Application of dezocine in preparation of nicotinamide phosphoribosyltransferase inhibitor
A technology of phosphoribosyl and nicotinamide, applied in the field of anti-tumor drugs, can solve the problem of not achieving tumor suppressive effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In vitro CNBr-activated Sepharose 4B beads pull down experiment
[0053] The reagent used in embodiment 1 is composed as follows:
[0054] Buffer I: 0.1M NaHCO 3 and 0.5M NaCl, pH 8.3.
[0055] Buffer II: 0.1M Tris-HCl, pH 8.0.
[0056] Buffer III: 0.1M acetic acid and 0.5M NaCl, pH 4.0.
[0057] Buffer IV: 0.1M Tris-HCl and 0.5M NaCl, pH 8.
[0058] Lysis buffer: 50mmol / L Tris (Tris, pH7.5), 5mmol / L ethylenediaminetetraacetic acid (EDTA), 150mmol / L NaCl, 1mmol / L dithiothreitol (DTT), 0.01% ethylphenyl polyethylene glycol (NinidetP-40), 0.02 mmol / L phenylmethylsulfonyl fluoride (PMSF), 4 μg / mL bovine serum albumin (BSA) and protease inhibitors.
[0059] Wash buffer: 50mmol / L tris (Tris, pH7.5), 5mmol / L ethylenediaminetetraacetic acid (EDTA), 150mmol / L NaCl, 1mmol / L dithiothreitol (DTT), 0.01% ethylphenyl polyethylene glycol and 0.02mmol / L phenylmethylsulfonyl fluoride (PMSF).
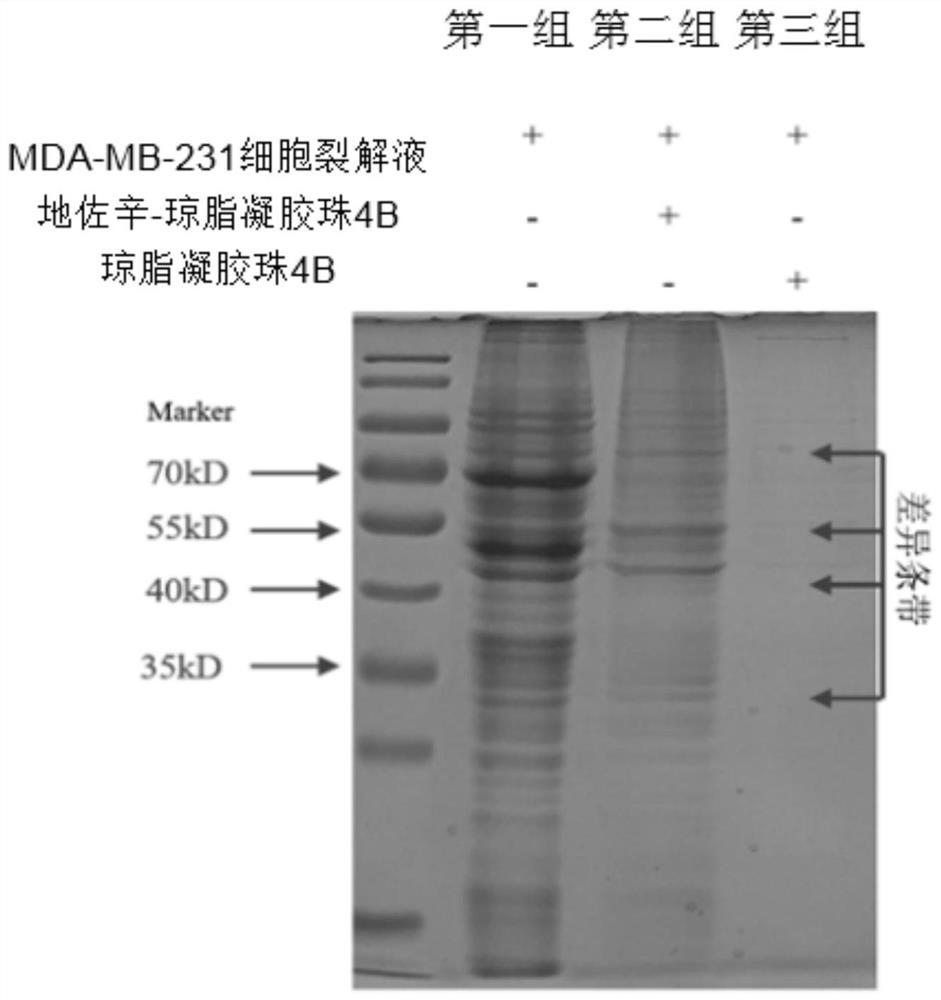
[0060] Example 1 There are three groups of experiments. The first group uses MDA-MB-23...
