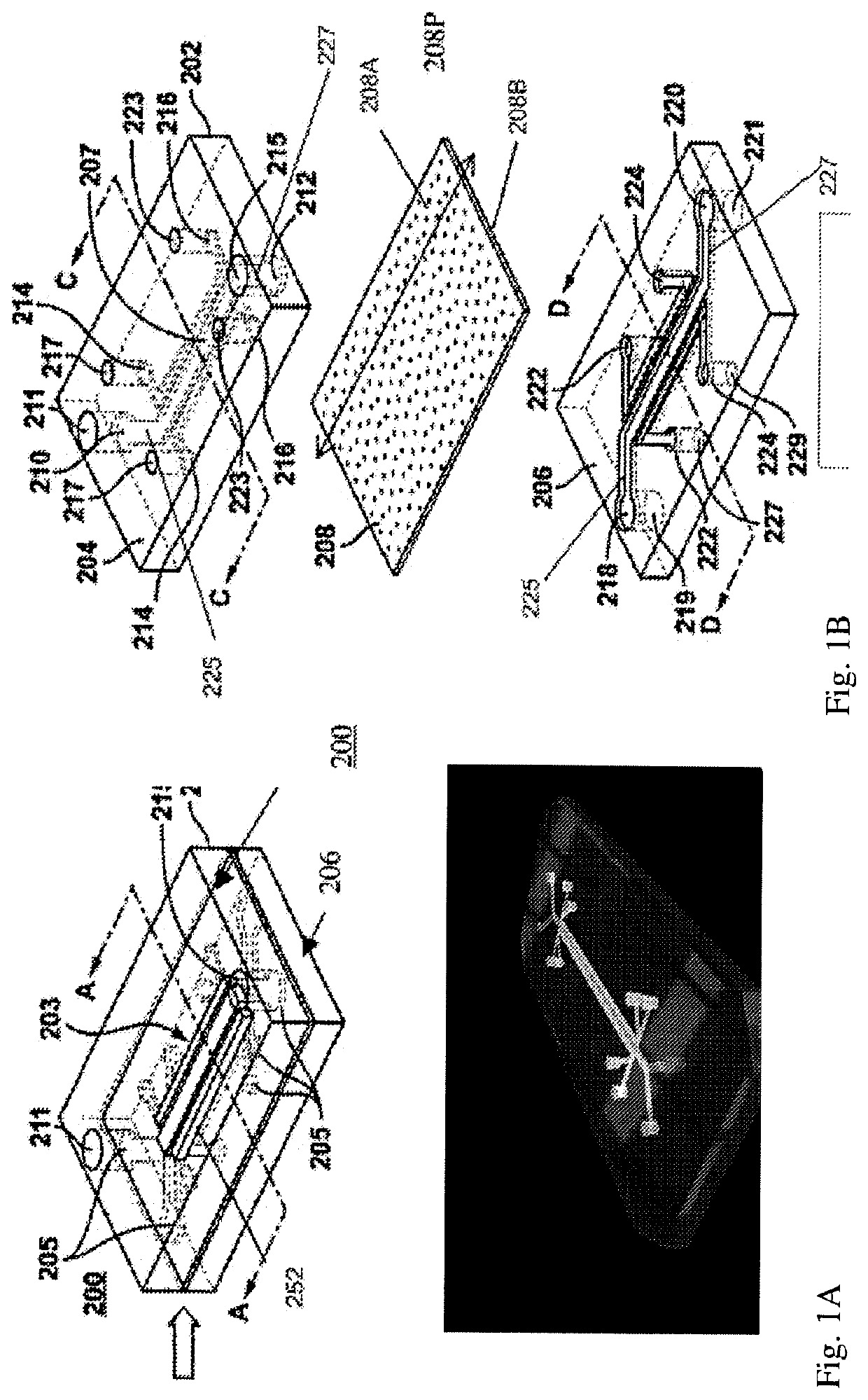
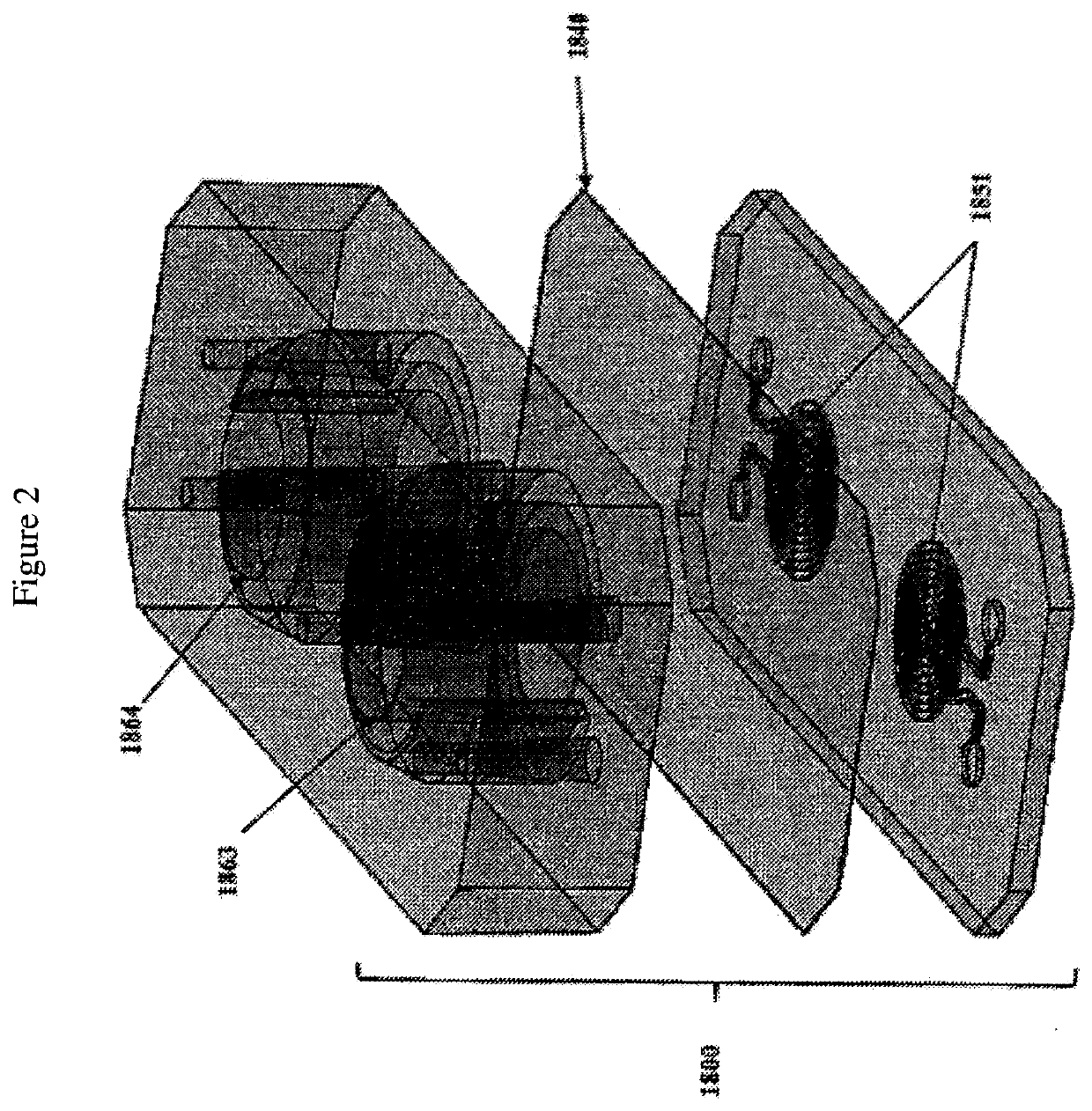
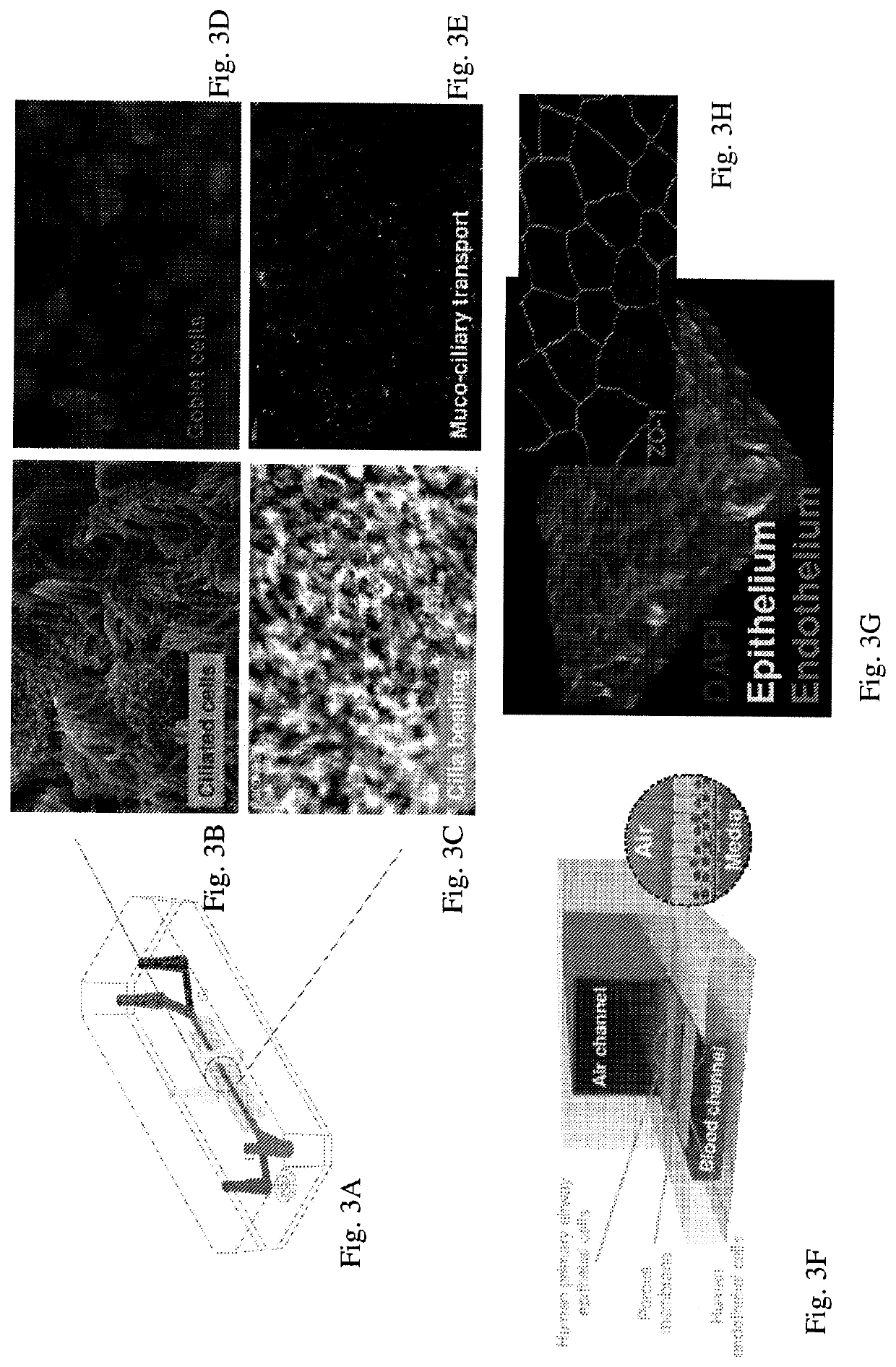
Advanced pulmonary models
The present invention relates to microfluidic fluidic systems and methods for the in vitro modeling diseases of the lung and small airway. In one embodiment, the invention relates to a system for testing responses of a microfluidic Small Airway-on-Chip infected with one or more infectious agents (e.g. respiratory viruses) as a model of respiratory disease exacerbation (e.g. asthma exacerbation). In one embodiment, this disease model on a microfluidic chip allows for a) the testing of anti-inflammatory and / or anti-viral compounds introduced into the system, as well as b) the monitoring of the participation, recruitment and / or movement of immune cells, including the transmigration of cells. In particular, this system provides, in one embodiment, an in-vitro platform for modeling severe asthma as “Severe Asthma-on-Chip.” In some embodiments, this invention provides a model of viral-induced asthma in humans for use in identifying potentially effective treatments.
