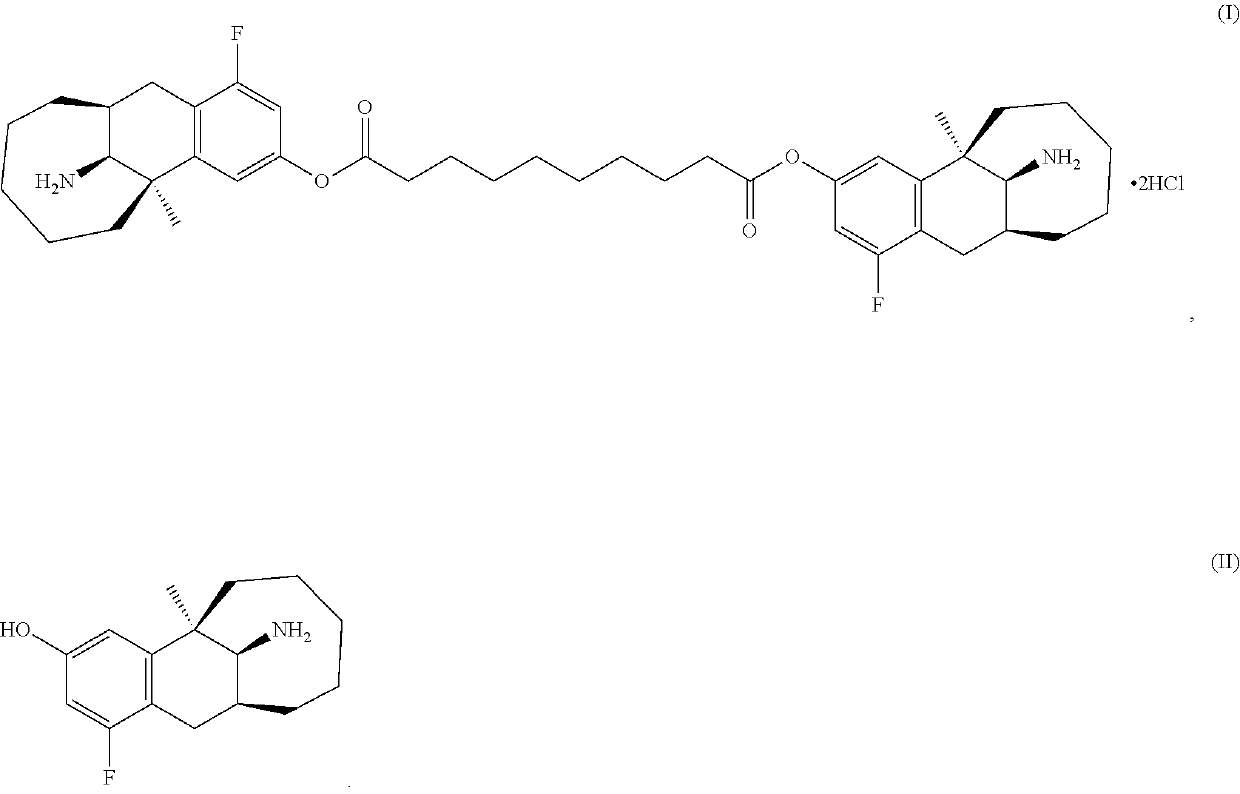
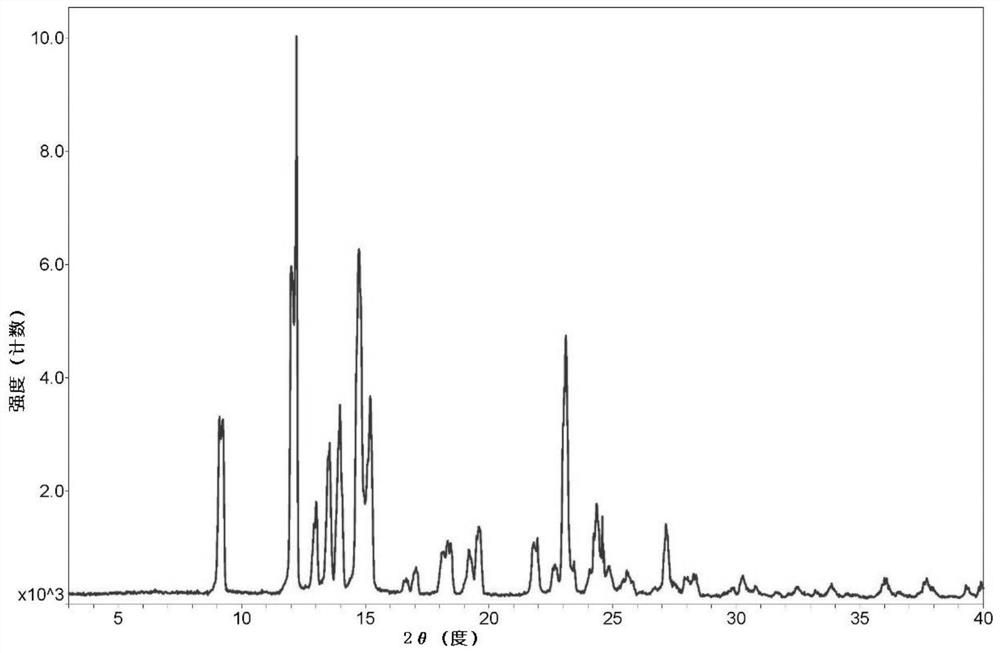
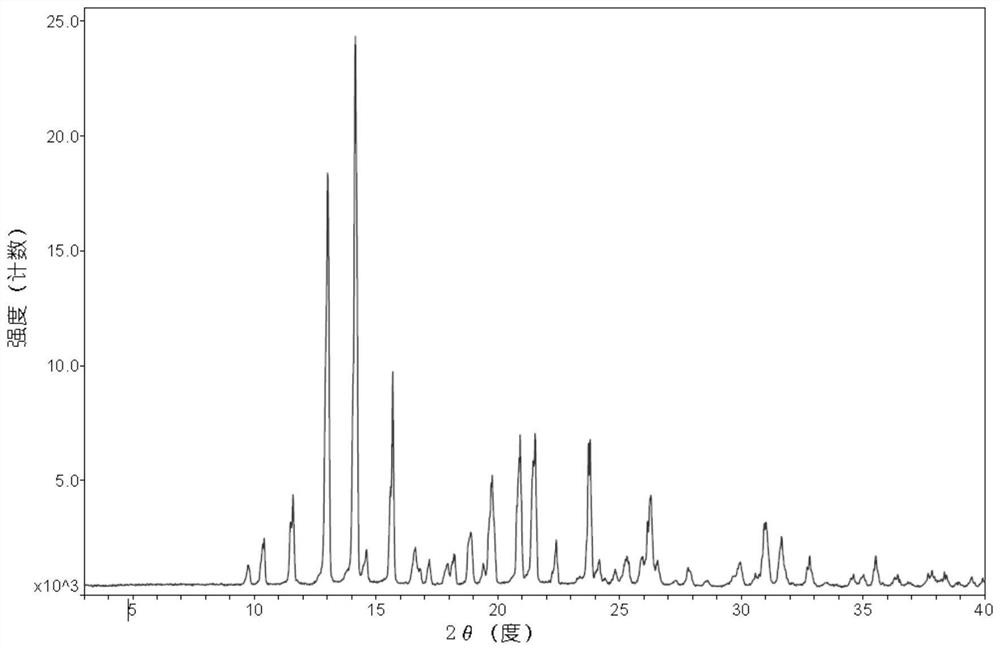
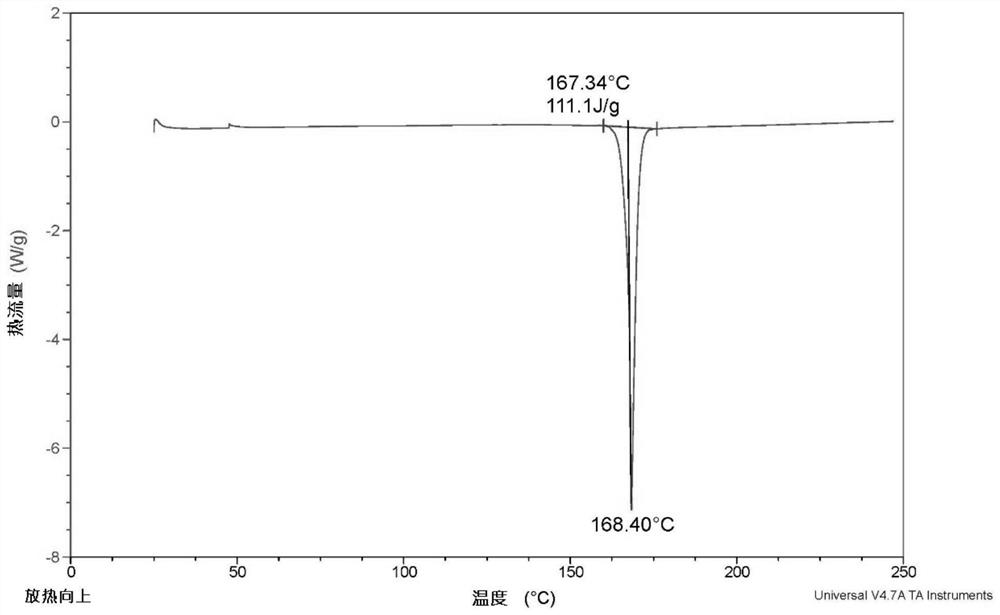
Patents
Literature
36 results about "Dezocine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
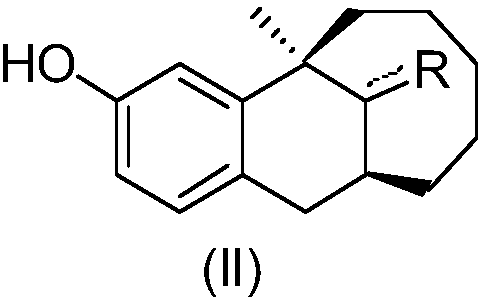
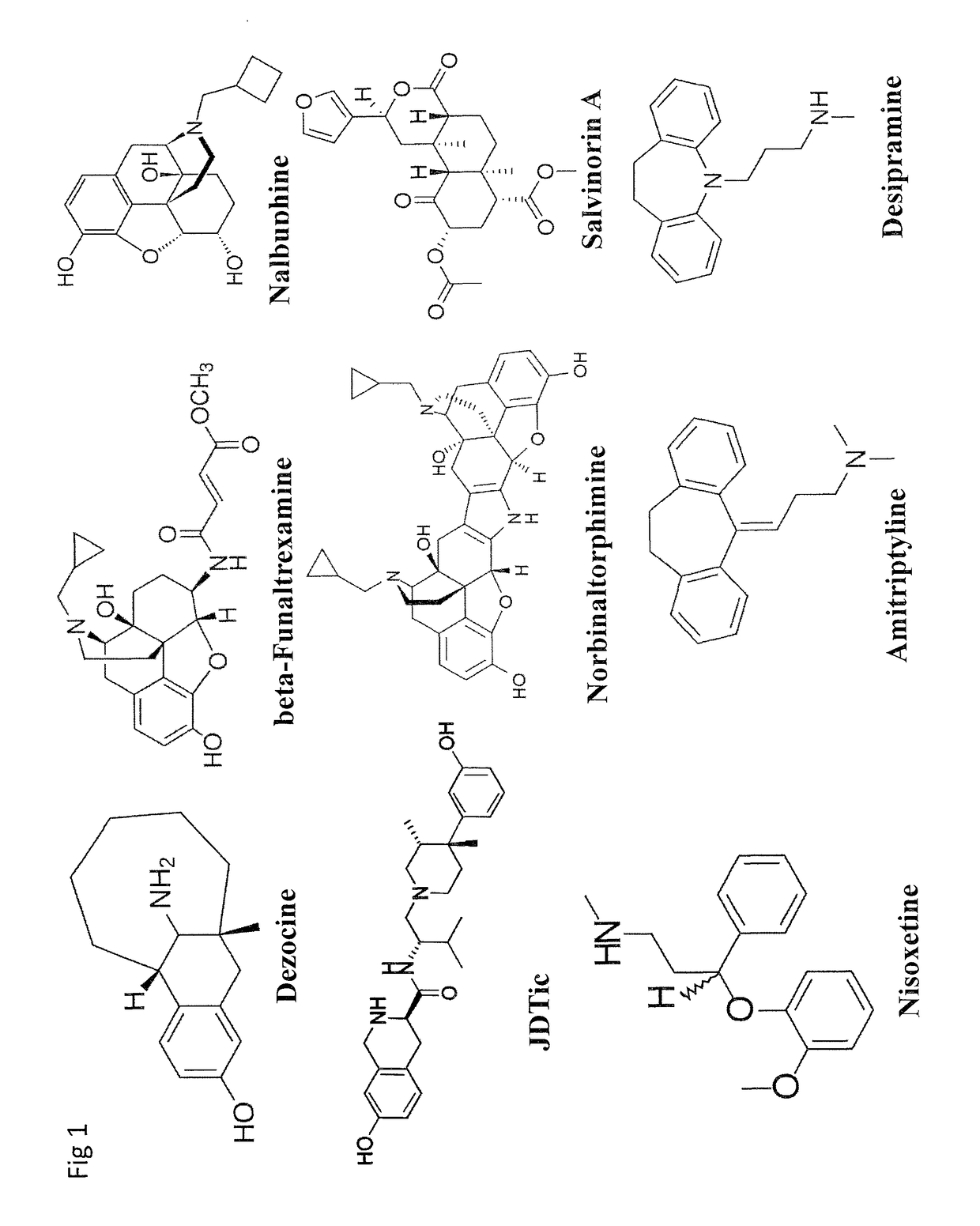
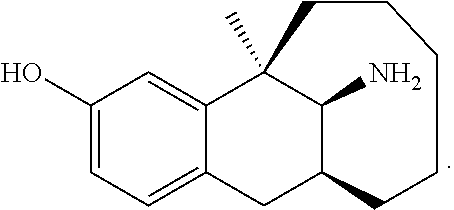
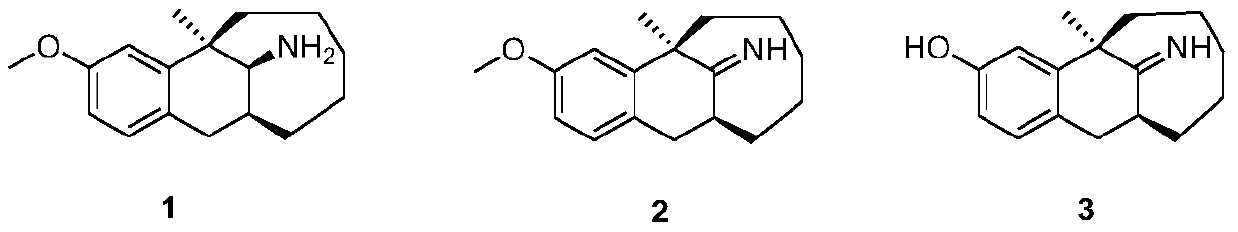
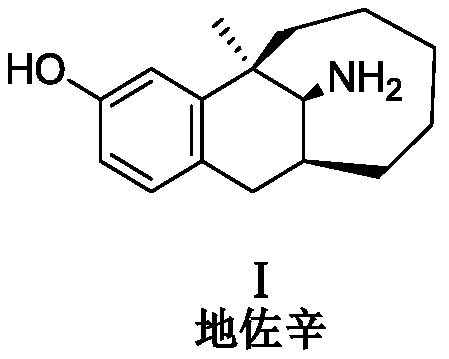
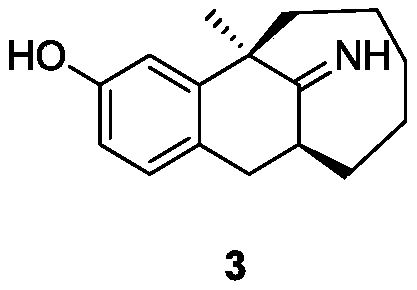
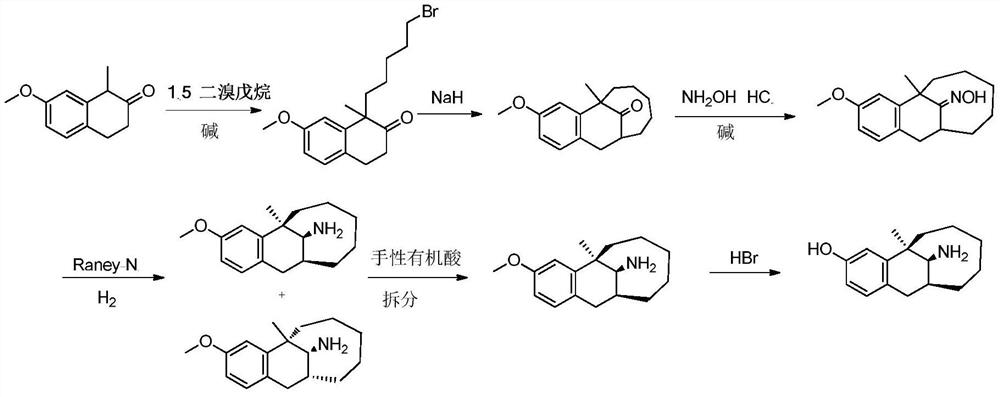
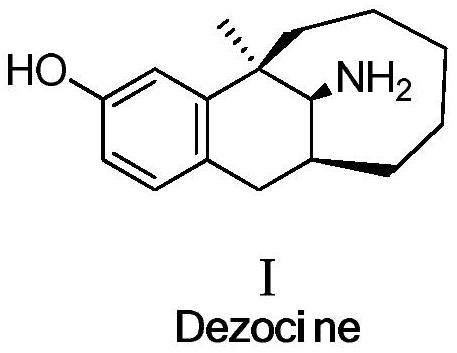
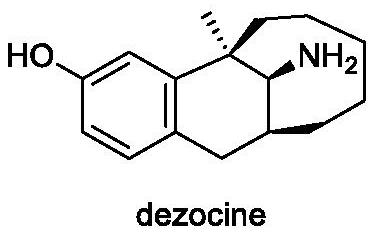
Dezocine (INN, USAN) (brand name Dalgan) is a marketed opioid analgesic of the benzomorphan group. First synthesized in 1970, it acts as a modulator of mu-, delta-, and kappa-opioid receptors. Dezocine is a mixed agonist/antagonist of opioid receptors. It is related to other benzomorphans such as pentazocine, with a similar profile of effects that include analgesia and euphoria. Unlike many other benzomorphans however, it is a silent antagonist of the κ-opioid receptor, and in accordance, does not produce side effects such as dysphoria or hallucinations at any dose.
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
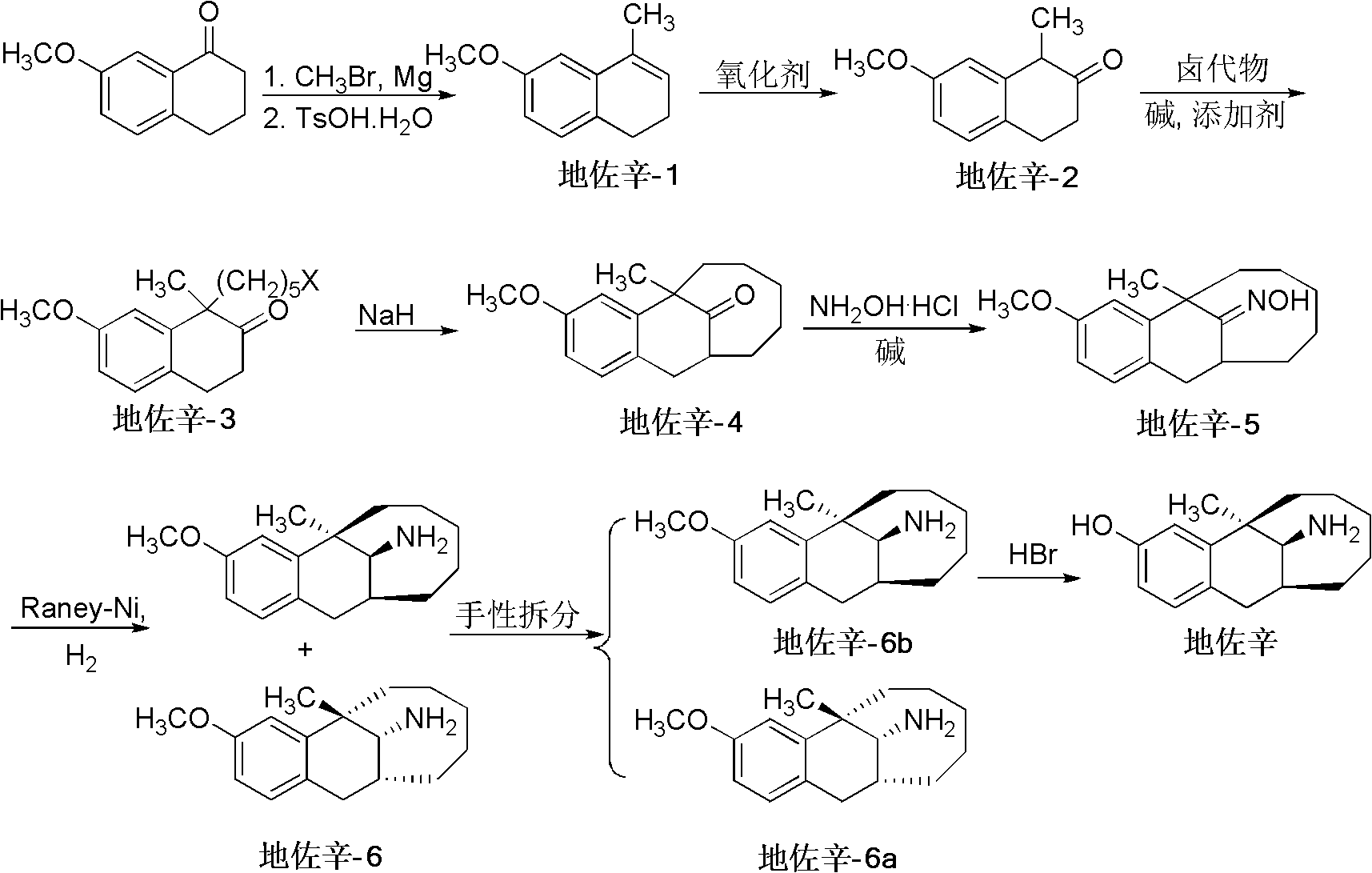
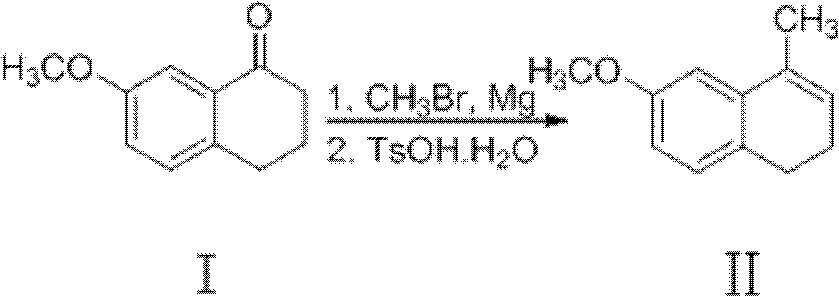
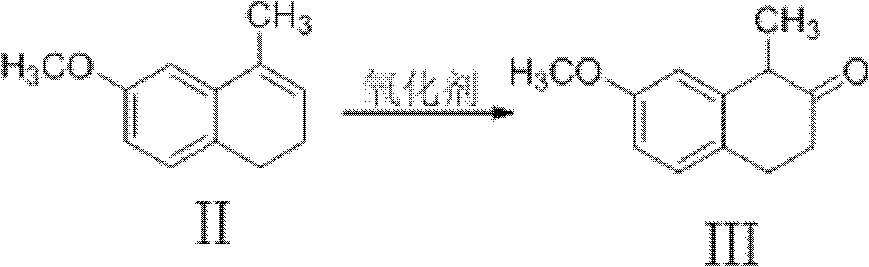
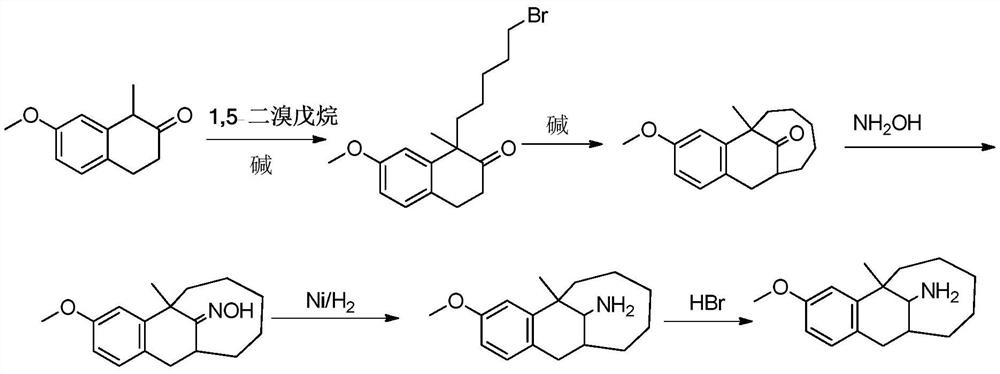
Preparation method of dezocine
ActiveCN102503840ASimple post-processingHigh optical purityOrganic compound preparationAmino-hyroxy compound preparationOrganic acidAlcohol