Embodiment 2
[0079] Liquid chromatography tandem mass spectrometry (LC-MS) analyzes the differential bands of Example 1
[0080] (1) Excise the Coomassie-stained spot on the polyacrylamide gel of the second group in Example 1, transfer it to a 1.5 mL microcentrifuge tube, and wash it twice with Mill-Q water. The blot was then destained with 100 μL of 50 mM ammonium bicarbonate / acetonitrile (1:1, v / v) and incubated by vortexing occasionally for 30 min depending on the staining intensity. The destained solution was then discarded, washed twice with 200 μL Mill-Q water, and 40 μL of 100% acetonitrile was added for 15 min to dry the gel spot in a Speedvac for 10 min. Finally, spot the gel in a minimum volume of 20 μg / mL sequencing-grade porcine trypsin or chymotrypsin (Promega, USA) in 25 mM NH 4 HCO 3 rehydrate and incubate overnight at 37°C. Transfer the supernatant to a 200 μL microcentrifuge tube and extract the gel once with extraction buffer (67% acetonitrile with 1% trifluoroacetic a...
Embodiment 3
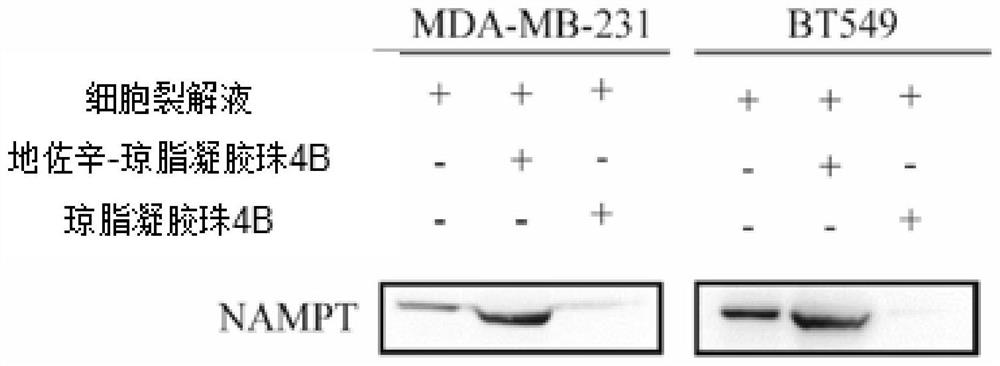
[0085] Perform western blot verification on the pull down results
[0086] Specifically, the pulldown product of MDA-MB-231 cell lysate and dezocine-coupled agar gel beads 4B and the cell lysate of human breast ductal carcinoma cell BT549 and dezocine-coupled agar gel The pulldown products of beads 4B were subjected to western blot using NAMPT antibody as the primary antibody to verify that dezocine can directly bind to NAMPT. The operation of western blot is as follows:
[0087] (1) Preparation of separation gel: Take 10% separation gel as an example, add the ingredients in Table 3 to a 50mL centrifuge tube in turn, shake well, add to a glass plate, and then add 1mL of isopropanol to seal the liquid surface.
[0088] Table 3 10% separation gel preparation (20mL)
[0089]
[0090] (2) Prepare 5% concentrated gel: After the separation gel is solidified, pour off the isopropanol and dry it with filter paper, then prepare the concentrated gel according to Table 4, mix well a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com