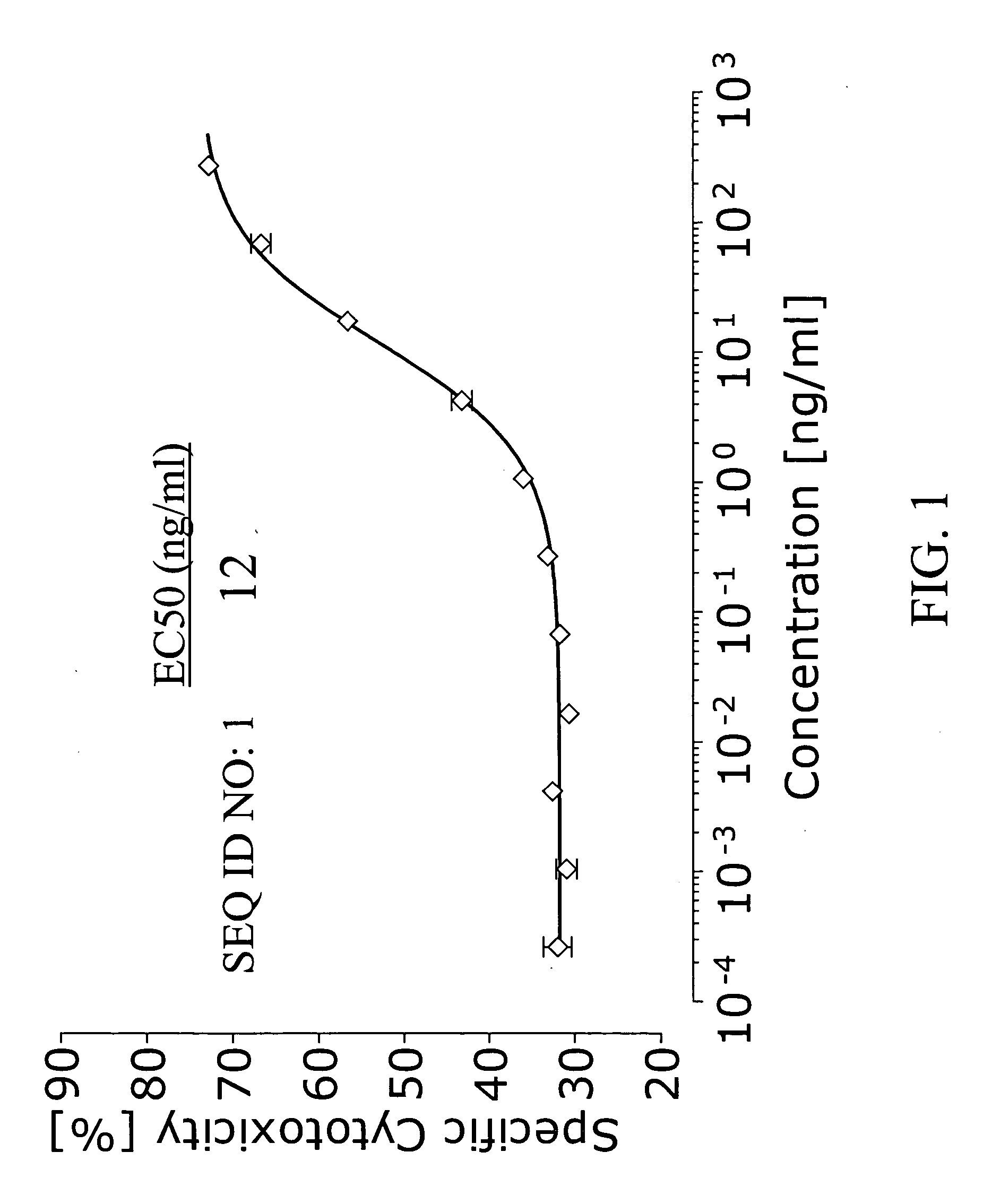
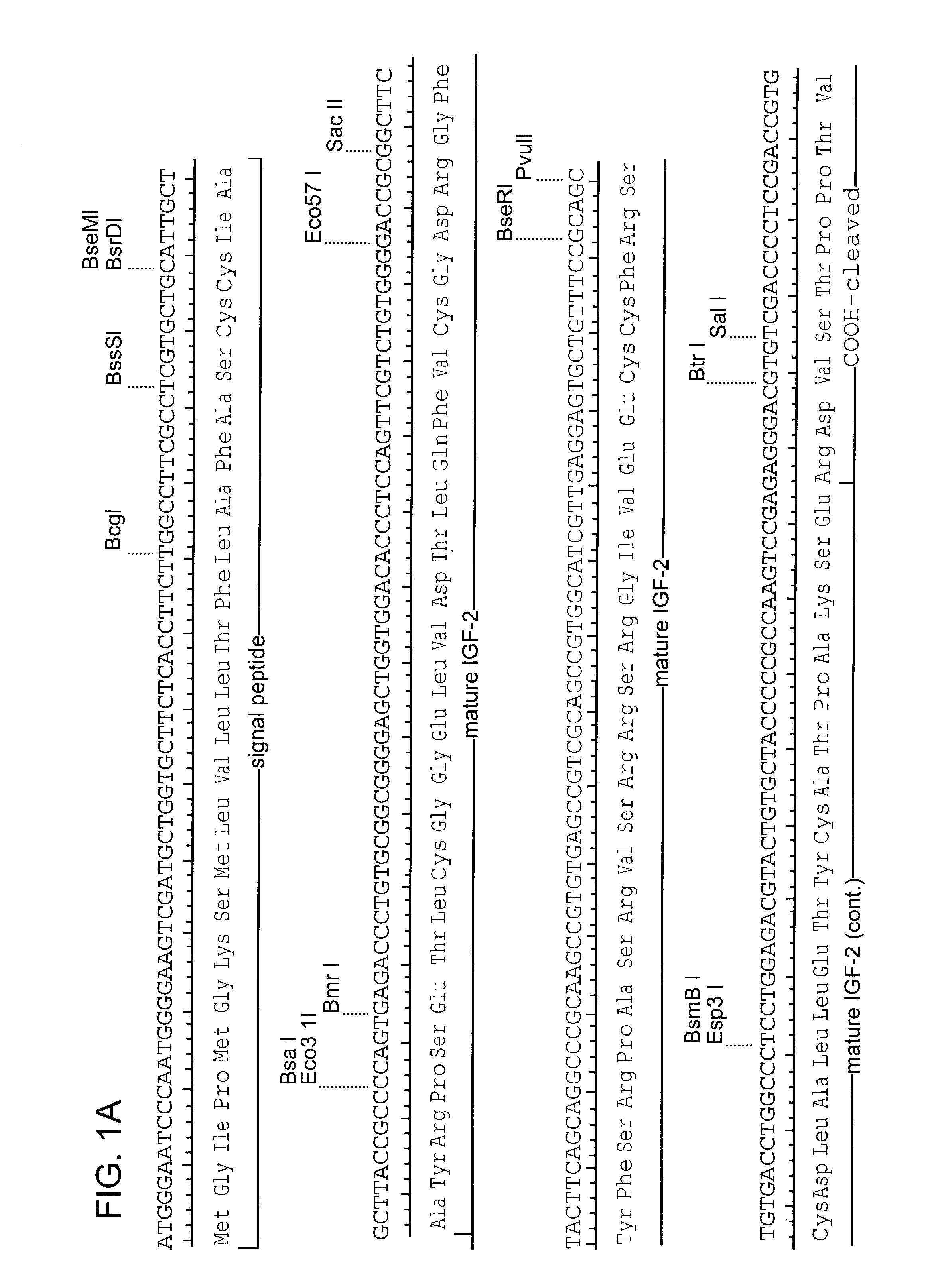
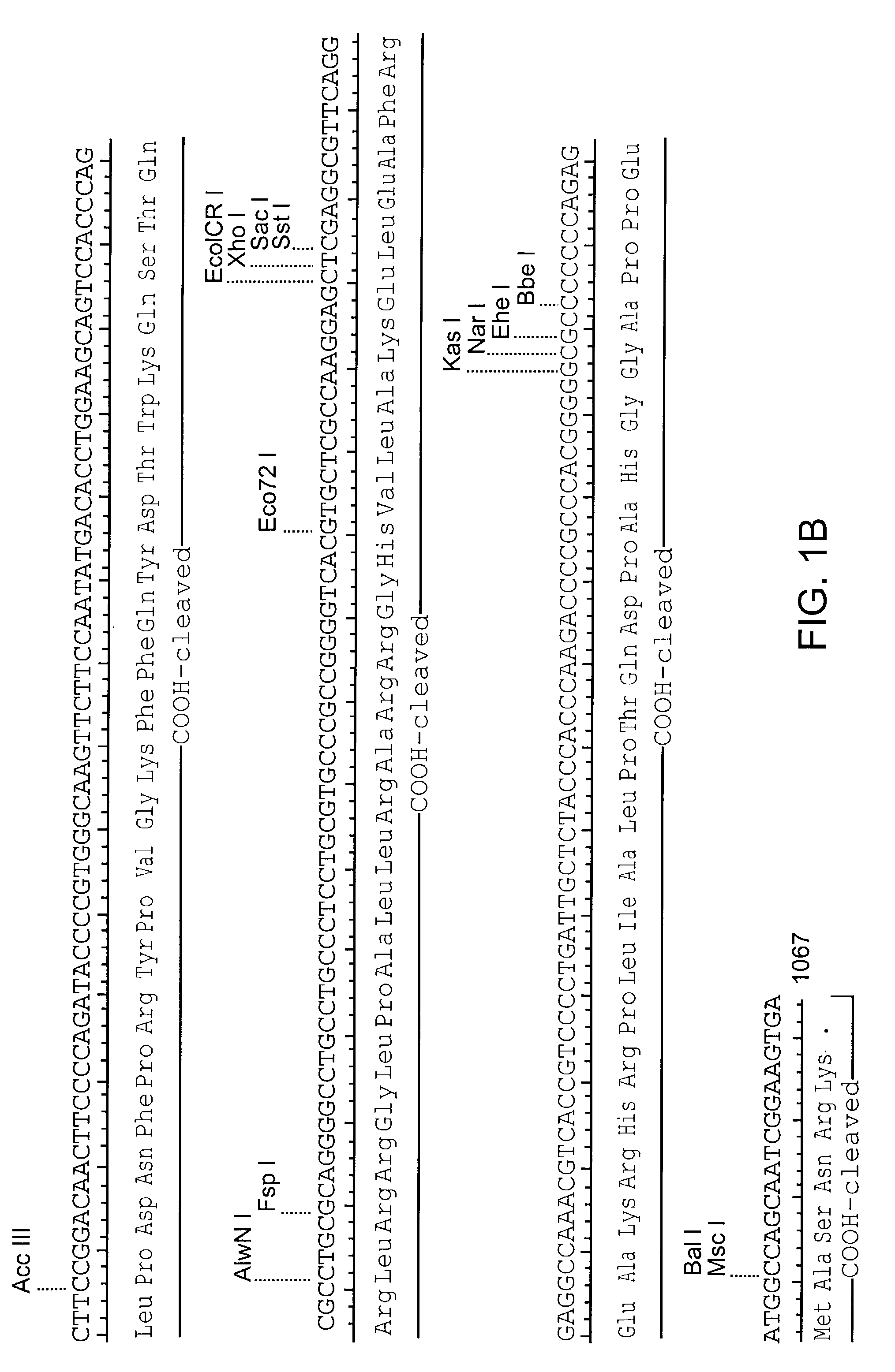
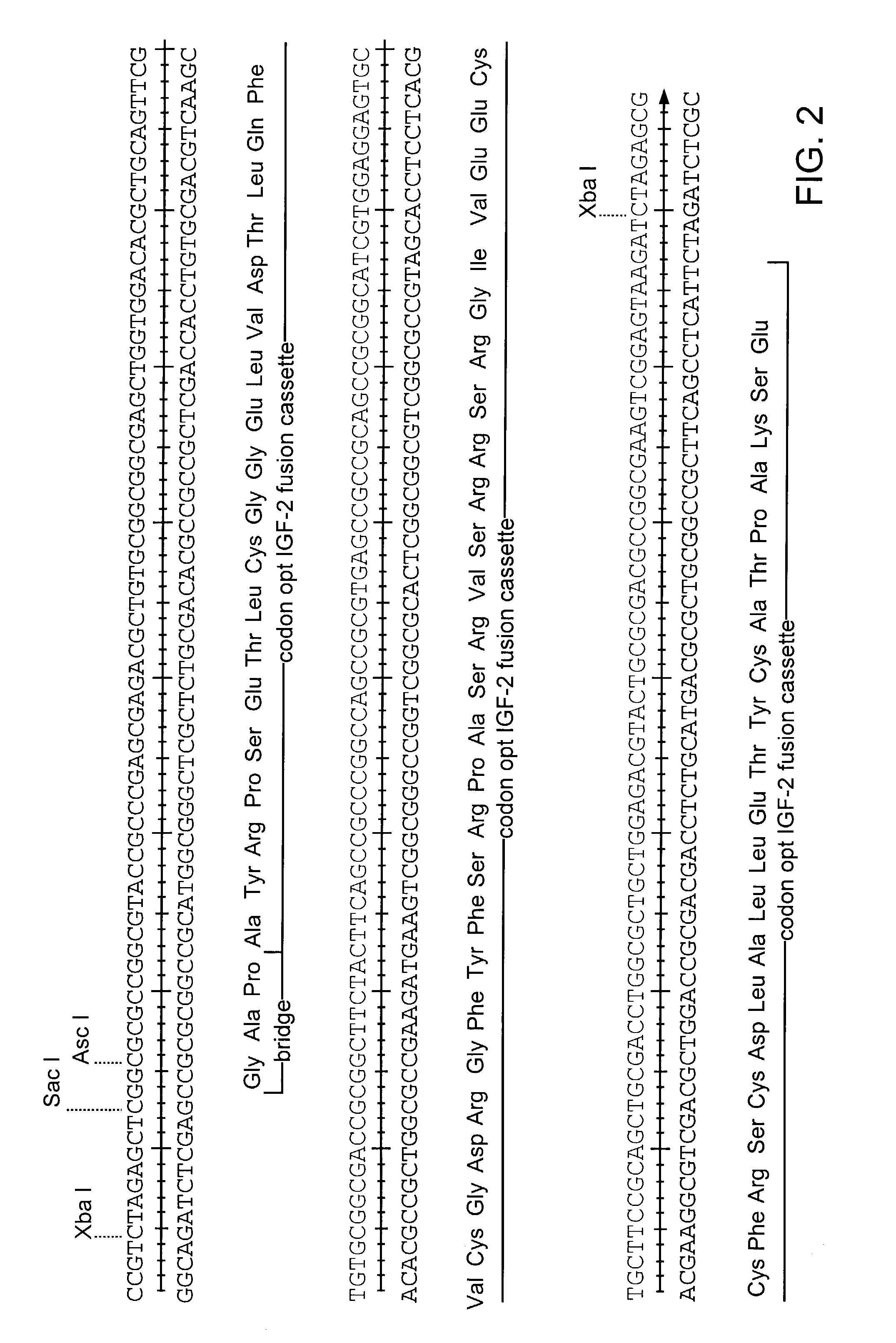
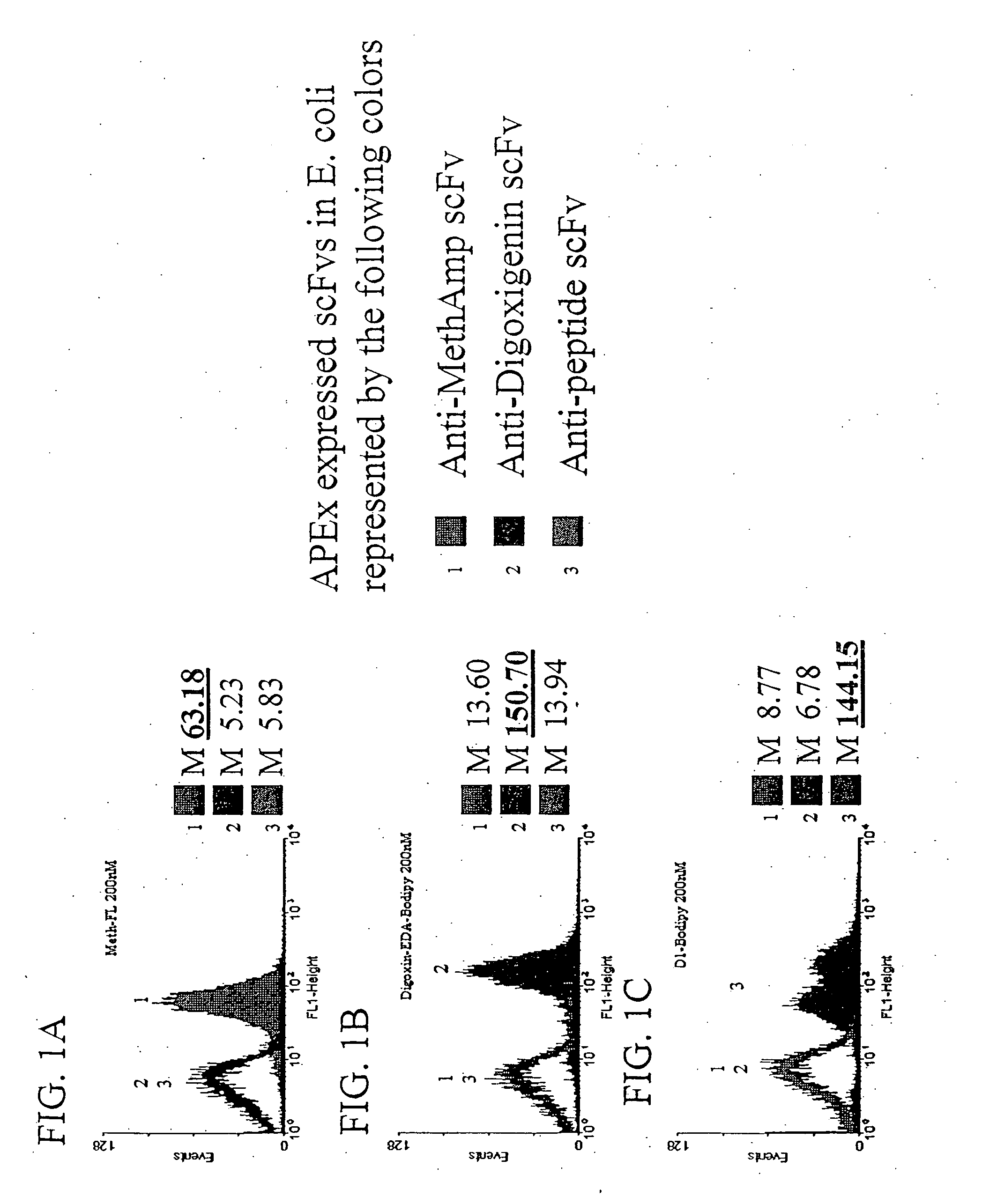
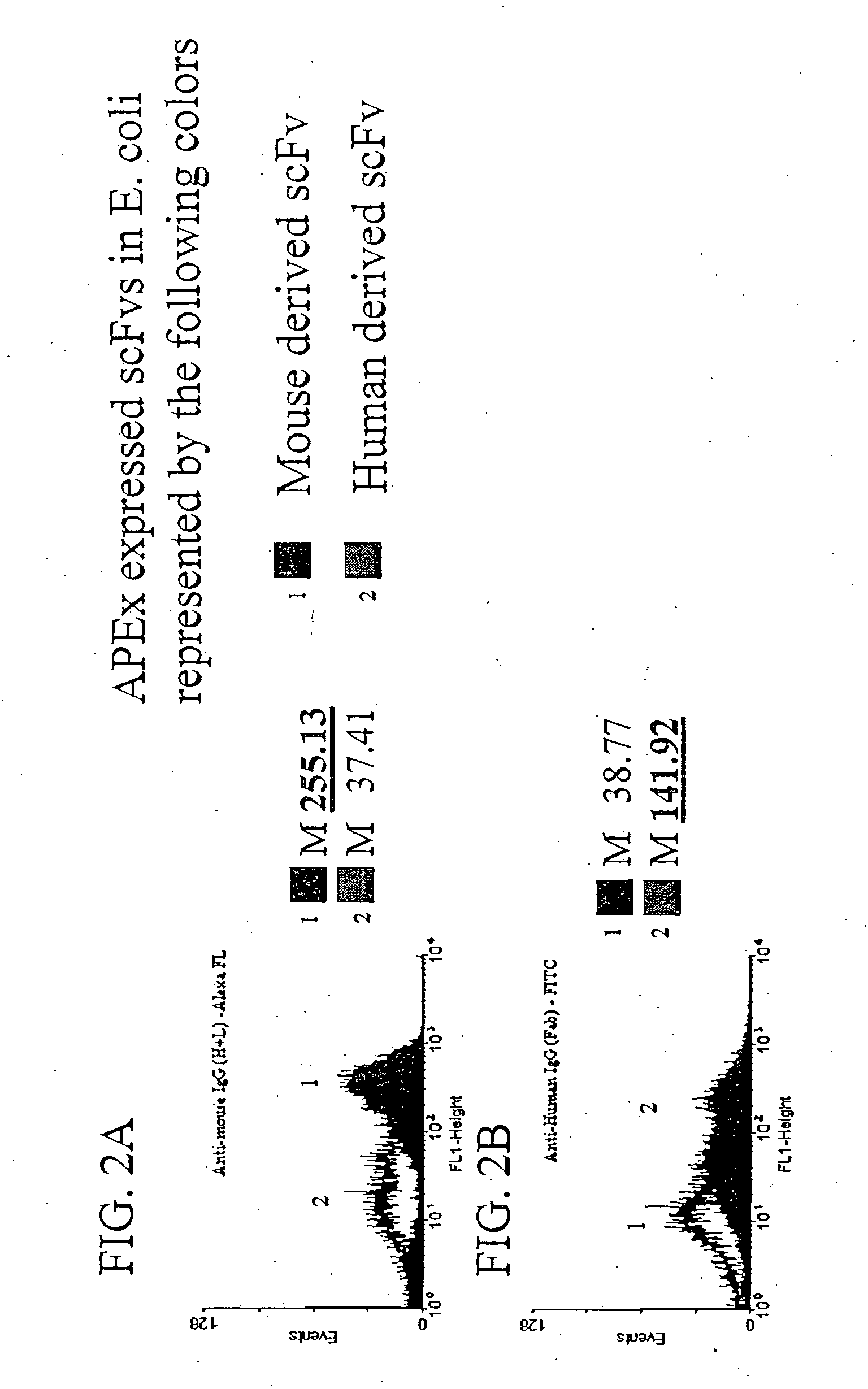