The invention provides a preparation method of dezocine, which has the following advantages: (1) when an intermediate which is dezocine-1 is used as a raw material to prepare another intermediate which is dezocine-2, the obtained oxidants are acid peroxide, alcohol peroxide or hydrogen peroxide which have low cost and are easy to obtain and post-process; (2) when the intermediate which is dezocine-2 is used as a raw material to prepare an intermediate which is dezocine-3, the obtained bases are bases which have low cost and are easy to obtain; (3) when an intermediate which is dezocine-6 is used as a raw material to prepare an intermediate which is dezocine-6b, an optically pure chiral organic acid and an racemic intermediate which is the dezocine-6 react for generate a pair of diastereomeric salts, and then the pair of diastereomeric salts are separated through recrystallization to obtain the intermediate which is the dezocine-6 with high optical purity; and (4), the operation is simple and efficient, the reaction condition is mild, and the used reagents are cheap, readily available, highly safe and suitable for industrial mass production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
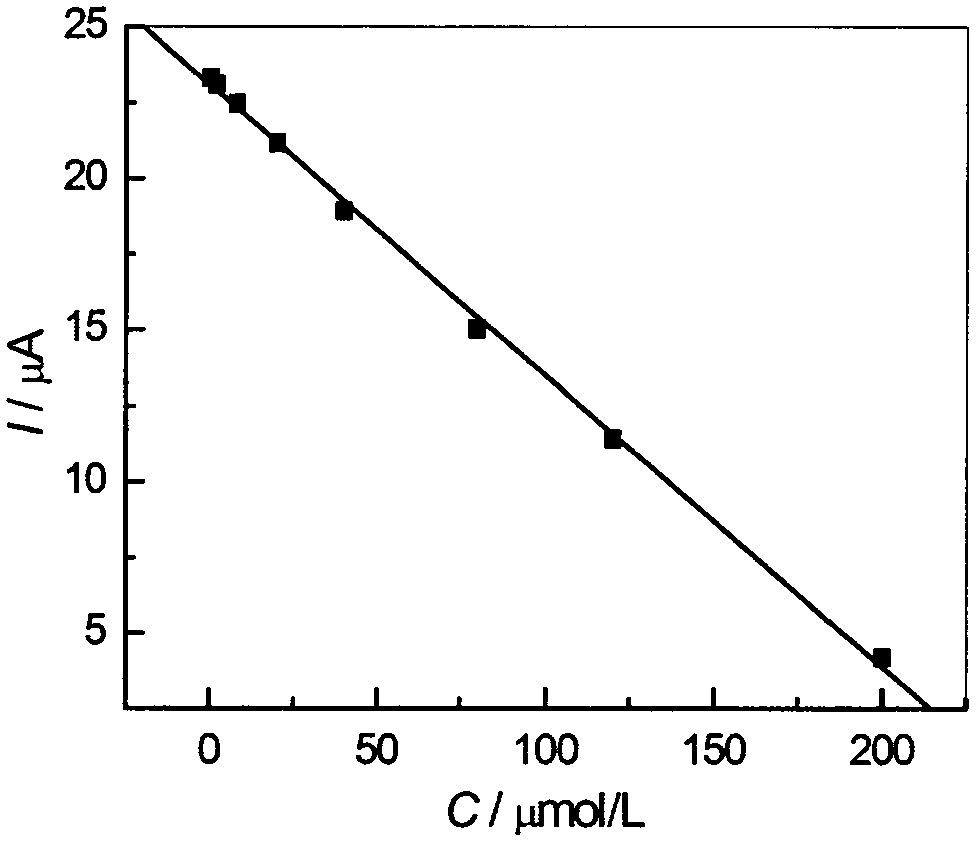
Method for preparing high-sensitivity nano-zirconia-doped dezocine molecular imprinting electrochemical sensor
The present invention discloses a method for preparing a high-sensitivity nano-zirconia-doped dezocine molecular imprinting electrochemical sensor, the high-sensitivity nano-zirconia-doped dezocine molecular imprinting electrochemical sensor is prepared by use of dezocine as a template molecule, (5s, 8s)-3-(4'-chloro-3'-fluoro-4-propenyl-biphenyl-3-yl)-4-hydroxy-8-methoxy-1-aza-spiro [4.5] dec-3-ene-2-one as a single function monomer, azobisisobutyronitrile as an initiator, nano-zirconia as a dopant and maleic rosin ethylene glycol acrylate as a crosslinking agent, wherein the maleic rosin ethylene glycol acrylate is prepared from rosin as a raw material, and the method is simple and practical, and overcomes the shortcomings that a conventional analysis method is complicated, expensive in equipment, and low in sensitivity.
Owner:GUANGXI UNIV FOR NATITIES
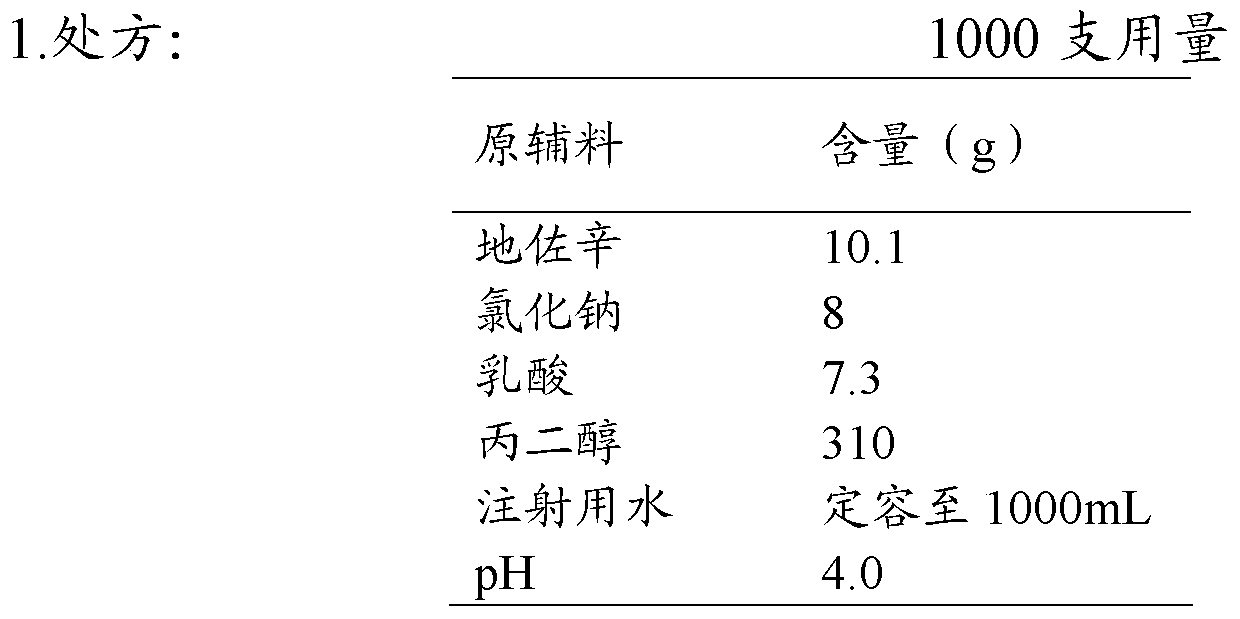
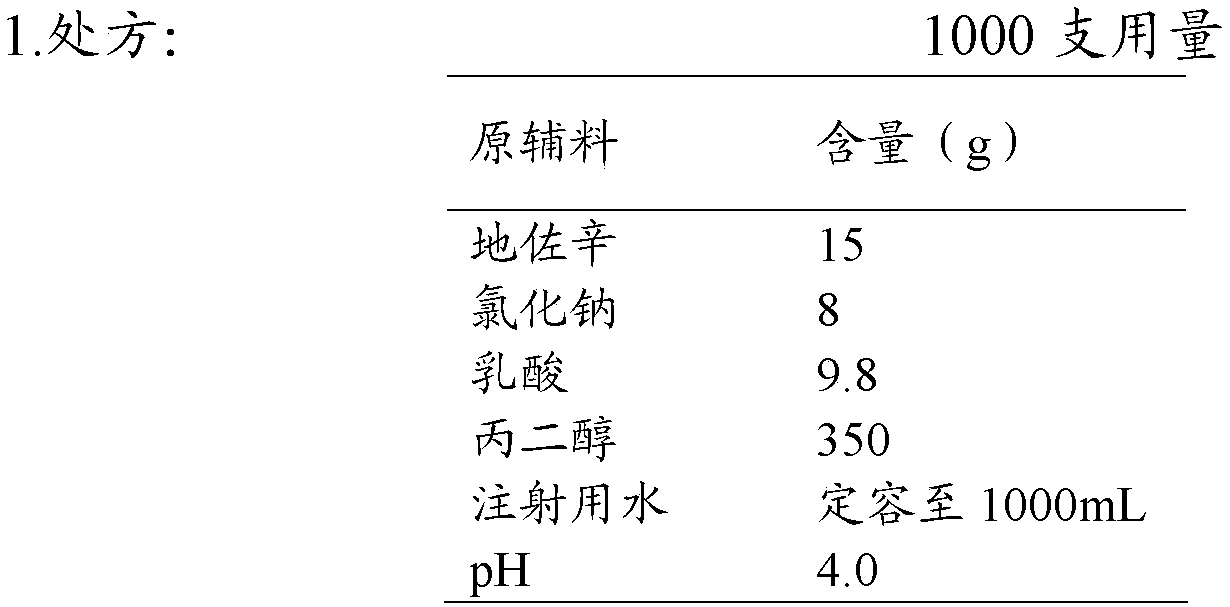
Injection of dezocine and preparation method of injection
ActiveCN104523584AImprove stabilityQuality improvementOrganic active ingredientsNervous disorderAqueous sodium hydroxideAdditive ingredient
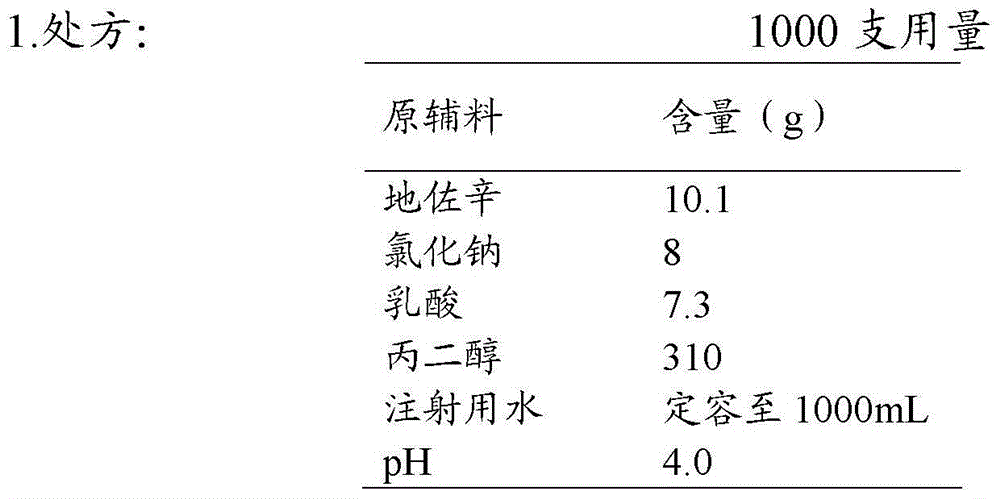
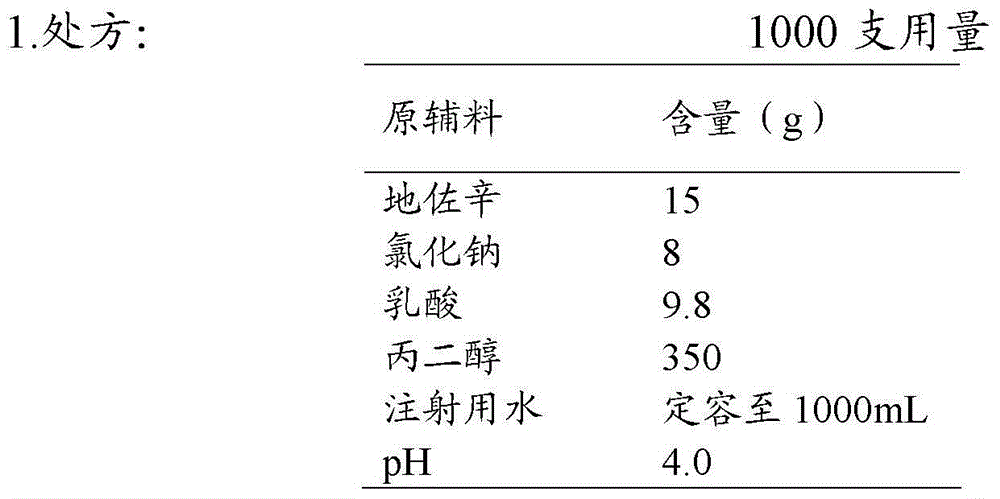
The invention discloses an injection of dezocine. Every milliliter of the injection consists of the following ingredients: 10.1mg to 25mg of dezocine, 1mg to 15mg of sodium chloride, 5mg to 20mg of lactic acid, 300mg to 450mg of propanediol, sodium hydroxide and the balance of nitrogen saturated injection water, wherein the consumption of the sodium hydroxide is determined as follows: the dezocine, sodium chloride, lactic acid and propanediol are dissolved by utilizing 30 to 70 percent by volume of the nitrogen saturated injection water to obtain a solution 1, and the pH value of the solution 1 is adjusted by utilizing a sodium hydroxide aqueous solution to 3.5 to 5.0. The injection is good in stability, stable in quality and suitable for being stored for a long time.
