Dezocine crystal form and preparation method thereof
A technology of dezocine and crystal form, applied in the field of medicine, can solve the problems of unfavorable preparation development, drug absorption, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
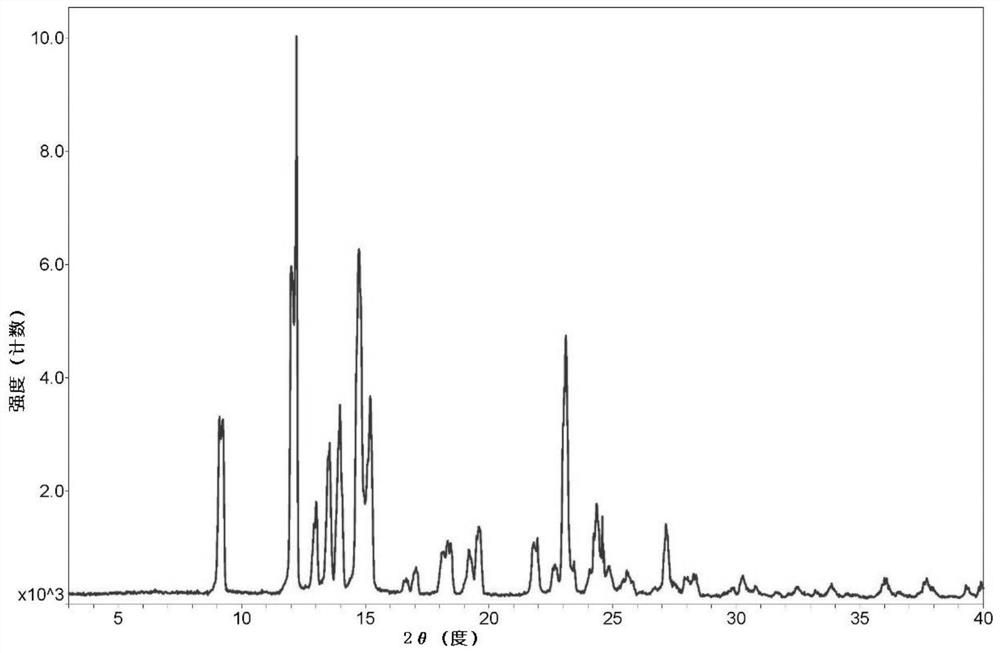
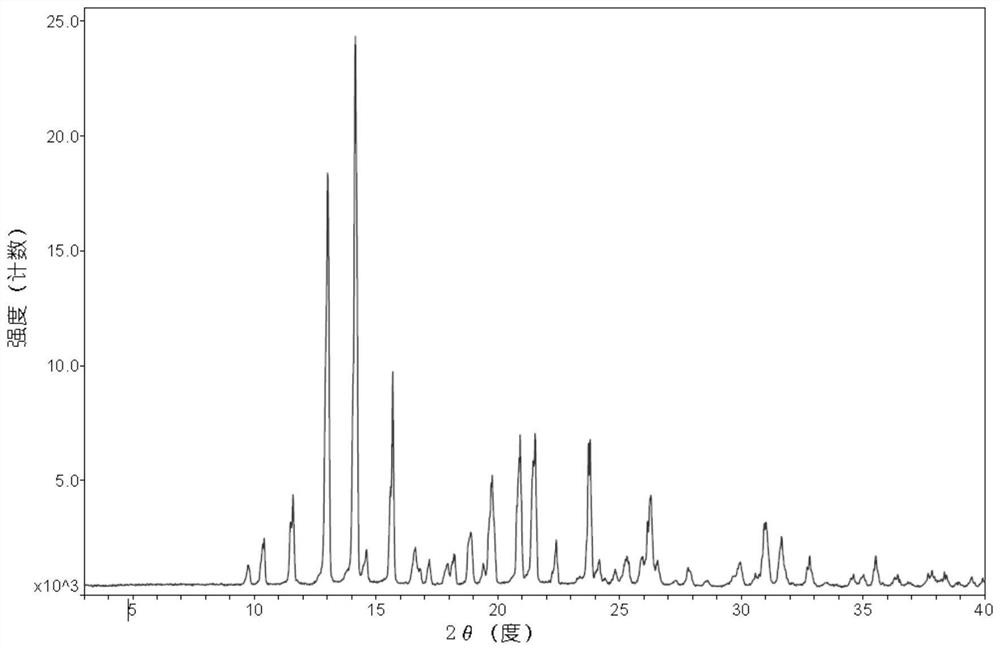
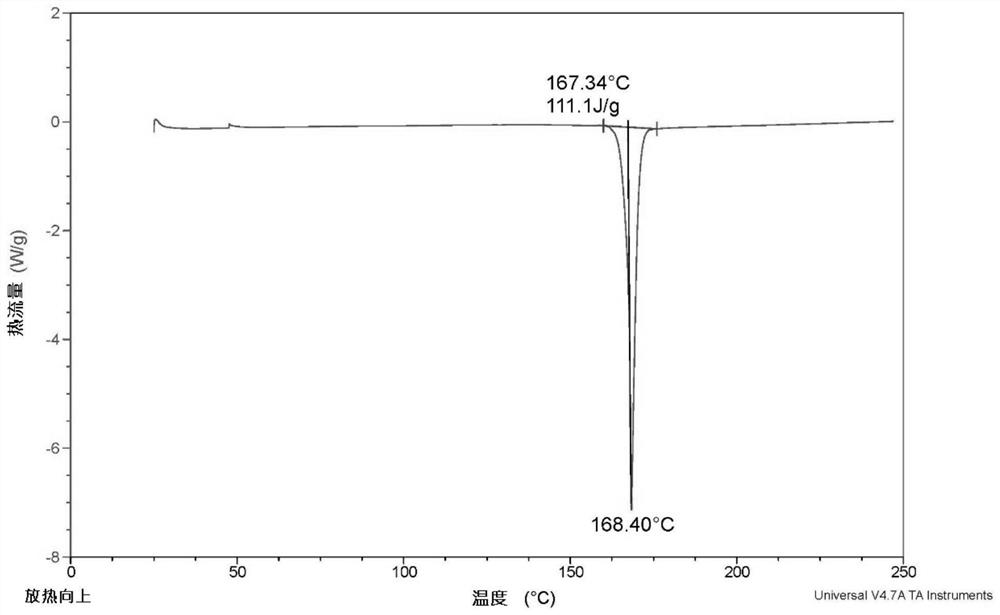
[0069] Add 2g of dezocine and 7.5ml of dioxane into a clean 50ml reaction bottle, stir and heat to 80°C for complete dissolution, then add 7.5ml of acetonitrile, cool down to about 5°C for crystallization for 2h, filter to obtain a solid, 80 Vacuum drying at °C gave 1.7 g of dry dezocine with a yield of 85%. Measure the XRD collection of patterns of this dezocine crystal form as Figure 1a As shown, the DSC spectrum is as Figure 2a As shown, the IR spectrum is as image 3 As shown, the TGA spectrum is as Figure 4 shown.
Embodiment 2
[0071] Add 2g of dezocine and 9ml of dioxane to a clean 50ml reaction bottle, stir and heat to 60°C for complete dissolution, then add 6ml of petroleum ether, cool down to about 5°C for crystallization for 2 hours, filter to obtain a solid, 60°C After vacuum drying, 1.9 g of dry dezocine was obtained with a yield of 95%.
Embodiment 3
[0073] Add 2g of dezocine into a clean 50ml reaction bottle, add 15ml of ethyl acetate, stir and heat to 60°C for complete dissolution, then add 6ml of petroleum ether, cool down to about 15°C for crystallization for 2 hours, filter to obtain a solid, at 80°C After vacuum drying, 1.3 g of dry dezocine was obtained, with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com