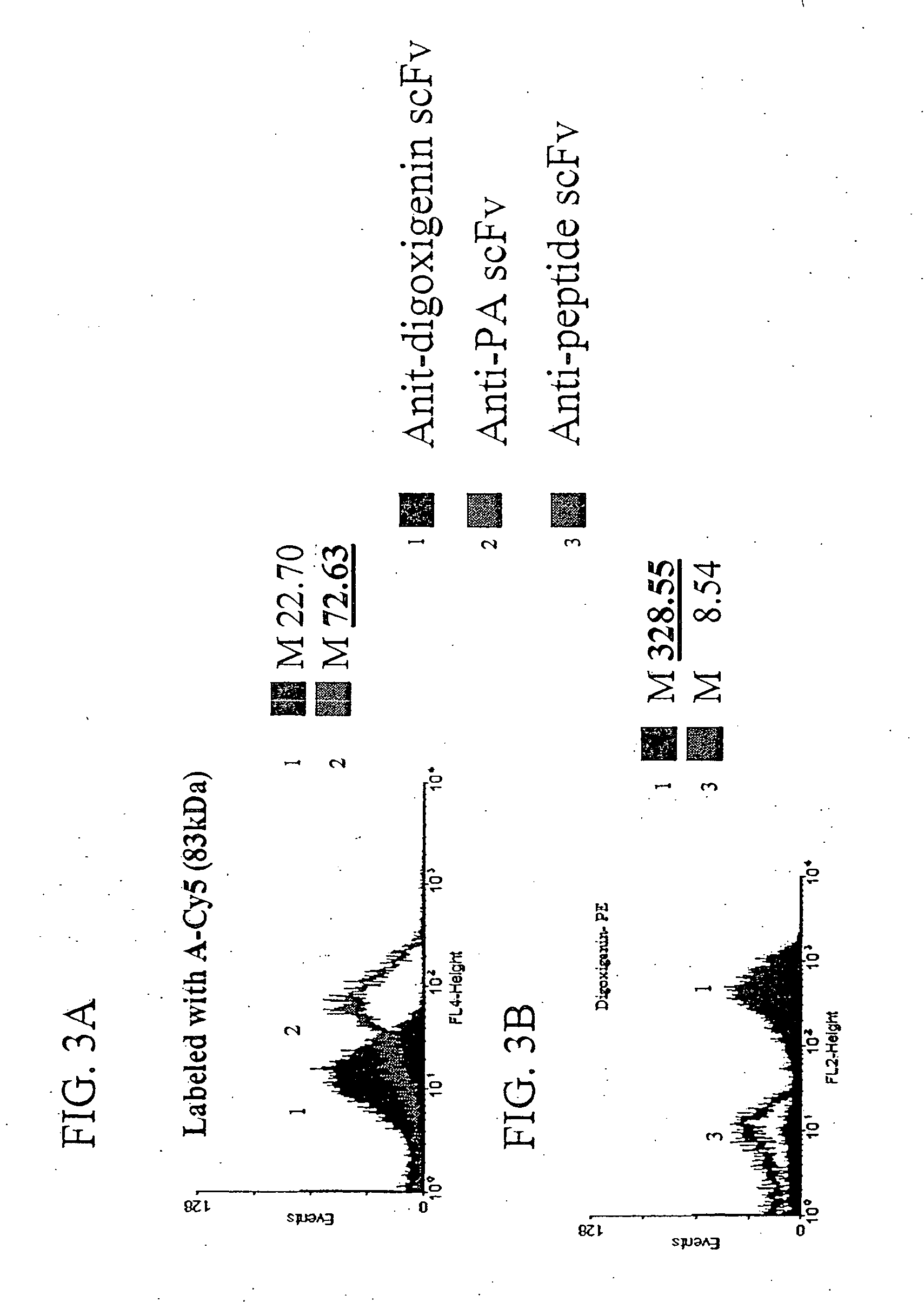
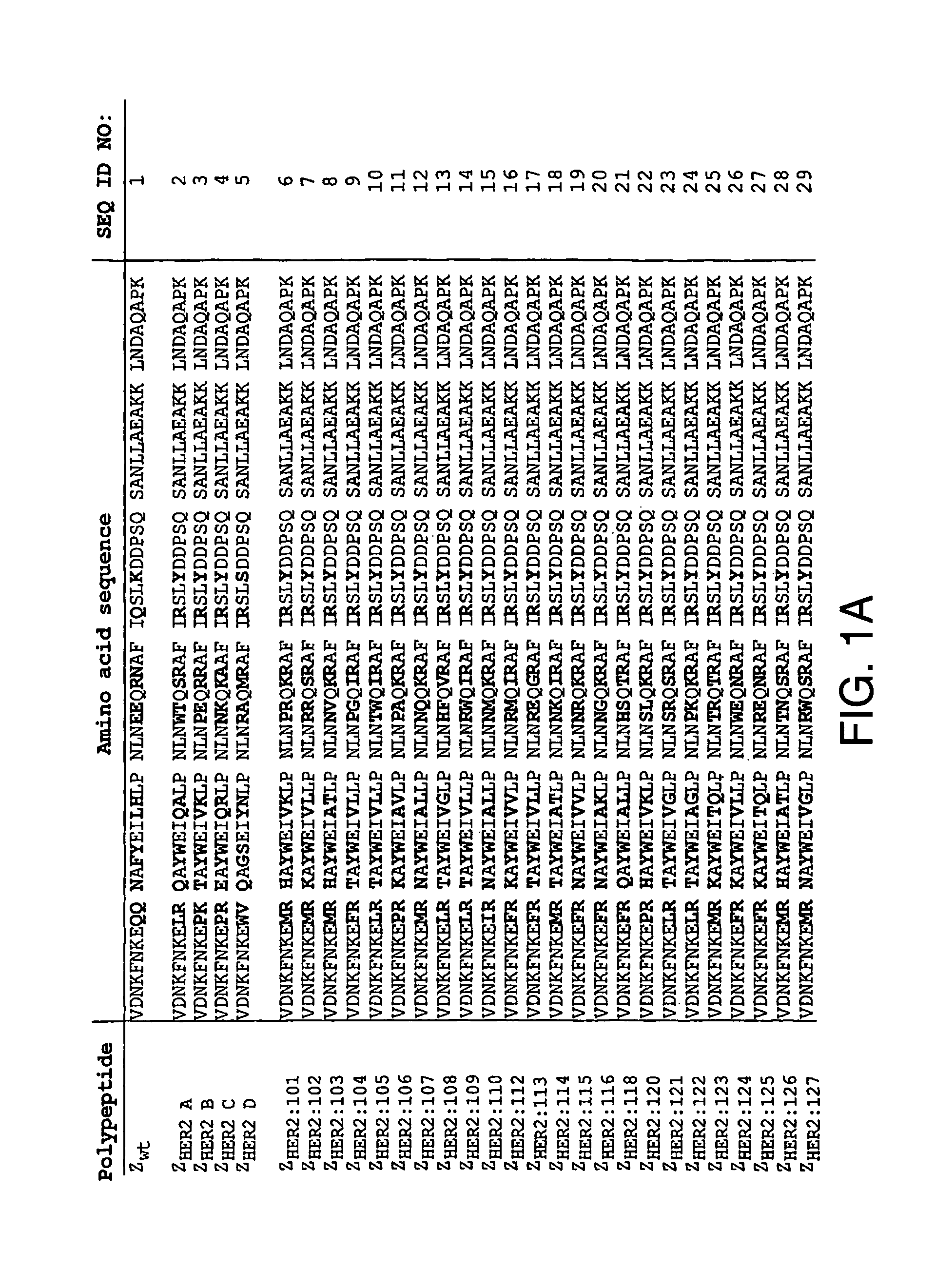
Patents
Literature
1757results about "Polypeptide with His-tag" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bispecific antibodies
ActiveUS7235641B2Improve productivityImprove