Owner:YANGTZE RIVER PHARM GRP CO LTD
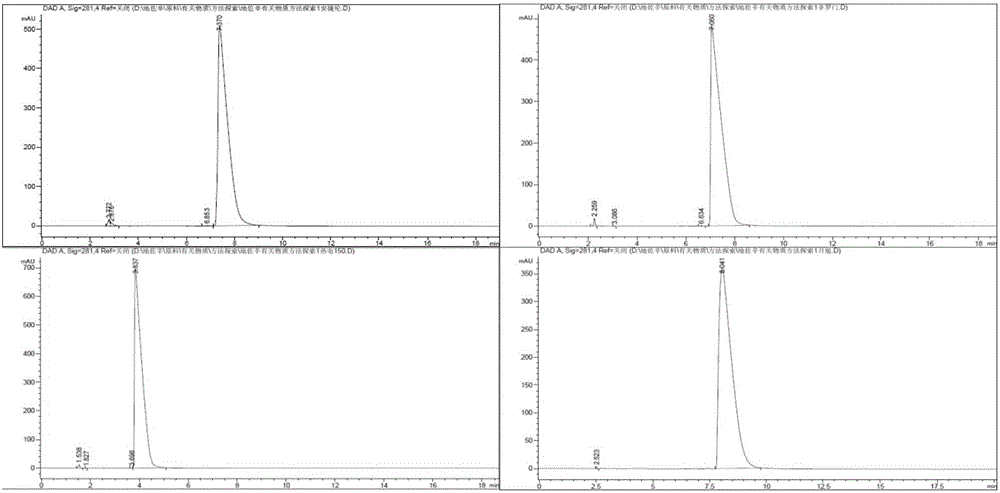
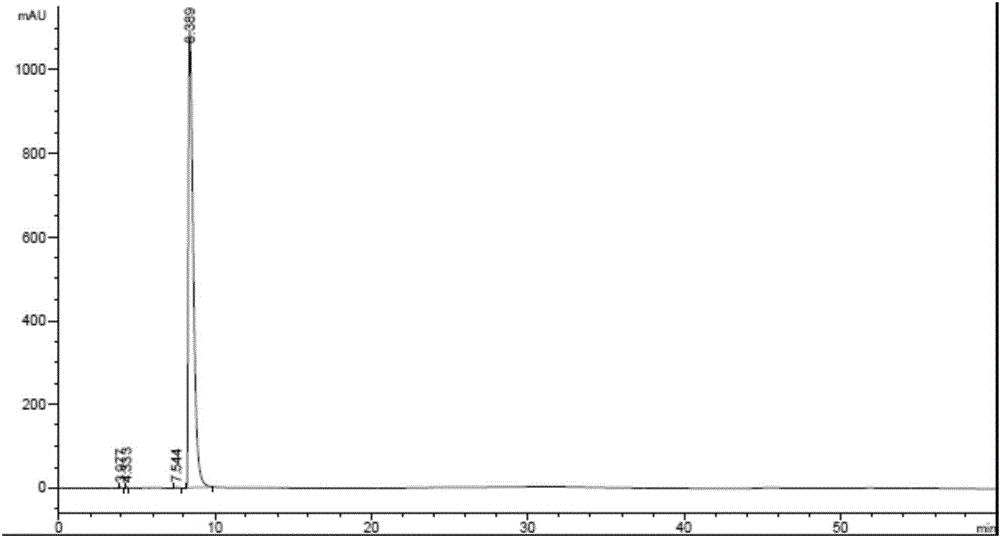
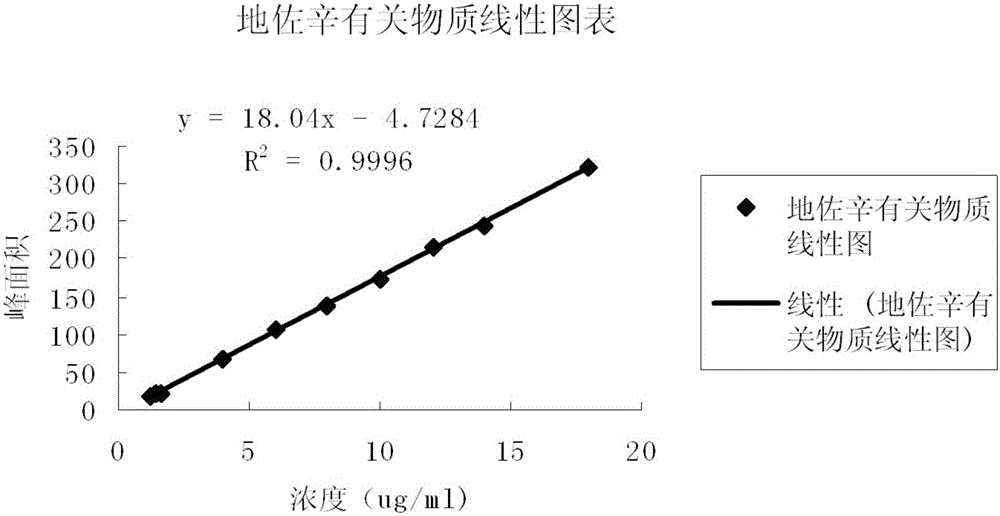
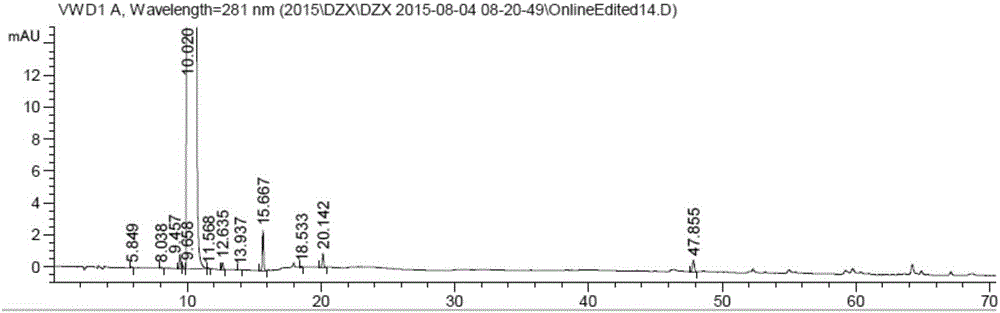
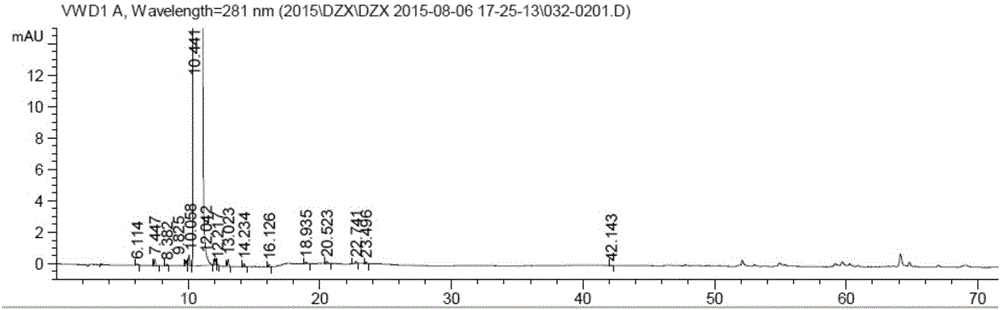
Detection method for dezocine related substances
InactiveCN106018584AIncrease concentrationImprove elutionComponent separationO-Phosphoric AcidSilica gel
The invention provides a detection method for dezocine related substances. Octadecyl silane bonded silica gel is adopted as a chromatographic column of a filling agent, and an acetonitrile-triethylamine solution is adopted as a mobile phase, wherein the acetonitrile-triethylamine solution is obtained by taking 1000 ml of water, adding 5 ml of triethylamine and 2 ml of a tetrabutyl ammonium hydroxide water solution, and adjusting pH to 3.2 through diluted phosphoric acid (1-5), and the ratio of acetonitrile to triethylamine is 200:800; the flow rate is 0.6 ml per min, and the detection wavelength is 281 nanometers. According to the detection method for dezocine related substances, the concentration of buffer salts is increased, elution is enhanced, an ion-pairing agent is added, the peak shape is improved, and the problems that due to the fact that the number of theoretical plates of an original method is small and a main peak cannot be rapidly eluted, the peak width is large and trailing is serious are solved.
