A kind of injection of dezocine and preparation method thereof
A technology of xin injection and dezocine, which is applied in the field of medicine and can solve the problems of increased degree, uncontrollable, and excessive degradation B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
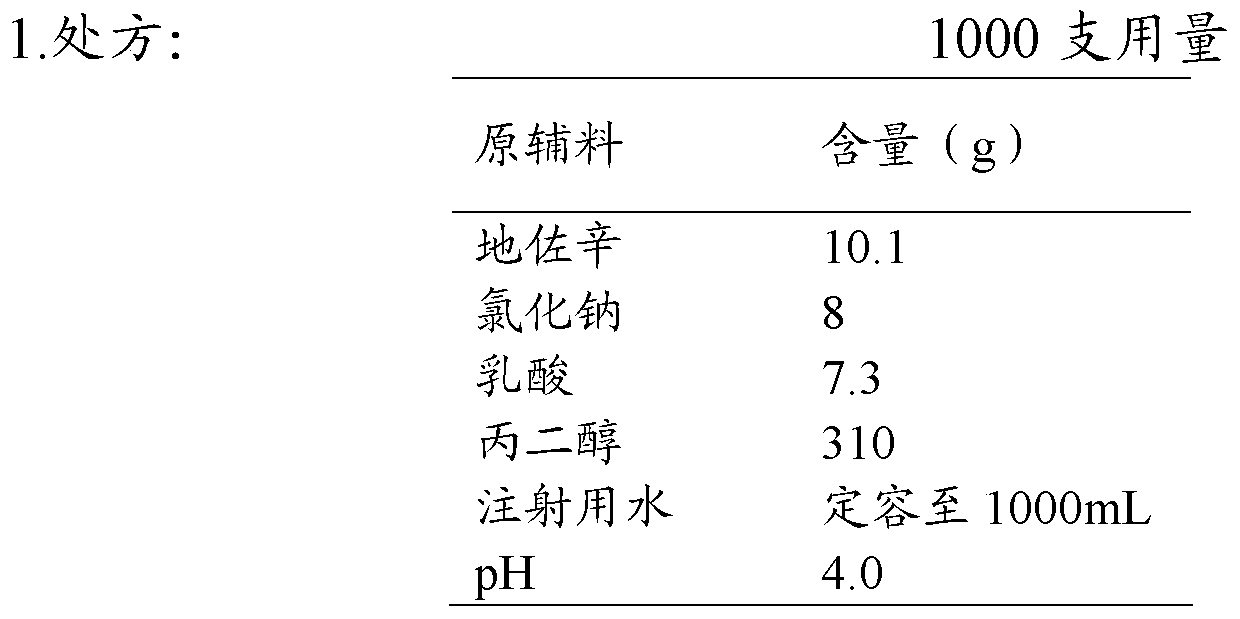
[0068]
[0069] 2. Preparation process
[0070] Take 50% of the prescription amount of nitrogen-saturated water for injection, add lactic acid, dezocine, propylene glycol, and sodium chloride successively, stir to dissolve, and obtain solution 1; adjust the pH value of the solution to 4.0 with 4% by weight sodium hydroxide solution; add Dilute the above solution to 1000ml with nitrogen-saturated water for injection; add medicinal charcoal according to 0.02%-0.5% (W / V) of the total volume, keep warm at 30°C for 10 minutes, and filter through pre-filtration and sterilizing filter; The above-mentioned medicinal solution was divided into ampoules, 1ml / bottle, filled with nitrogen, and melt-sealed; put the above-mentioned ampoules containing the medicinal solution into an autoclaving cabinet for sterilization, and the sterilization conditions were 121°C for 12min; Inspection, labeling, packaging, and the finished product is obtained.