Owner:EMULATE INC
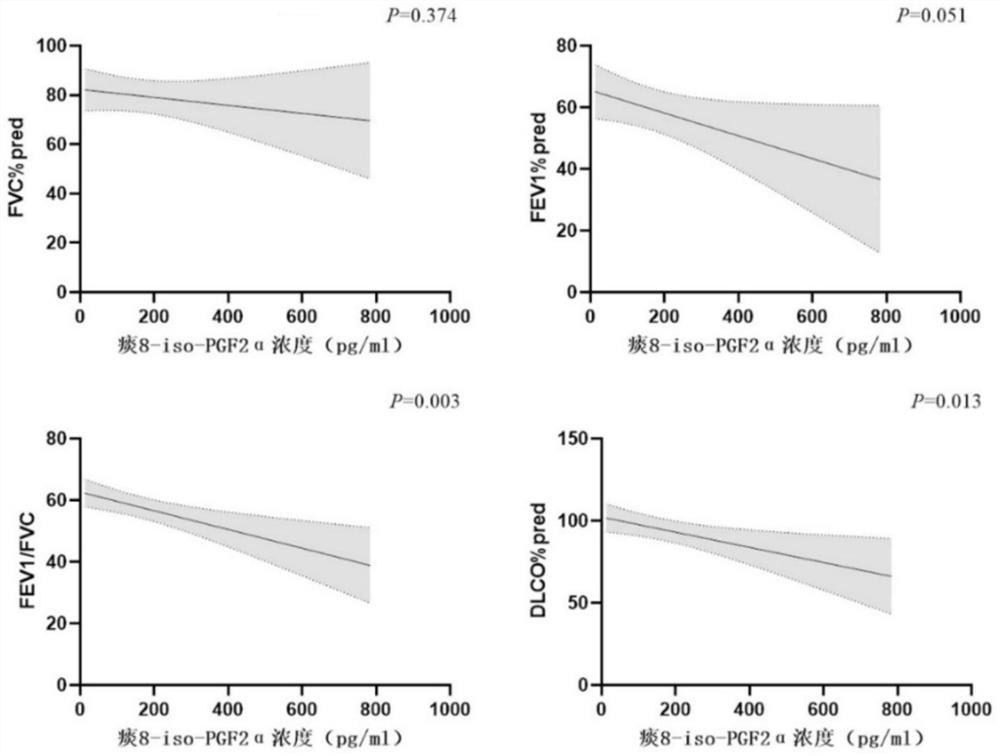
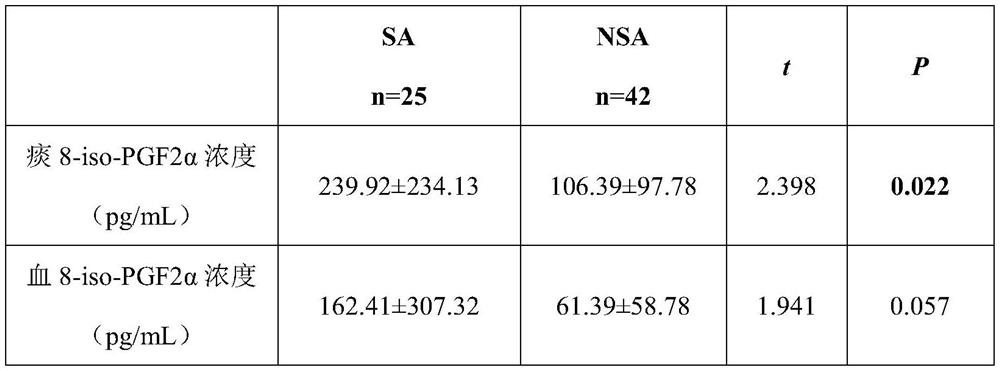
Application of 8-isoprostaglandin F2alpha in diagnosis of severe asthma
PendingCN114705871AEasy diagnosisEasy to detectDisease diagnosisBiological testingAssayAIDS diagnosis
The invention discloses an application of 8-isoprostaglandin F2alpha in diagnosis of severe asthma. The invention provides an application of 8-isoprostaglandin F2alpha as a biomarker in preparation of a product for diagnosis or auxiliary diagnosis of severe asthma, which comprises the following steps: detecting the concentration of 8-isoprostaglandin F2alpha in plasma and induced sputum supernatant of an asthma patient; the concentration of 8-isoprostaglandin F2alpha is found to have significant difference in detection samples of severe asthma patients (SA) and non-severe asthma patients (NSA), and meanwhile, by detecting lung function indexes of the asthma