Owner:JINAN LIMIN PHARMA
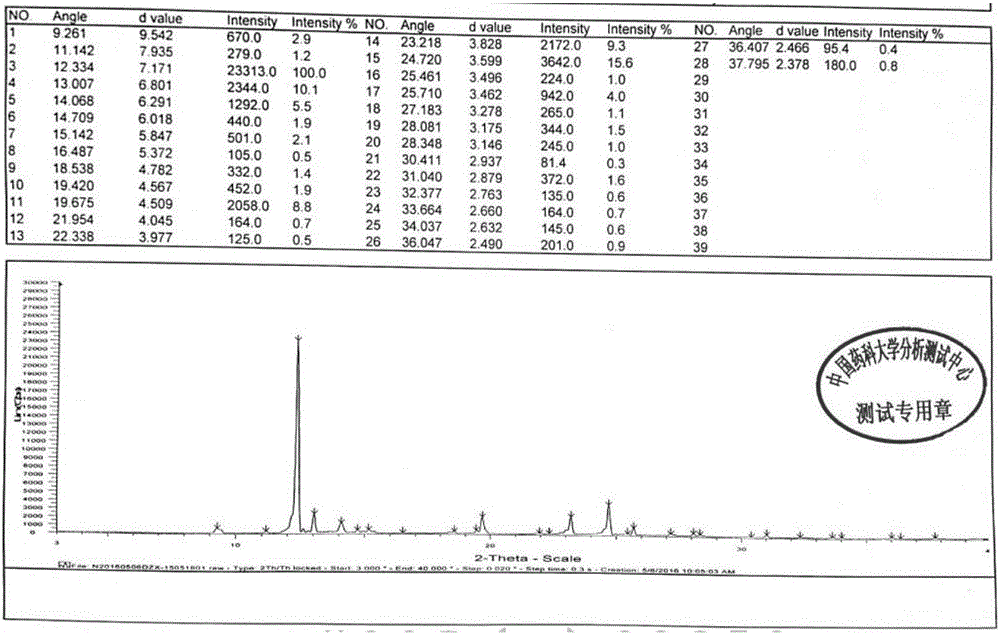
Dezocine A crystal form and preparation method thereof
InactiveCN107522625AHigh purityImprove bioavailabilityOrganic active ingredientsNervous disorderEnantiomerBioavailability
The invention provides a dezocine A crystal form. Cu-Kalpha ray radiation, X-ray powder diffraction represented by an angle 2theta has characteristic peaks at 9.261+ / -0.1, 11.142+ / -0.1, 12.334+ / -0.1, 13.007+ / -0.1, 14.068+ / -0.1, 14.709+ / -0.1, 15.142+ / -0.1, 18.538+ / -0.1, 19.420+ / -0.1, 19.675+ / -0.1, 23.218+ / -0.1, 24.720+ / -0.1, 25.710+ / -0.1, 27.183+ / -0.1, 28.081+ / -0.1, 28.348+ / -0.1 and 31.040+ / -0.1. The invention further provides a preparation method of the dezocine A crystal form. The dezocine A crystal form disclosed by the invention has extremely high bioavailability. The preparation method of the high-purity dezocine A crystal form disclosed by the invention has the technical effects that the impurity content is reduced, the individual impurity content is reduced from 0.15% to 0.05% or less, the total impurity content is reduced from 1.0% to 0.3% or less, the enantiomer content is not more than 0.2%, and the technical effect of simple and convenient operation is achieved.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
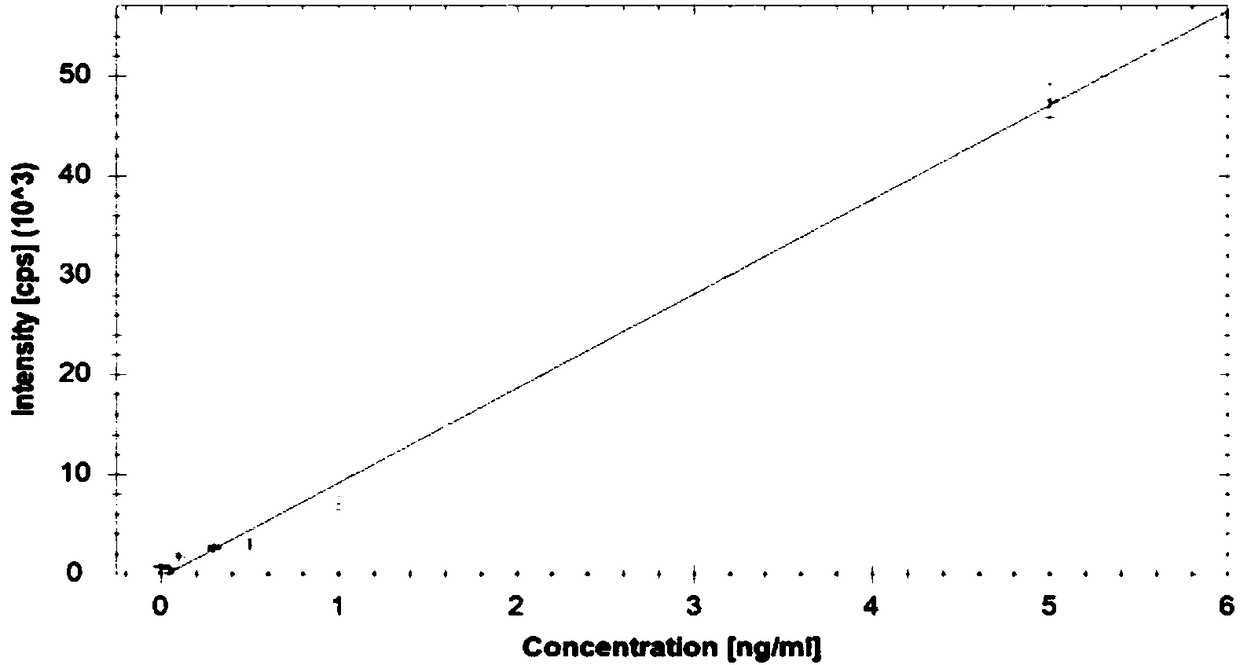
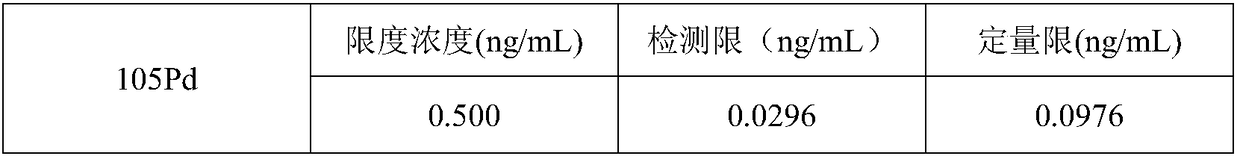
Method for determining palladium metal residues in dezocine raw materials
The invention provides a method for determining palladium metal residues in dezocine raw materials and specifically relates to the field of chemical detection. According to the method, the content ofpalladium in the dezocine raw materials is controlled, a sample is directly dissolved with an acetonitrile water liquor with proper concentration, a liquor is diluted with 2% HNO3 to required concentration, and the content of palladium elements is determined with a one-point external standard method by use of an ICP-MS (inductively coupled plasma source mass spectrometer). Compared with microwavedigestion or graphite digestion, the treatment method is safer and time-saving.
Owner:南京明捷生物医药检测有限公司
Preparation method of dezocine
ActiveCN102503840BSimple post-processingHigh optical purityOrganic compound preparationAmino-hyroxy compound preparationOrganic acidAlcohol
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
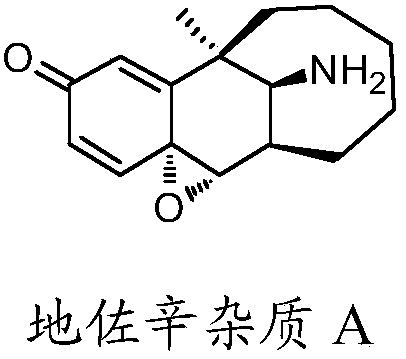
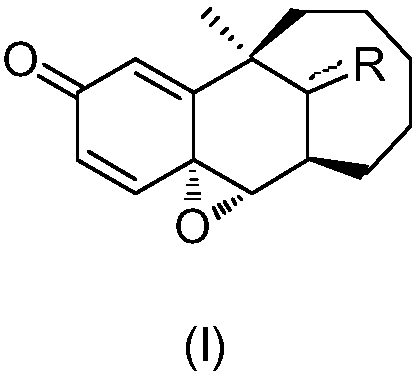
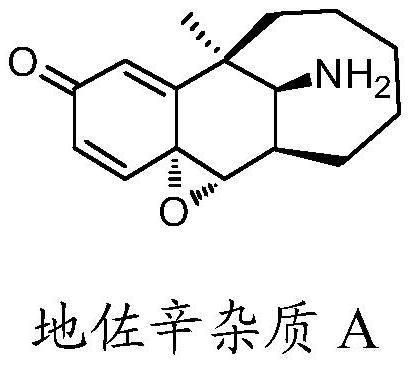
Preparation method of dezocine impurity A and homologues thereof
The invention discloses a preparation method of a dezocine impurity A and homologues thereof. The method includes the steps of: taking dezocine and homologues thereof as the raw material, adopting a transition metal as the catalyst and using a peroxide as the oxidizing agent, and carrying out reaction to obtain the dezocine impurity A and homologues thereof. The invention has the advantages that:a new chemical method is provided to convert phenolic substances into epoxides, at the same time the used reagents are simple and easily available, the operation is simple, and parts of the unreactedraw materials can be recovered.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Dezocine in-situ gel composition and application thereof
ActiveCN113679659AModerate viscosityHelps maintain analgesic effectOrganic active ingredientsAntipyreticDrug reservoirUse medication
The invention discloses a dezocine in-situ gel composition and a preparation thereof, the composition comprises the following components: dezocine, a biodegradable carrier and a non-aqueous solvent, and the dezocine in-situ gel composition has proper viscosity, can be used for injection, and is gelatinized at a medication site to form a semi-solid drug reservoir. The composition and the preparation disclosed by the invention not only can continuously deliver drugs and is beneficial to maintaining an analgesic effect, but also have excellent biocompatibility and safety.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of surface anesthesia drug composition, microemulsion and its preparation method and application
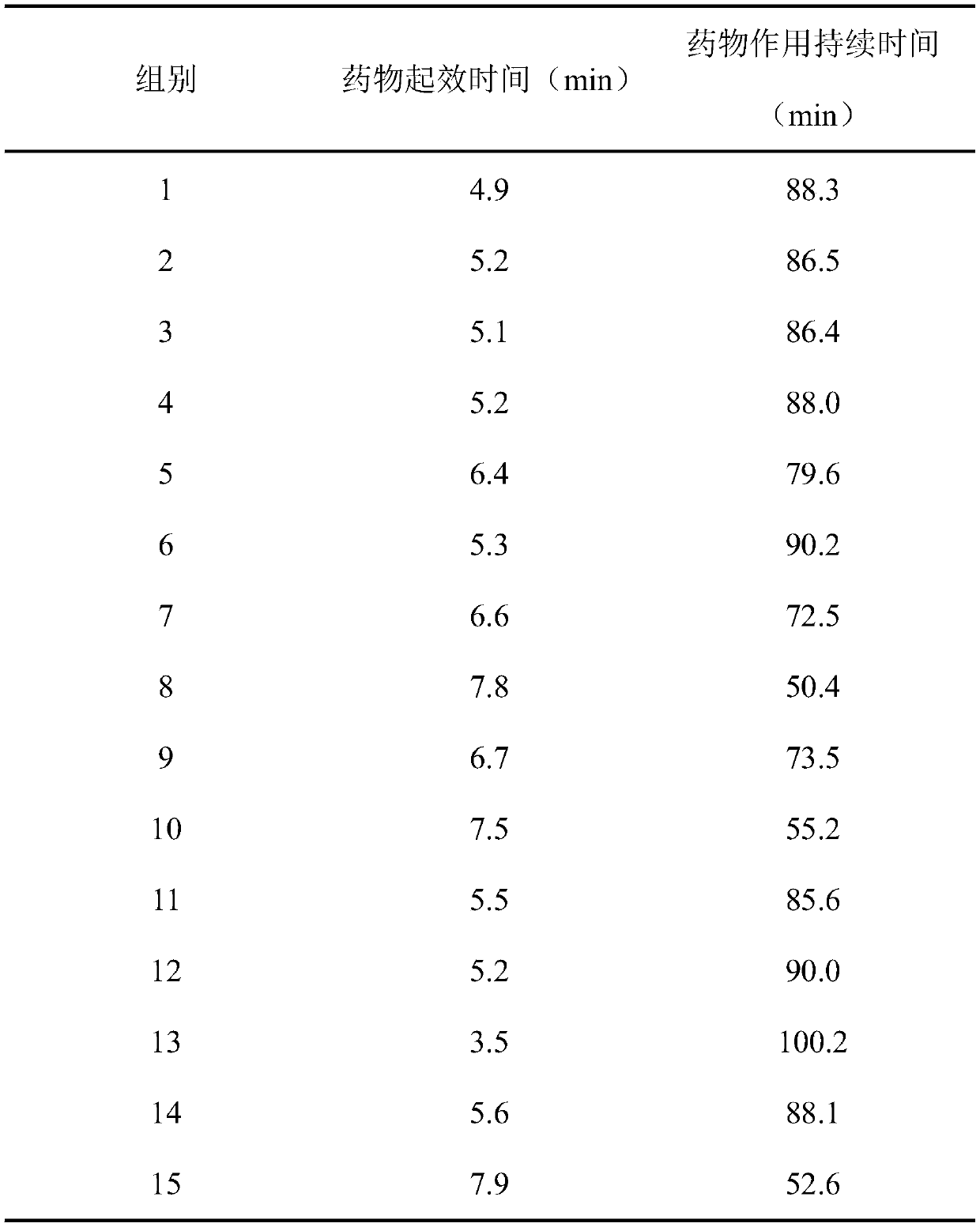
ActiveCN108653446BFast onset of anesthesiaLong duration of anesthesiaAnaestheticsEmulsion deliveryPharmaceutical drugNarcotic