efficiencyAntipyreticAntibody mimetics/scaffoldsBispecific antibodyImmune effector cell
Owner:AMGEN RES (MUNICH) GMBH
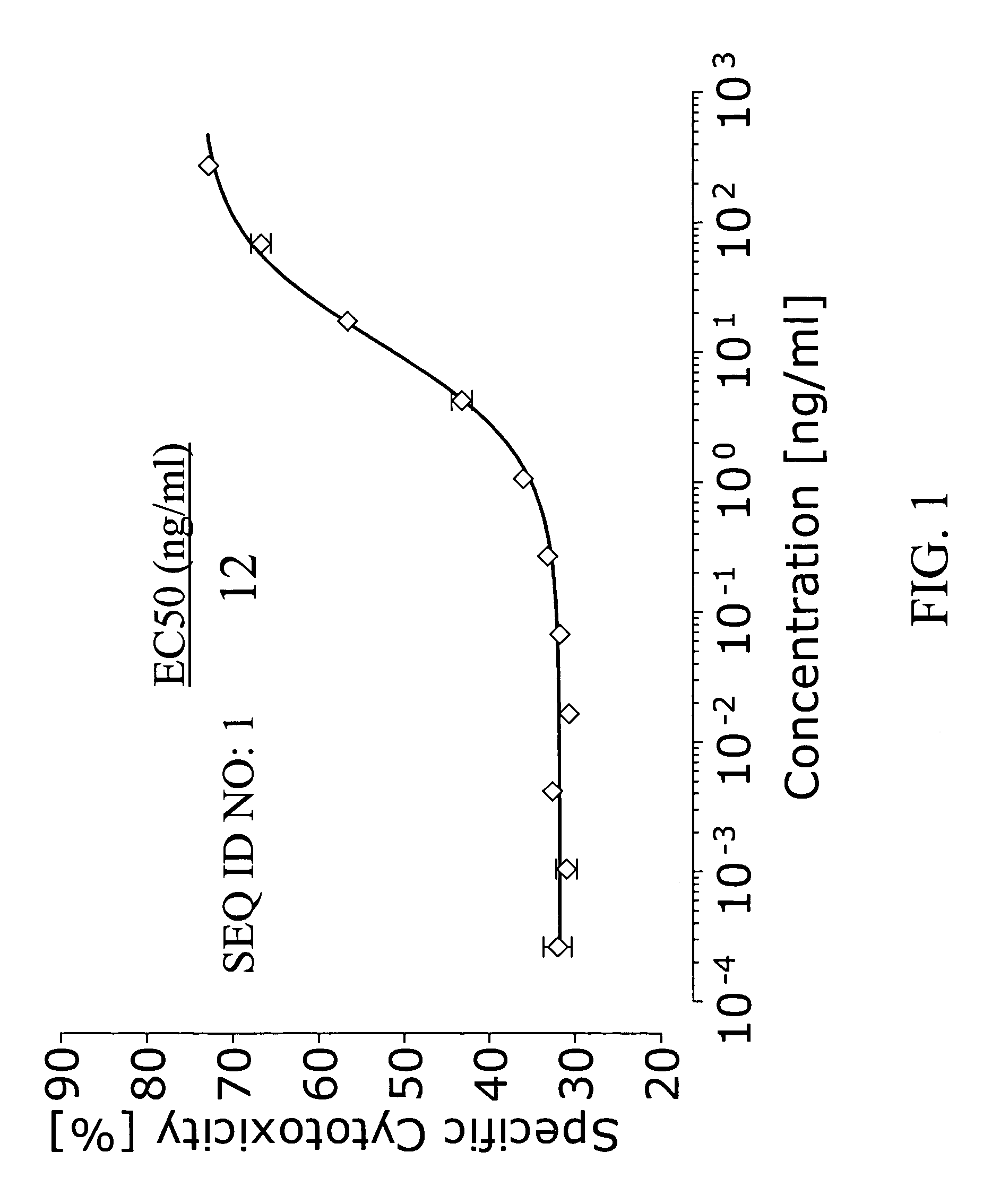
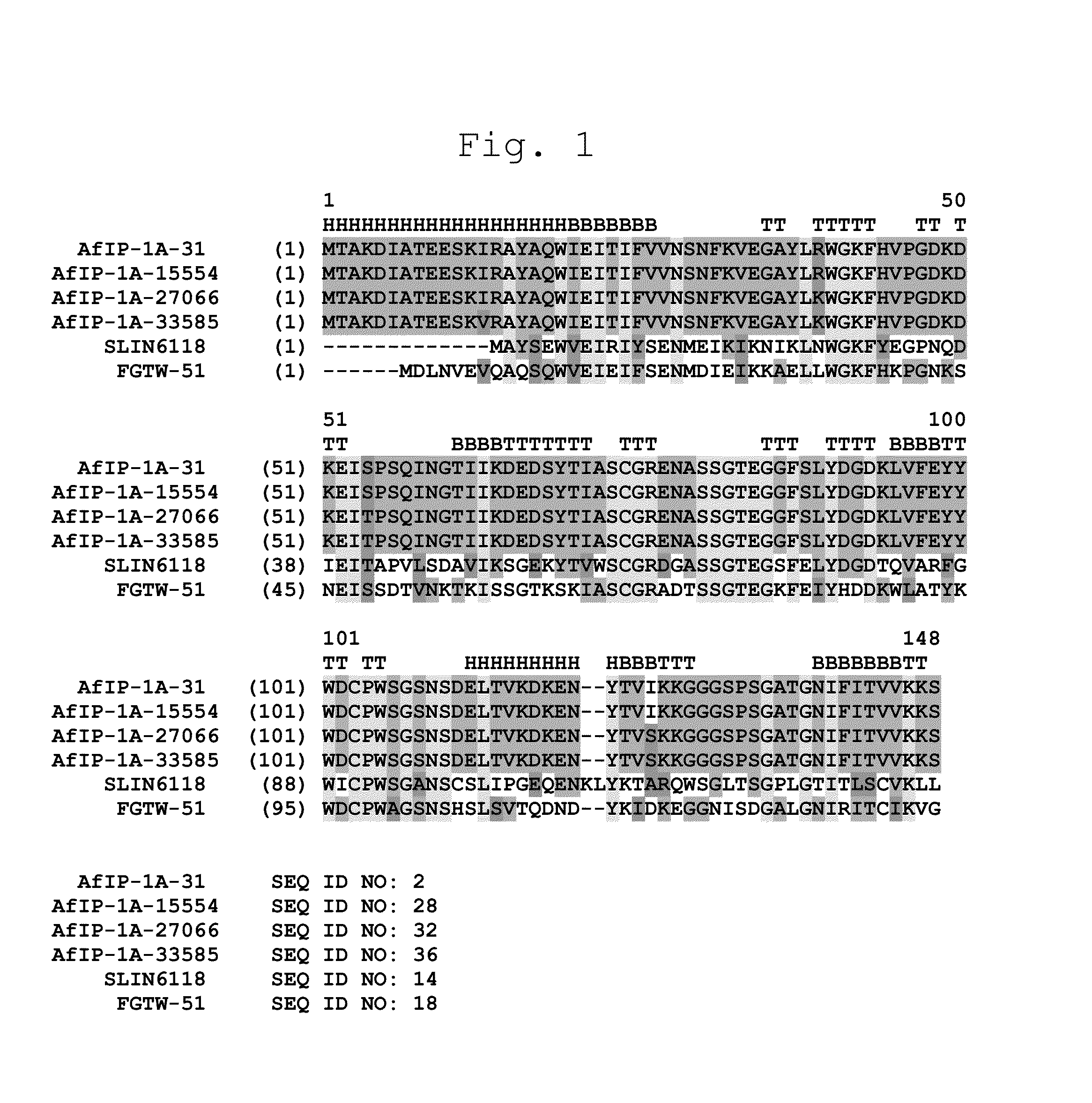
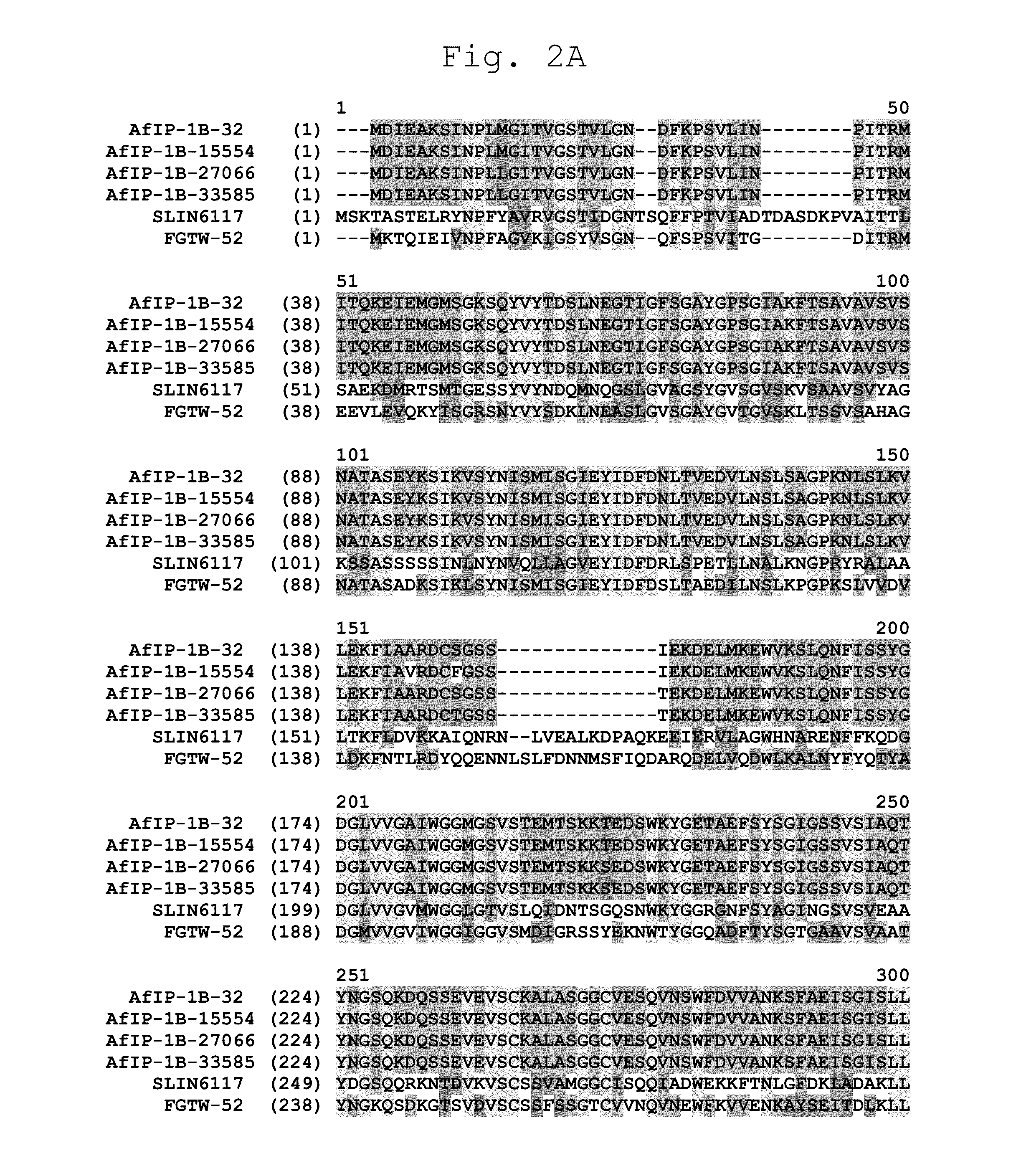