patients, the concentration of 8-isoprostaglandin F2alpha in the detection samples is found to have a negative correlation trend with the lung function indexes; the biomarker 8-iso-PGF2 alpha provided by the invention can be conveniently detected through ELISA (Enzyme-Linked Immunosorbent Assay), and the severe asthma can be diagnosed in a simpler and more convenient manner.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
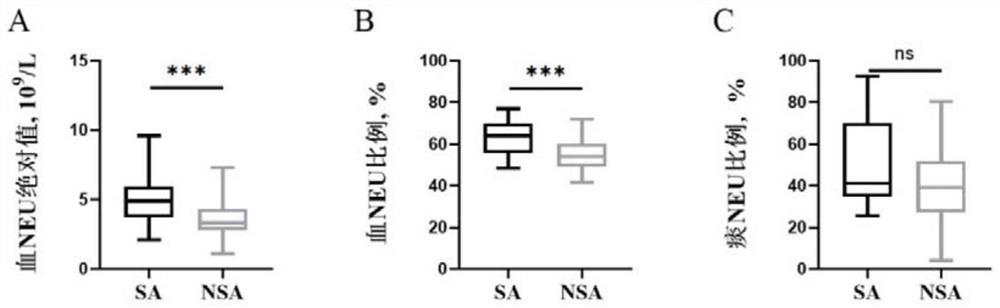
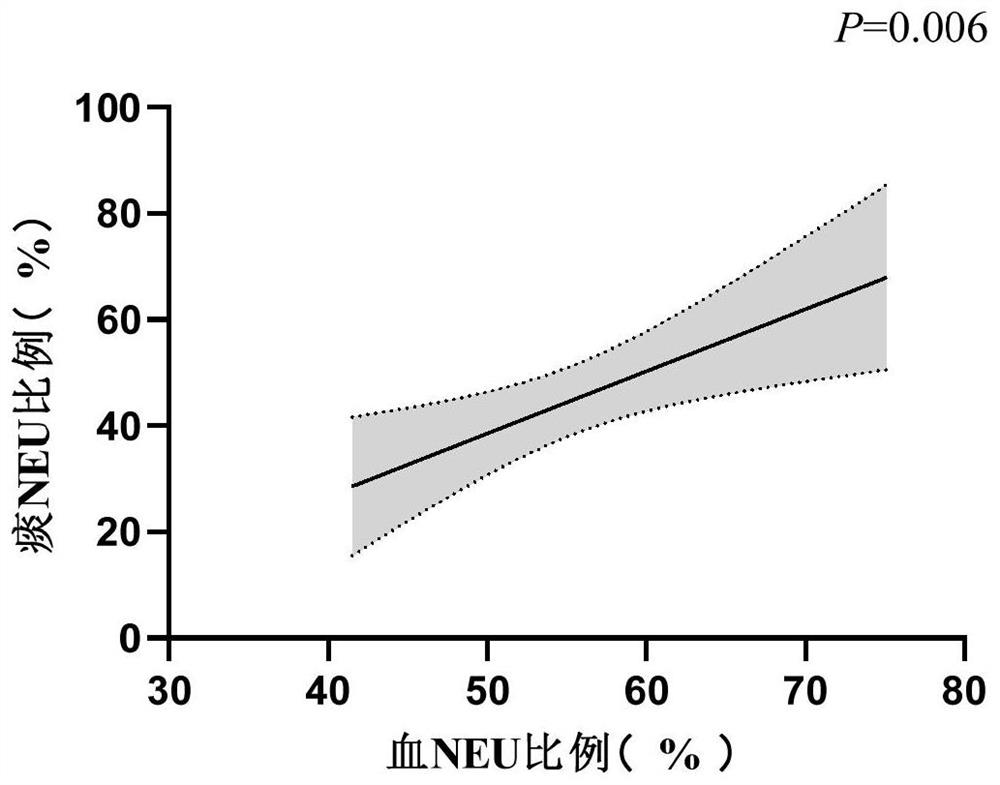
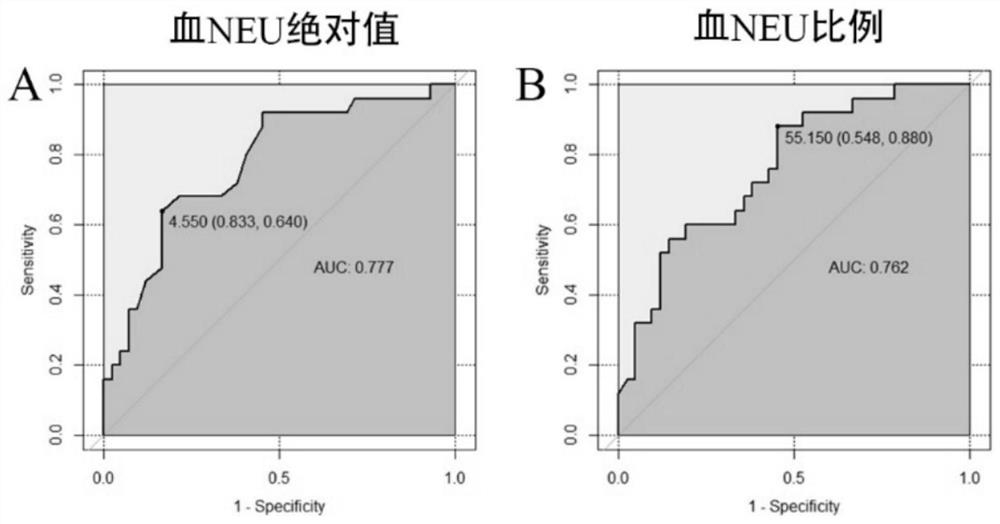
Application of peripheral blood neutrophil as severe asthma diagnostic marker
PendingCN114755428AEasy and fast detectionDisease diagnosisBiological testingBiochemistrySevere asthma
The invention discloses application of peripheral blood neutrophil as a severe asthma diagnosis marker. The invention provides application of peripheral blood neutrophil as a marker in preparation of a product for diagnosing severe asthma. According to the present invention, the determination index of severe asthma is obtained by detecting the absolute value and / or the ratio of the peripheral blood neutrophil through the blood routine test of the asthma patient, such that the determination index of severe asthma is determined as follows: the absolute value gt of the peripheral blood neutrophil; 4.55 * 10 < 9 > / L (the sensitivity is 83.3%, and the specificity is 64.0%) or the percentage gt of the neutrophils in the peripheral blood; when the sensitivity is 54.8% and the specificity is 88.0%, the severe asthma is judged, and the AUC of the ROC curve is 0.777 and 0.762 respectively. Results of the invention show that compared with sputum induction, the peripheral blood neutrophil which is more rapid and convenient to detect has a good application prospect in the diagnosis of severe asthma.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com