The invention provides surface anesthesia medicine composition. The medicine composition is prepared from components in parts by weight as follows: 2-3 parts of lidocaine, 0.5-1 part of dezocine, 1-2parts of scopolamine and 0.3-0.5 parts of a radix zanthoxyli ethanol extract. The invention also provides a corresponding preparation of the medicine composition, a preparation method of the preparation as well as an application of the medicine composition in preparation of a surface anesthesia medicine. The medicine composition has the effects that the medicine composition takes anesthesia effectquickly, has long anesthesia holding time and can prevent shiver after anesthesia. The obtained microemulsion has small particle size, narrow particle size distribution and good transdermal effects.The medicine composition has good prospects in clinical application of surface anesthesia.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
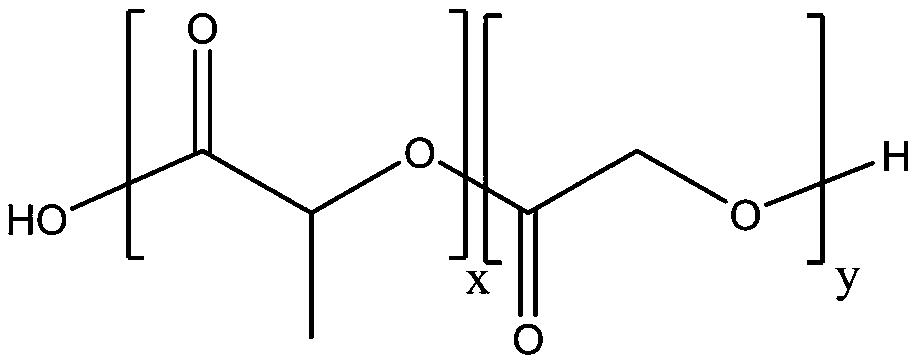
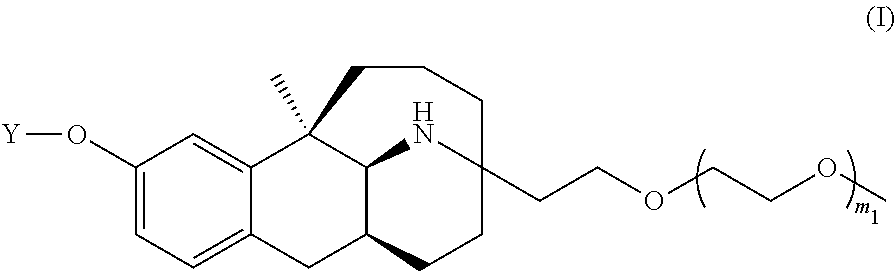
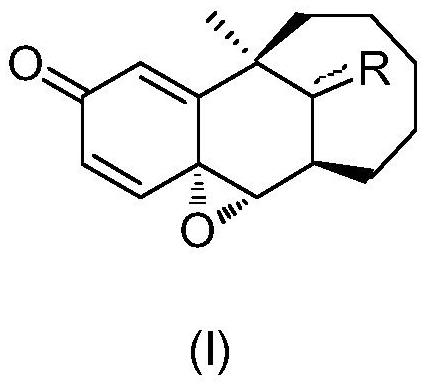
Sustained release suspension containing dezocine analogue ester and preparation method therefor
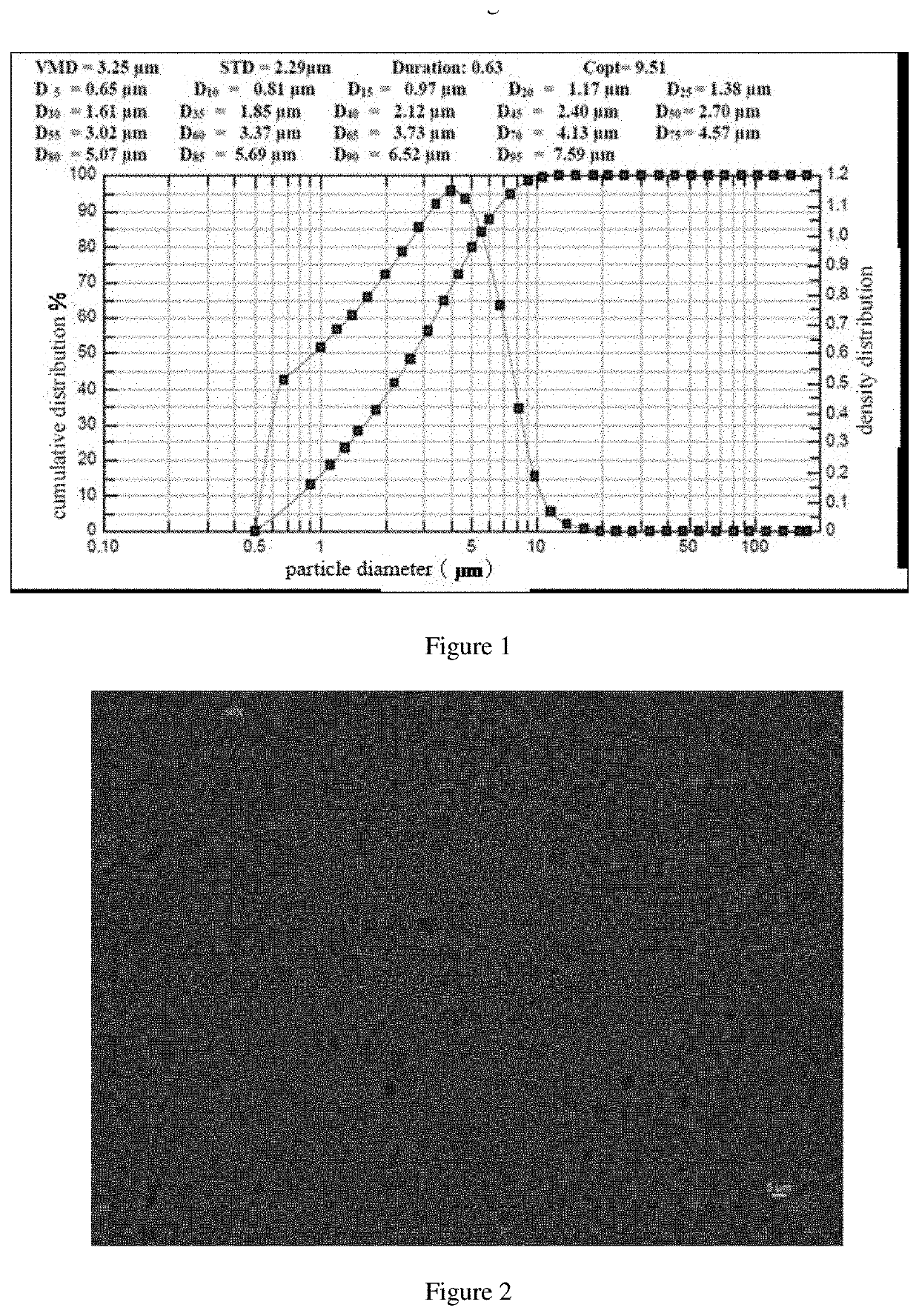
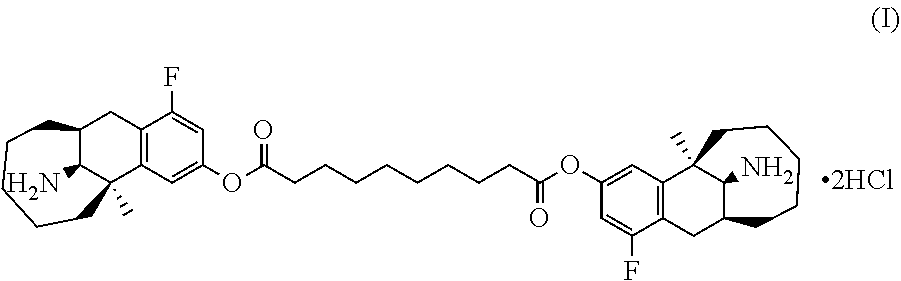
ActiveUS20200188342A1Simple compositionMaintain stable propertiesSolution deliveryEmulsion deliverySolventOrganic chemistry
Disclosed are a sustained release suspension containing dezocine analogue ester and a preparation method therefor. According to the present invention, an injectable oily auxiliary material which may pharmaceutically serve as a sustained-release depot and an excipient are selected, and micronization technology is used jointly with high pressure homogenization technology and an anti-solvent technique to prepare an injectable long-acting suspension. The main components of the suspension are a compound represented by formula (I), the injectable oil, and the like.
Owner:SHANDONG DANHONG PHARMA
Separation and purification method of dezocine
PendingCN114380702APurification and preparation are efficient and stableThe synthesis steps are simpleOrganic compound preparationOrganic chemistry methodsSimple Organic CompoundsBiochemical engineering
The invention discloses a dezocine separation and purification method, and relates to the technical field of medicine production, separation and purification are carried out according to organic compounds, and the dezocine separation and purification method comprises the following steps: step 1, preparing materials and reagents; 2, carrying out sample treatment; and step 3, separation and purification of dezocine. According to the invention, dezolamide salt, melizocine salt and dezocine are purified and prepared, dezocine is stored, and synthesis steps and reaction conditions are optimized, so that the synthesis period is shortened, the purification preparation route is simple, the steps are few, the yield is high, the operation is simple, the synthesis period is greatly shortened, the reaction conditions are mild, the process safety is improved, the raw material cost is low, and industrialization is facilitated; key process parameters and product quality standards are determined, so that key control points in the dezocine purification process can be quickly mastered, dezocine purification preparation can be efficiently and stably carried out, and convenience is brought to operation.
Owner:TAIZHOU DANDING BIOTECH CO LTD
Dezocine crystal form and preparation method thereof
InactiveCN111606816AImprove solubilityImprove stabilityOrganic compound preparationOrganic chemistry methodsPhysical chemistryPharmaceutical drug
The invention discloses a dezocine crystal form. An X-ray powder (XRD) diffraction pattern of the dezocine crystal form is measured by using Cu / K-alpha1, the dezocine crystal form has diffraction peaks at 2 theta values of 9.1+ / -0.2 and 12.2+ / -0.2, and the height percentages of the diffraction peaks are greater than 20. In addition, the invention also discloses a preparation method, a pharmaceutical composition and application of the crystal form. The dezocine crystal form is not reported yet, and is good in solubility, simple in preparation method operation, good in reproducibility, suitablefor industrial production and the like.