Embodiment 2
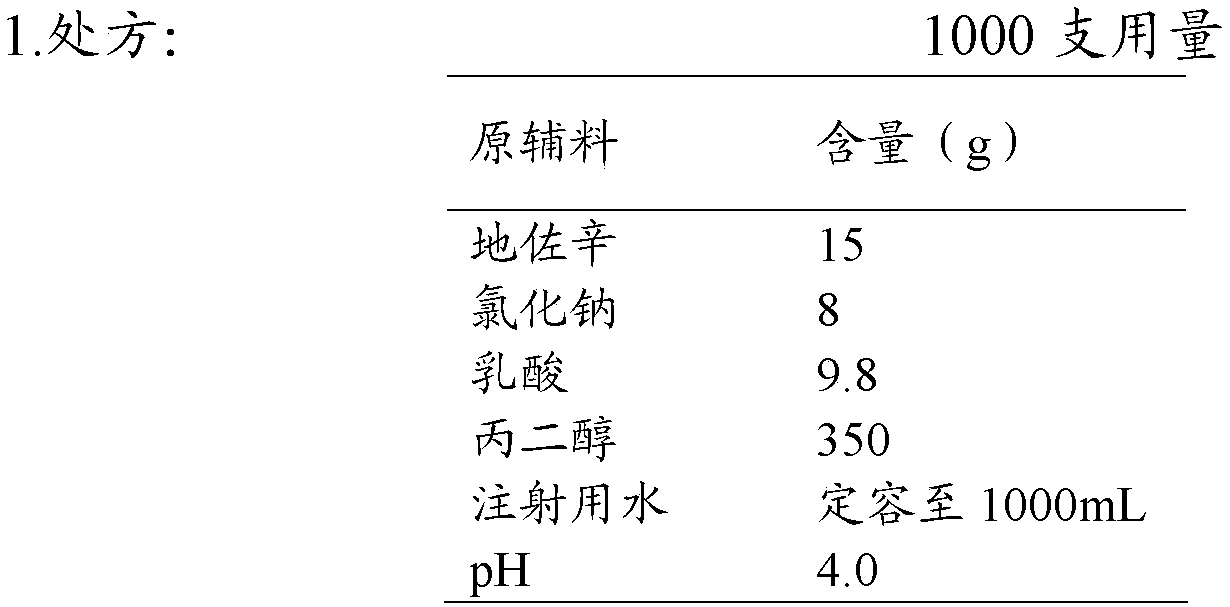
[0072]
[0073] 2. Preparation process
[0074] Take 50% of the prescription amount of nitrogen-saturated water for injection, add lactic acid, dezocine, propylene glycol, and sodium chloride successively, stir to dissolve, and obtain solution 1; adjust the pH value of the solution to 4.0 with 4% by weight sodium hydroxide solution; add Dilute the above solution to 1000ml in nitrogen-saturated water for injection; add medicinal charcoal according to 0.02%-0.5% (W / V) of the total volume, keep warm at 40°C for 15 minutes, and filter through pre-filtration and sterilizing filter; The above-mentioned medicinal solution was divided into ampoules, 1ml / bottle, filled with nitrogen, and melt-sealed; put the above-mentioned ampoules containing the medicinal solution into an autoclaving cabinet for sterilization, and the sterilization conditions were 121°C for 12min; Inspection, labeling, packaging, and the finished product is obtained.
Embodiment 3
[0076]
[0077]
[0078] 2. Preparation process
[0079] Take 50% of the prescription amount of nitrogen-saturated water for injection, add lactic acid, dezocine, propylene glycol, and sodium chloride successively, stir to dissolve, and obtain solution 1; adjust the pH value of the solution to 4.0 with 4% by weight sodium hydroxide solution; add Dilute the above solution to 1000ml with nitrogen-saturated water for injection; add medicinal charcoal according to 0.02%-0.5% (W / V) of the total volume, keep warm at 30°C for 10 minutes, and filter through pre-filtration and sterilizing filter; The above-mentioned medicinal solution was divided into ampoules, 1ml / bottle, filled with nitrogen, and melt-sealed; put the above-mentioned ampoules containing the medicinal solution into an autoclaving cabinet for sterilization, and the sterilization conditions were 121°C for 12min; Inspection, labeling, packaging, and the finished product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com