Owner:YANGTZE RIVER PHARM GRP CO LTD
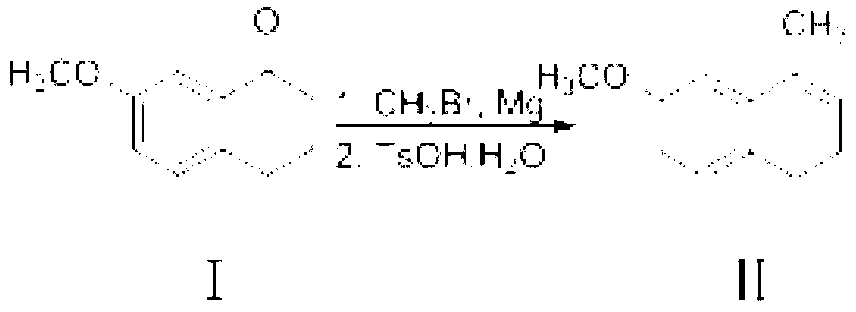
A kind of preparation method of dezocine key intermediate
ActiveCN104910002BImprove securityLow costOrganic compound preparationCarbonyl compound preparationAlkyl transferSynthesis methods
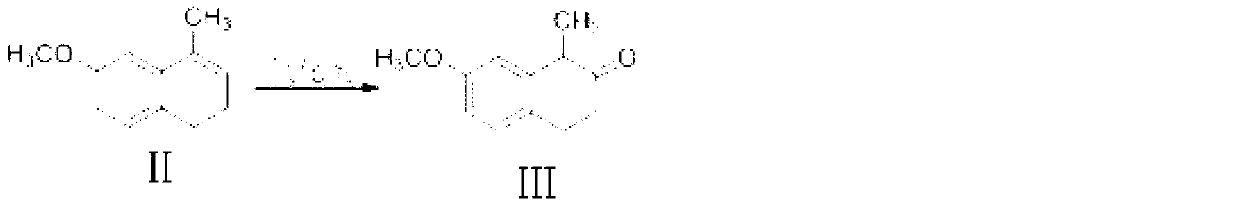
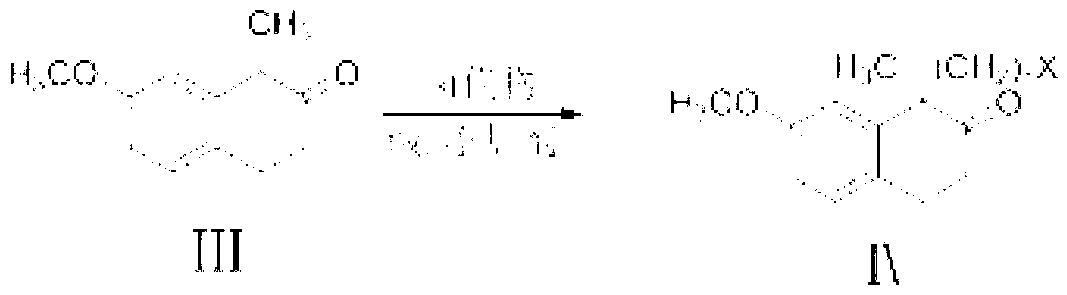
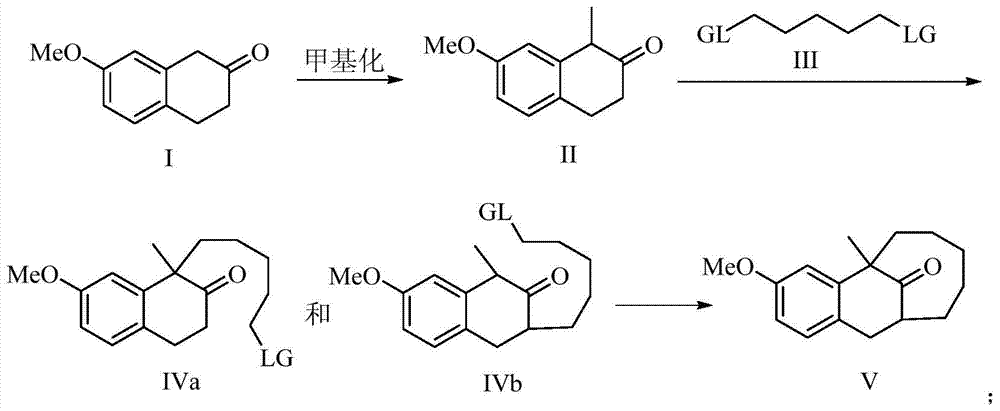
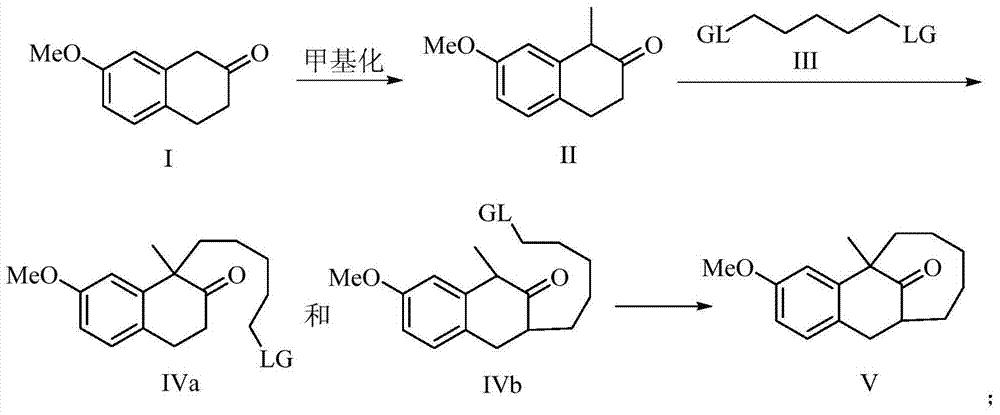
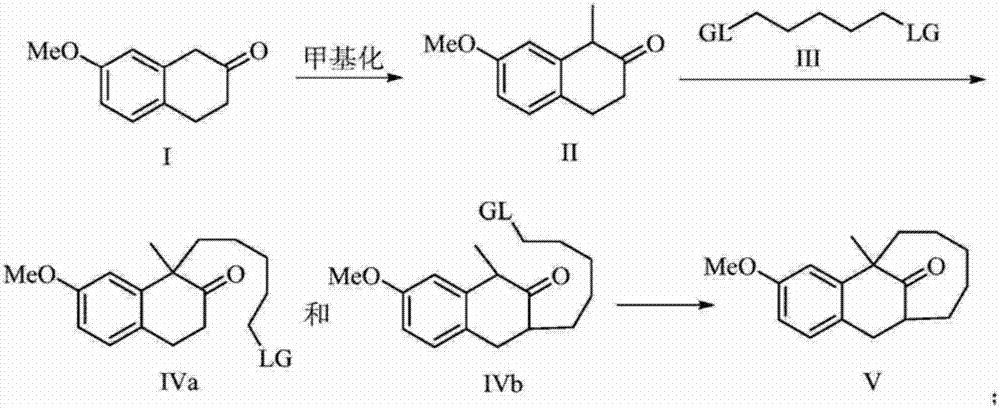
The present invention discloses a dezocine key intermediate preparation method, 7-methoxy-2-tetralone is used as a starting material for first benzyl-site methylation and then ortho alkylation of the carbonyl group, the dezocine key intermediate can be prepared by three strategies of stepwise synthesis, two-step and one-pot methods. The dezocine key intermediate preparation method has the advantages of simple synthetic route, less steps, high yield simple operation, great reduction of the synthesis cycle, mild reaction conditions, and improvement of the safety of the technology, the low raw material cost and easy industrialization. The preparation cost is greatly reduced by the simple synthesis method, the patient dosage cost is reduced, the national social security spending can be reduced to some extent, and some of the social and economic benefits are produced.
Owner:WENZHOU MEDICAL UNIV
Application of dezocine to prepare medicament for alleviating hyperpathia related to opioids after operation
InactiveCN104784163AImprove satisfactionIdentifying the role of acute hyperalgesiaOrganic active ingredientsNervous disorderWhole bodyClinical tests
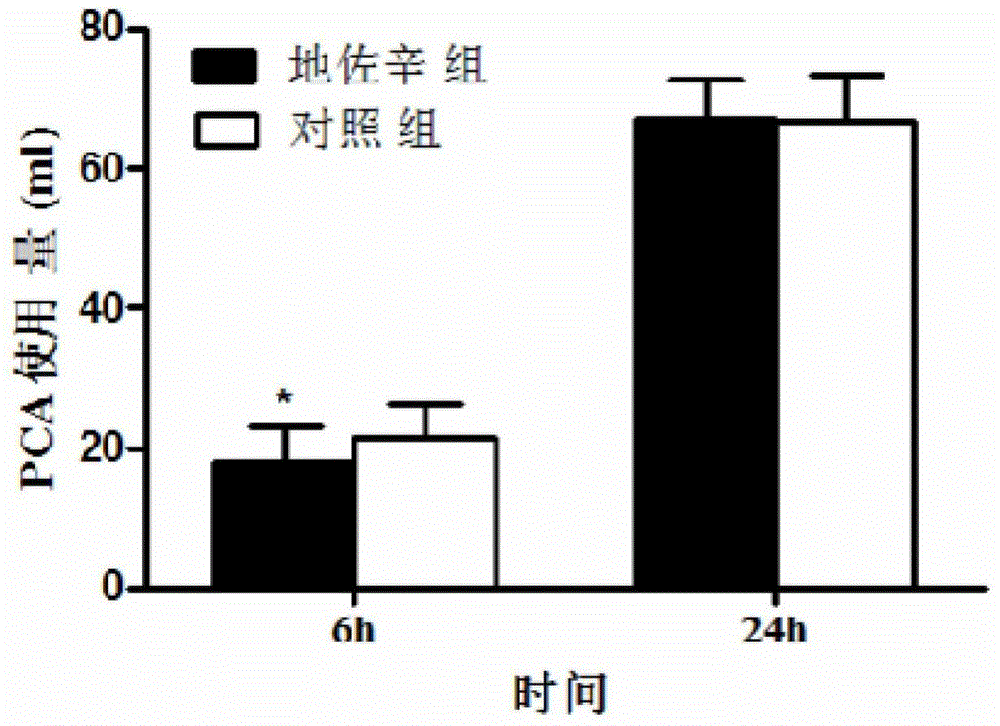
The invention discloses an application of dezocine to prepare medicament for alleviating hyperpathia related to opioids after operation, which belongs to the field of pharmaceutical chemistry. According to the invention, it is proved by clinical tests that the morbidity of hyperpathia related to opioids after operation of patients suffering morbidity can reach as high as 88%, and hyperpathia of the whole body of a patient after operation can be alleviated by intravenous injection of dezocine. The range of hyperpathia around an operative incision is decreased; the use amount of PCIA after operation is reduced; the satisfaction degree of pain control for the patients is high; and no server side effect due to single-dose intravenous injection of dezocine is found.
Owner:俞芳
Dezocine oral preparation
InactiveCN108403651AAchieve therapeutic effectAchieve the effect of injection drug deliveryOrganic active ingredientsNervous disorderBlood concentrationAdditive ingredient
The invention discloses a dezocine oral preparation. The dezocine oral preparation is suitable for unit preparations, and is prepared from 5-150 mg of dezocine and any pharmaceutically acceptable ingredients of the oral preparation. In addition, the invention further discloses a preparation method and application of the oral preparation. The clinical tests prove that within the dosage range, the dezocine oral preparation has the similar blood concentration compared with commercially available dezocine injection, the adverse reaction is slight, and drug administration is more convenient.
Owner:YANGTZE RIVER PHARM GRP CO LTD
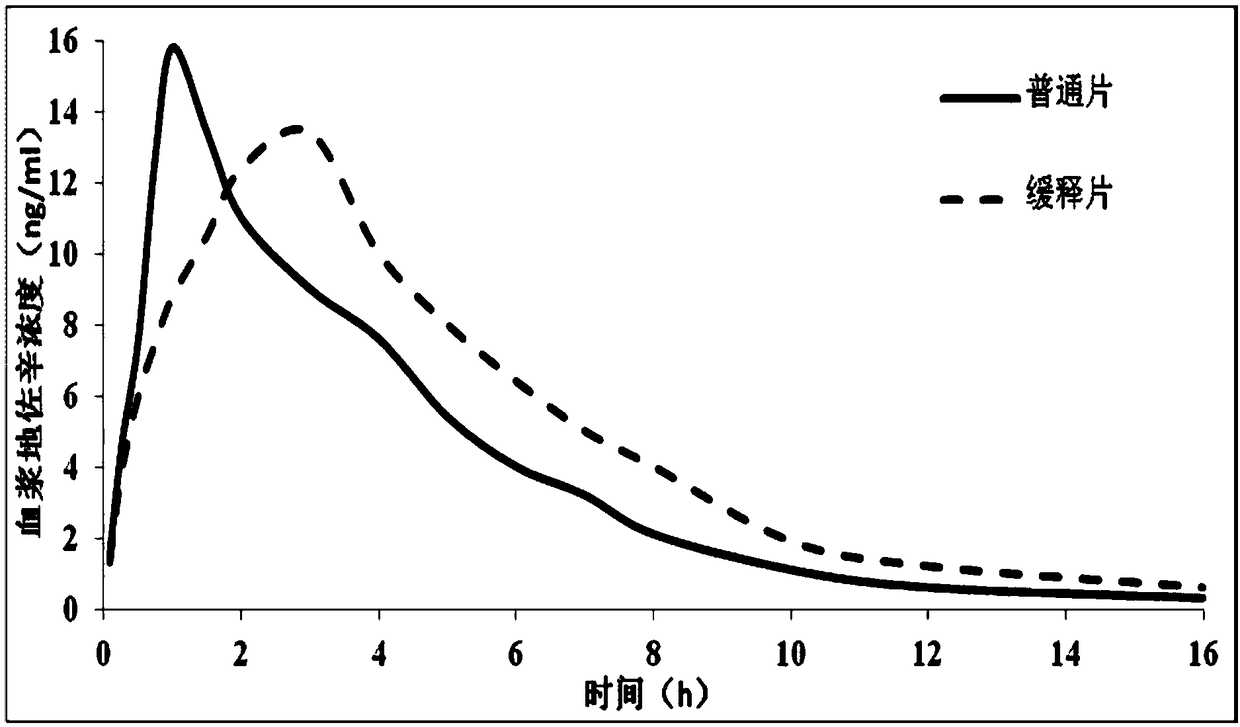
A drug sustained-release preparation containing dezocine and its preparation method
ActiveCN110101865BStable release rateRelieve painOrganic active ingredientsNervous disorderEngineeringDrug release
The invention provides a dizocine-containing medicine sustained-release preparation and a preparation method thereof. The dizocine-containing medicine sustained-release preparation is prepared from, by weight, 1-10 parts of dezocine, 1-10 parts of a polylactic acid-hydroxyacetic acid block copolymer, 1-10 parts of a dissolution aiding additive, 1-10 parts of an osmotic pressure regulator, 0.1-0.5part of a pH value stabilizer, 1-5 parts of a wetting agent, 1-10 parts of a freeze-dried powder protectant, 10-50 parts of an organic solvent and 100-1000 parts of water for injection. Compared withan existing dezocine injection reagent, the dezocine is encapsulated in a biocompatible high-molecular polymer, the medicine can be released slowly, the release speed of the medicine is stable, the stable blood concentration can be maintained for 8h or more after single-time administration, the pain of a patient can be effectively relieved, and the dezocine injection reagent can be used for relieving the pain of the patient after the operation.
Owner:TIANJIN CITY THIRD CENT HOSPITAL
Preparation method of a highly sensitive nano-zirconia-doped dezocine molecularly imprinted electrochemical sensor
The present invention discloses a method for preparing a high-sensitivity nano-zirconia-doped dezocine molecular imprinting electrochemical sensor, the high-sensitivity nano-zirconia-doped dezocine molecular imprinting electrochemical sensor is prepared by use of dezocine as a template molecule, (5s, 8s)-3-(4'-chloro-3'-fluoro-4-propenyl-biphenyl-3-yl)-4-hydroxy-8-methoxy-1-aza-spiro [4.5] dec-3-ene-2-one as a single function monomer, azobisisobutyronitrile as an initiator, nano-zirconia as a dopant and maleic rosin ethylene glycol acrylate as a crosslinking agent, wherein the maleic rosin ethylene glycol acrylate is prepared from rosin as a raw material, and the method is simple and practical, and overcomes the shortcomings that a conventional analysis method is complicated, expensive in equipment, and low in sensitivity.
Owner:GUANGXI UNIV FOR NATITIES
Compositions and methods for treating opioid receptor associated diseases
The invention relates to dezocine compositions and uses thereof. Specifically, the invention relates to dezocine compositions, including nano-dezocine compositions and methods for preventing or treating opioid receptor associated diseases, including neuropathic pain; addiction, such as opioid or cocaine addiction; and depression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Opioids composition for treating withdrawal symptoms of morphine addiction
InactiveCN107648234AReduce dosageAlleviate withdrawal symptomsOrganic active ingredientsNervous disorderSide effectQuality of life
The invention relates to the field of pharmaceutical technologies, in particular to an opioids composition for treating withdrawal symptoms of morphine addiction. The opioids composition comprises buprenorphine and dezocine. A weight ratio of the buprenorphine to the dezocine is 1:6-1:1. The opioids composition has the advantages that obvious effects of treating the morphine addiction can be realized by the opioids composition; the withdrawal symptoms can be obviously relieved, the opioids composition is little in side effect, secondary addiction can be prevented, and the quality of life can be effectively improved.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
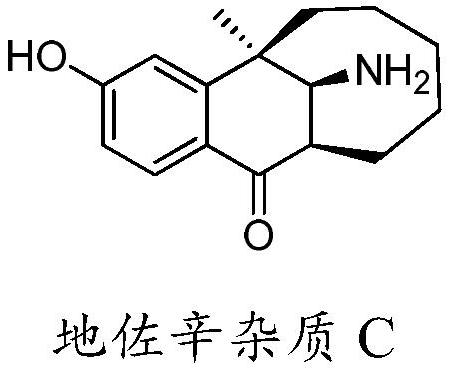
A kind of preparation method of dezocine impurity c
ActiveCN110483315BEfficient synthesisOrganic chemistryOrganic compound preparationPtru catalystBis epoxide
The invention discloses a preparation method of dezocine impurity C, which uses dezocine impurity A and its homologues as raw materials, undergoes a rearrangement reaction in the presence of a catalyst, and separates and prepares dezocine impurity C. The advantage of the present invention is that: using dezocine impurity A with epoxy structure or its homologues as raw materials, the simple and efficient synthesis of dezocine impurity C is realized by utilizing the rearrangement reaction of epoxides, and the reagents used are simple and easy to obtain , easy to operate.
Owner:YANGTZE RIVER PHARM GRP CO LTD
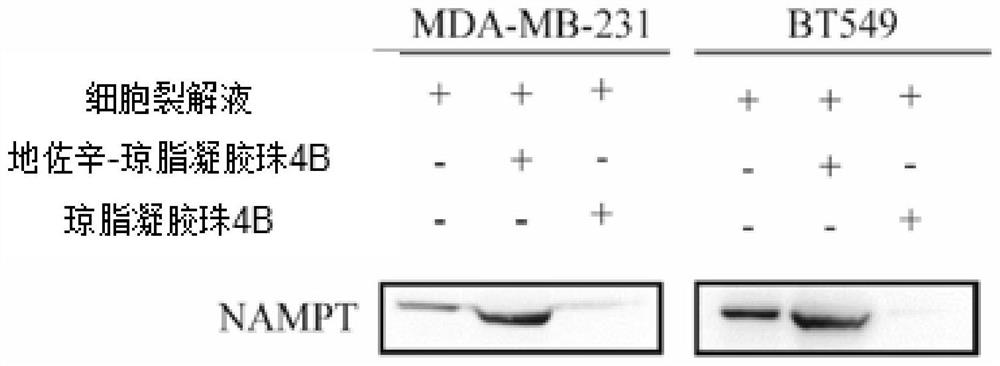
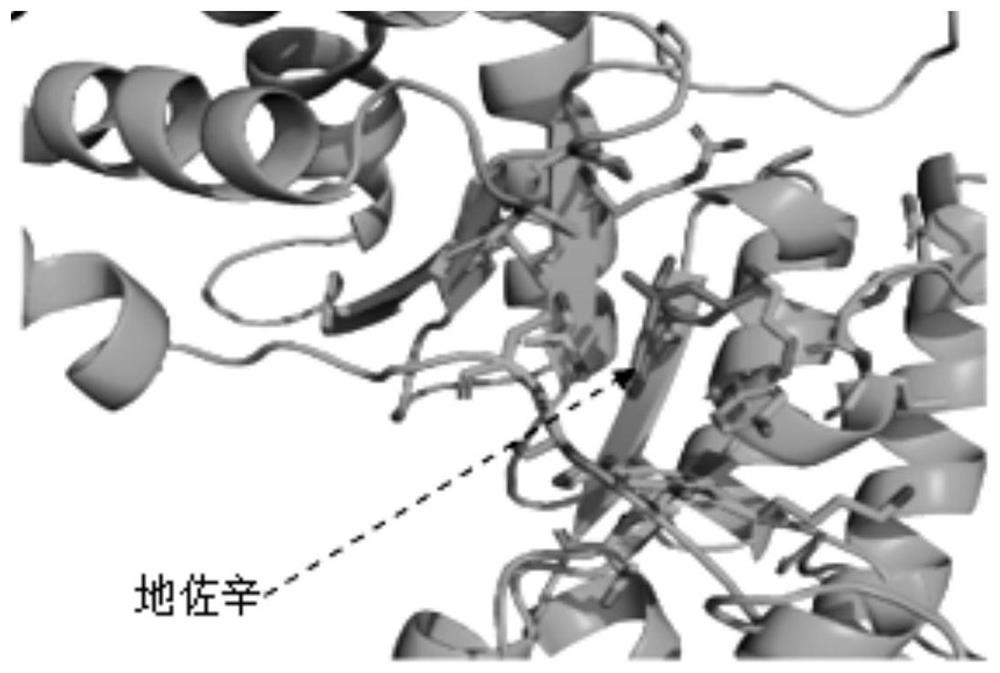
Application of dezocine in preparation of nicotinamide phosphoribosyltransferase inhibitor
InactiveCN111939145AOrganic active ingredientsAntineoplastic agentsNicotinamide phosphoribosyltransferaseAmidophosphoribosyltransferase
The invention relates to an application of dezocine in preparation of a nicotinamide phosphoribosyltransferase inhibitor. Tests prove that dezocine can be directly combined with nicotinamide phosphoribosyltransferase to inhibit the activity of nicotinamide phosphoribosyltransferase, and can be used as an active ingredient of a nicotinamide phosphoribosyltransferase inhibitor.
Owner:SHENZHEN UNIV
Conjugate of dezocine and polyethylene glycol
ActiveUS20190298845A1Improved pharmacokinetic propertiesHigh drug absorption degreeOrganic active ingredientsNervous disorderSide effectTolerability
The present invention relates to the technical field of medicine, in particular to a conjugate of dezocine and polyethylene glycol and a pharmaceutical composition thereof. The conjugate of dezocine and water-soluble oligomer provided by the present invention has better pharmacokinetic properties and a high drug absorption degree, may reduce the side effects of the drug, and achieve a smaller administration dosage and a more diverse mode of administration, such as oral administration, in clinic. Compared with dezocine, the conjugate of the present invention has a stronger analgesic effect and a longer analgesic duration, may reduce the frequency of drug administration, improve patient compliance, and has advantages in effectiveness and safety of the drug, as well as drug tolerance, etc.
Owner:JENKEM TECH
Preparation method of dezocine impurities
ActiveCN111170886AImprove medication safetyHigh purityImino compound preparationCombinatorial chemistryOrganic chemistry
The invention discloses a preparation method of a dezocine impurity. (5R,11S,13S)-3-methoxy-5-methyl-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methylbenzocyclononene-13-amine is used as an initial raw material to efficiently synthesize and prepare (5R,11S)-3-hydroxy-5-methyl-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methylbenzocyclononene-13-imine. The preparation method has the advantages of simple operation, easily available raw materials, mild reaction conditions, high product purity and high yield, and is suitable for large-scale preparation. The synthesized impurity can be used for qualitative and quantitative analysis of impurities, so the medication safety of dezocine can be improved.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of asymmetric synthesis method of dezocine key intermediate
ActiveCN108299173BOrganic compound preparationCarbonyl compound preparationTetraloneReaction intermediate
The invention discloses an asymmetric synthesis method of a dezocine key intermediate, namely, (5R,11S)-5,6,7,8,9,10,11,12-octahydro-3-methoxy-5-methyl-5,11-methylenebenzocyclodecene-13-one. The synthesis method comprises the steps as follows: 7-methoxy-1-methyl-2-tetralone is adopted as a raw material, and an alkylation reaction intermediate, namely, (1R)-1-(5-bormopentyl)-7-methoxy-1-methyl-tetralone, is synthesized in a stereoselective manner under the catalysis of a cinchonidine derivative; ring formation under the alkaline action is performed, and (5R,11S)-5,6,7,8,9,10,11,12-octahydro-3-methoxy-5-methyl-5,11-methylenebenzocycldecene-13-one with high chiral purity is obtained through recrystallization. The method is high in reaction yield, low in cost, mild in condition and suitable for efficient synthesis of dezocine on a large scale.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of injection of dezocine and preparation method thereof
ActiveCN104523584BImprove stabilityQuality improvementOrganic active ingredientsNervous disorderAqueous sodium hydroxide1-Propanol
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of preparation method of dezocine impurity a and its homologue
Owner:YANGTZE RIVER PHARM GRP CO LTD
Asymmetric synthesis method of dezocine key intermediate
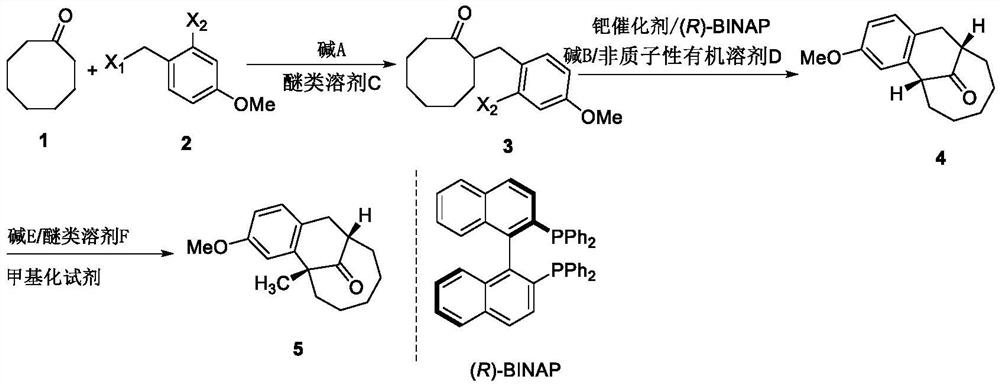
PendingCN113896621AStarting materials are cheap and readily availableShort and efficient preparation stepsOrganic compound preparationOrganic chemistry methodsPtru catalystPalladium catalyst
The invention discloses an asymmetric synthesis method of a dezocine key intermediate. The method comprises the following steps of carrying out nucleophilic reaction on a compound 1 and a compound 2 in an ether solvent C under the condition of alkali A to obtain a compound 3, carrying out coupling reaction on the compound 3 in an aprotic organic solvent D under the action of alkali B, a palladium catalyst and a chiral ligand (R)-BINAP at 80-110 DEG C to obtain a compound 4, wherein the palladium catalyst is selected from any one or more than two of Pd(OAc)2, Pd(dba)2 and Pd2(dba)3, and reacting the compound 4 in an ether solvent F under the action of alkali E and a methylation reagent to obtain a compound 5. The method is simple and easy to operate, the dezocine key intermediate with high optical purity can be obtained, and the method is suitable for industrial production.
Owner:宁波赜军医药科技有限公司
A compound drug sustained-release preparation containing dezocine and lidoine and its preparation method
ActiveCN110101660BStable release rateRelieve painOrganic active ingredientsPowder deliveryEngineeringDrug release
Owner:TIANJIN CITY THIRD CENT HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com