Novel Insecticidal Proteins and Methods for Their Use
ActiveUS20140033361A1Improve pest resistanceImprove toleranceBiocideAntibody mimetics/scaffoldsBiotechnologyOrder Lepidoptera
Compositions and methods for controlling pests are provided. The methods involve transforming organisms with a nucleic acid sequence encoding an insecticidal protein. In particular, the nucleic acid sequences are useful for preparing plants and microorganisms that possess insecticidal activity. Thus, transformed bacteria, plants, plant cells, plant tissues and seeds are provided. Compositions are insecticidal nucleic acids and proteins of bacterial species. The sequences find use in the construction of expression vectors for subsequent transformation into organisms of interest including plants, as probes for the isolation of other homologous (or partially homologous) genes. The pesticidal proteins find use in controlling, inhibiting growth or killing Lepidopteran, Coleopteran, Dipteran, fungal, Hemipteran and nematode pest populations and for producing compositions with insecticidal activity.
Owner:CORTEVA AGRISCIENCE LLC +1
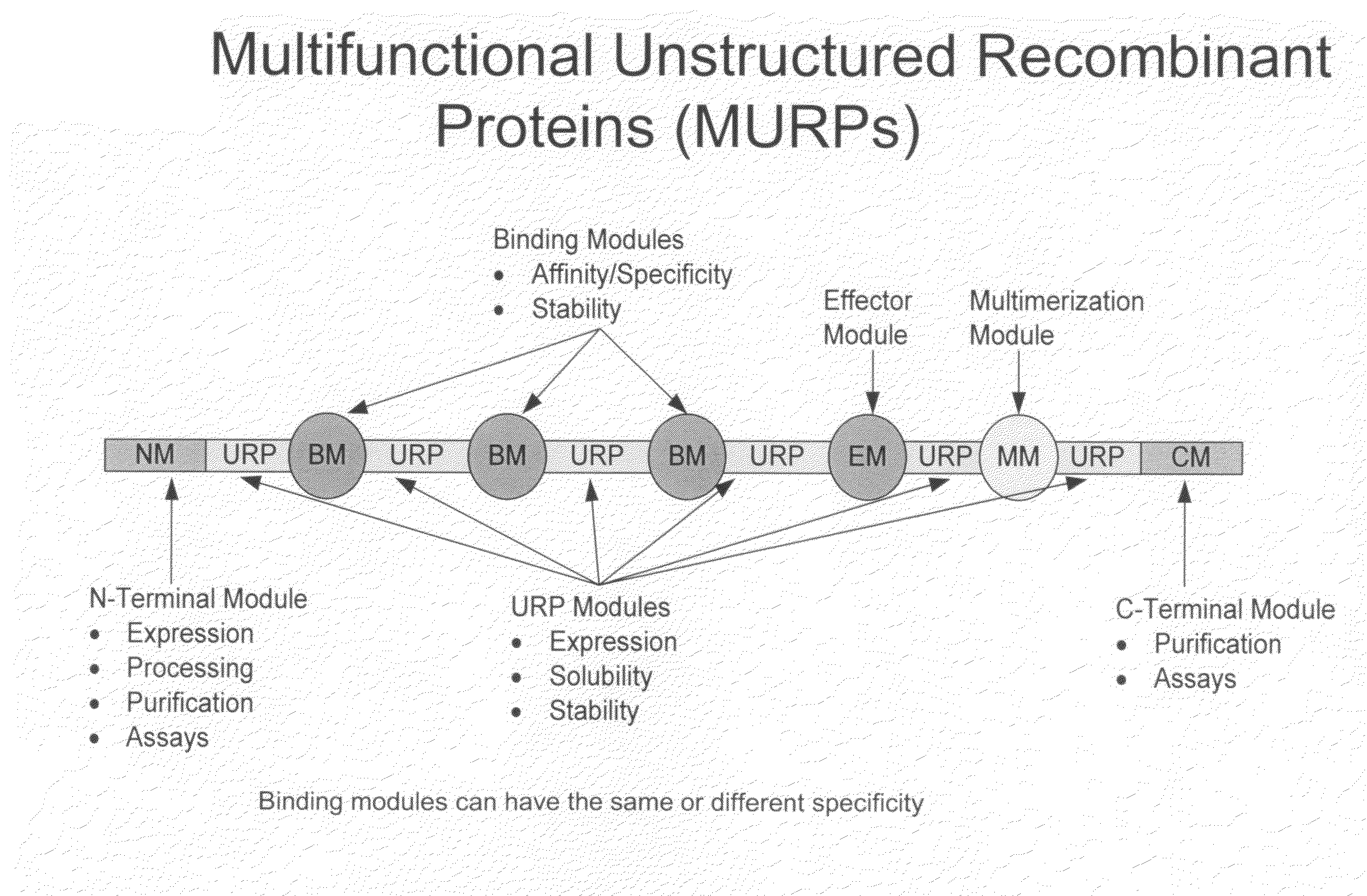
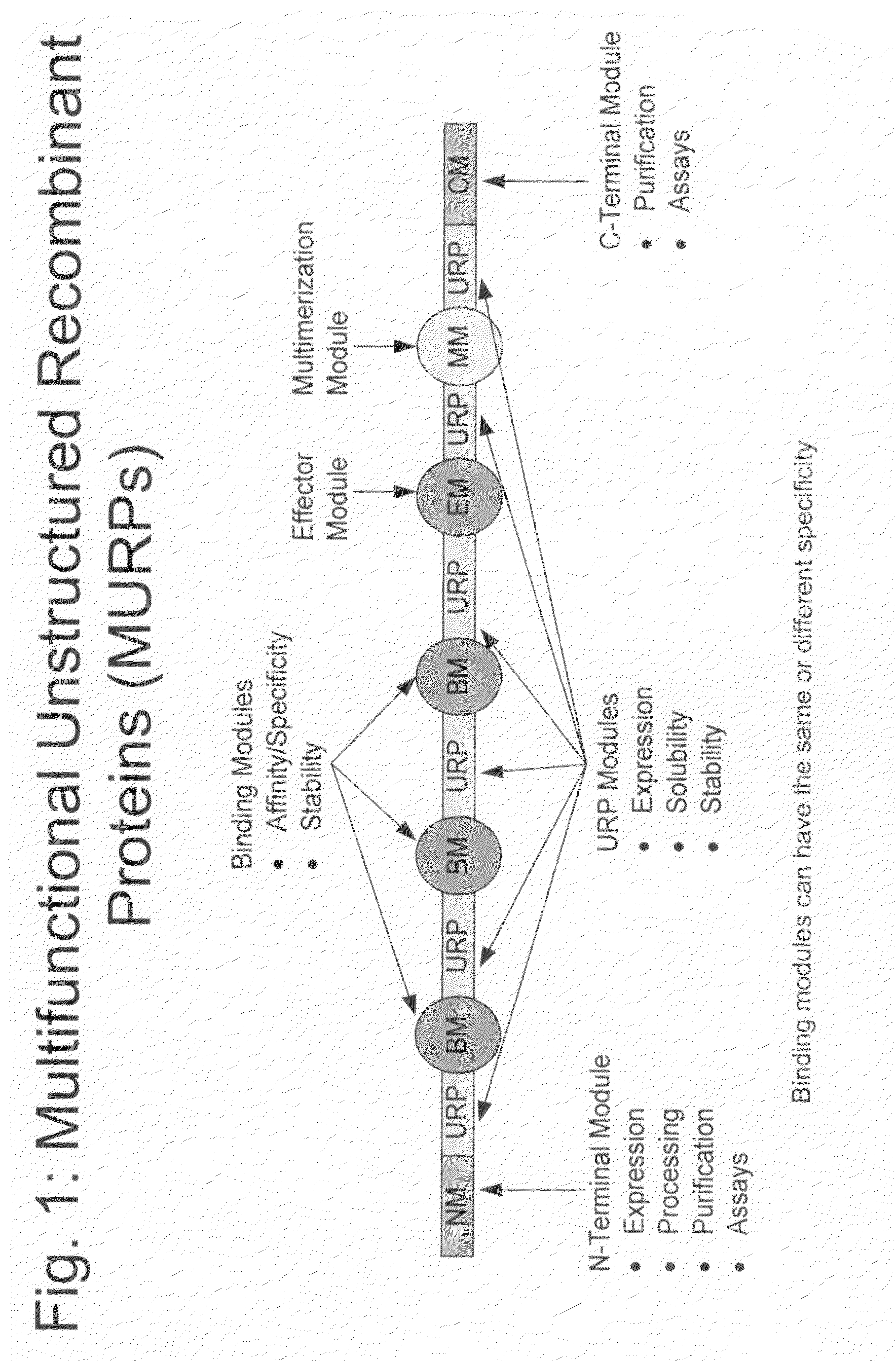
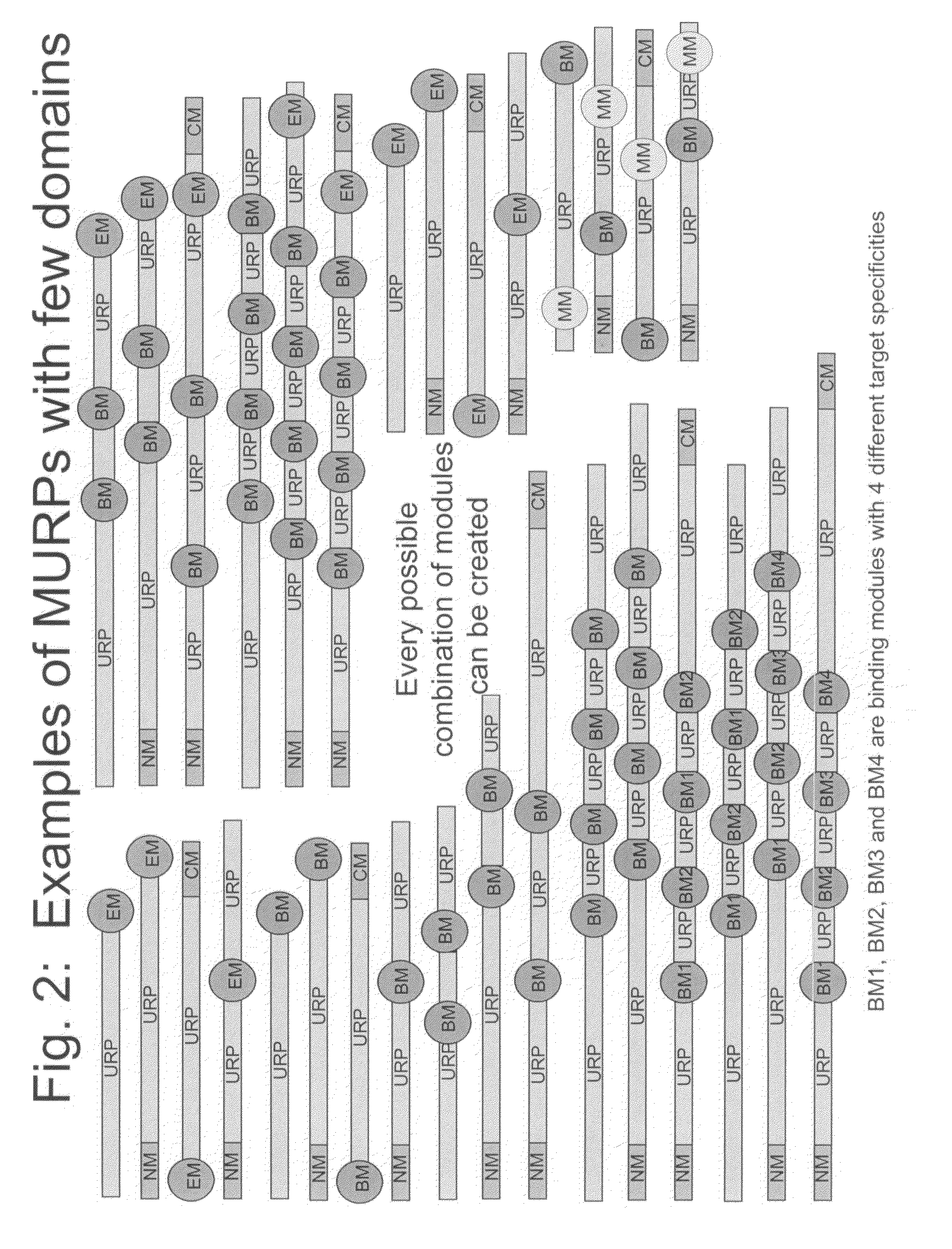
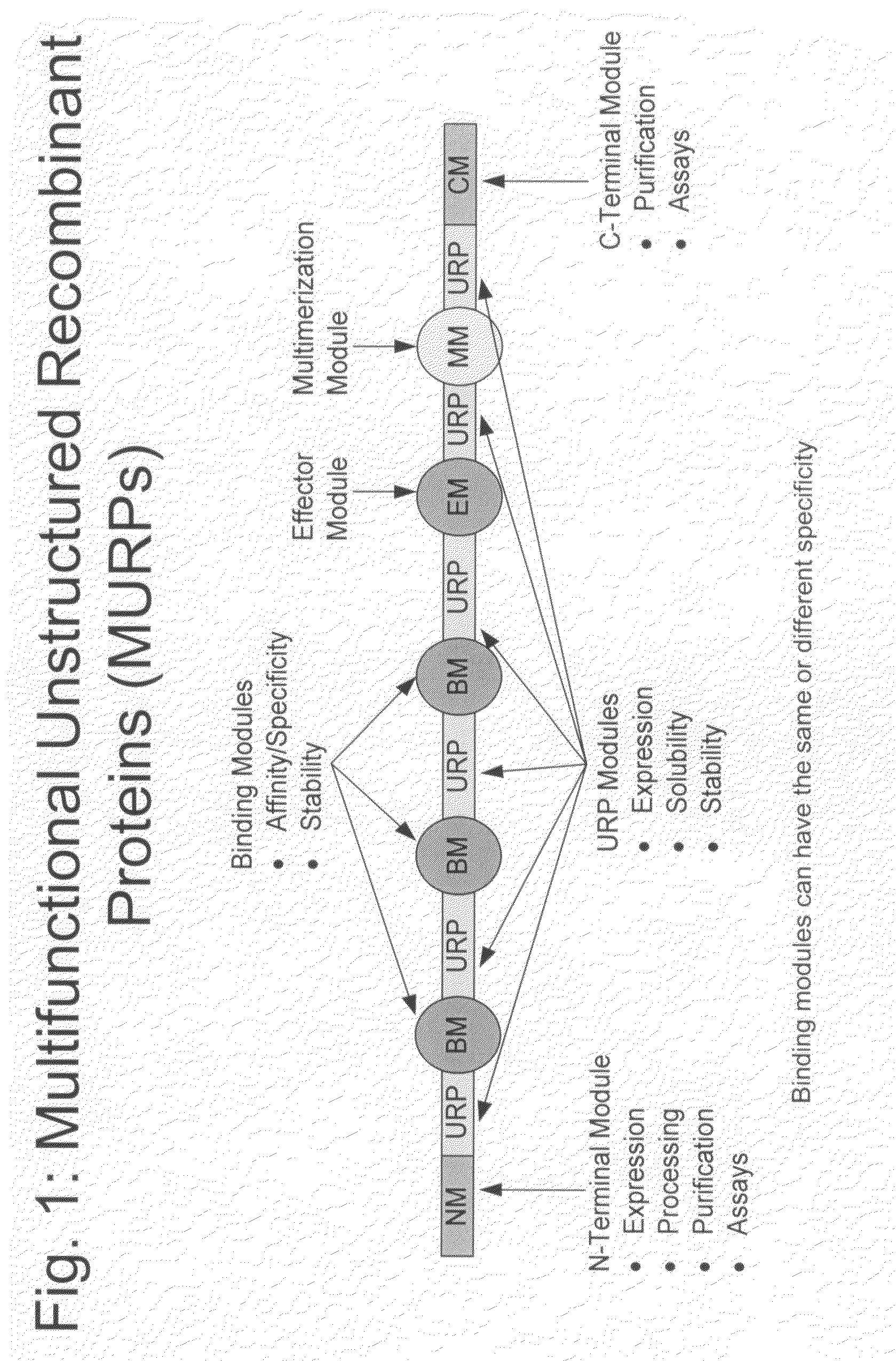
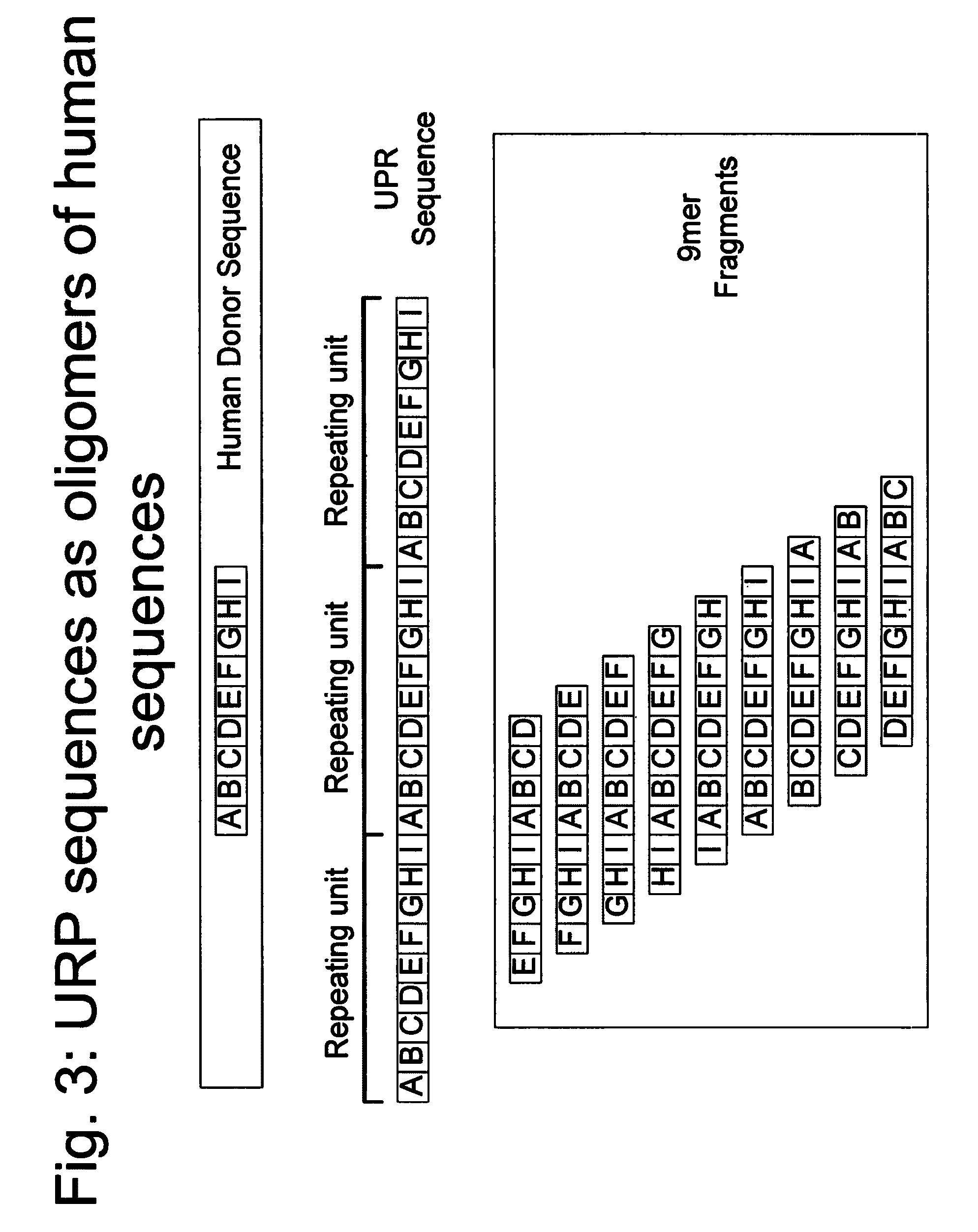
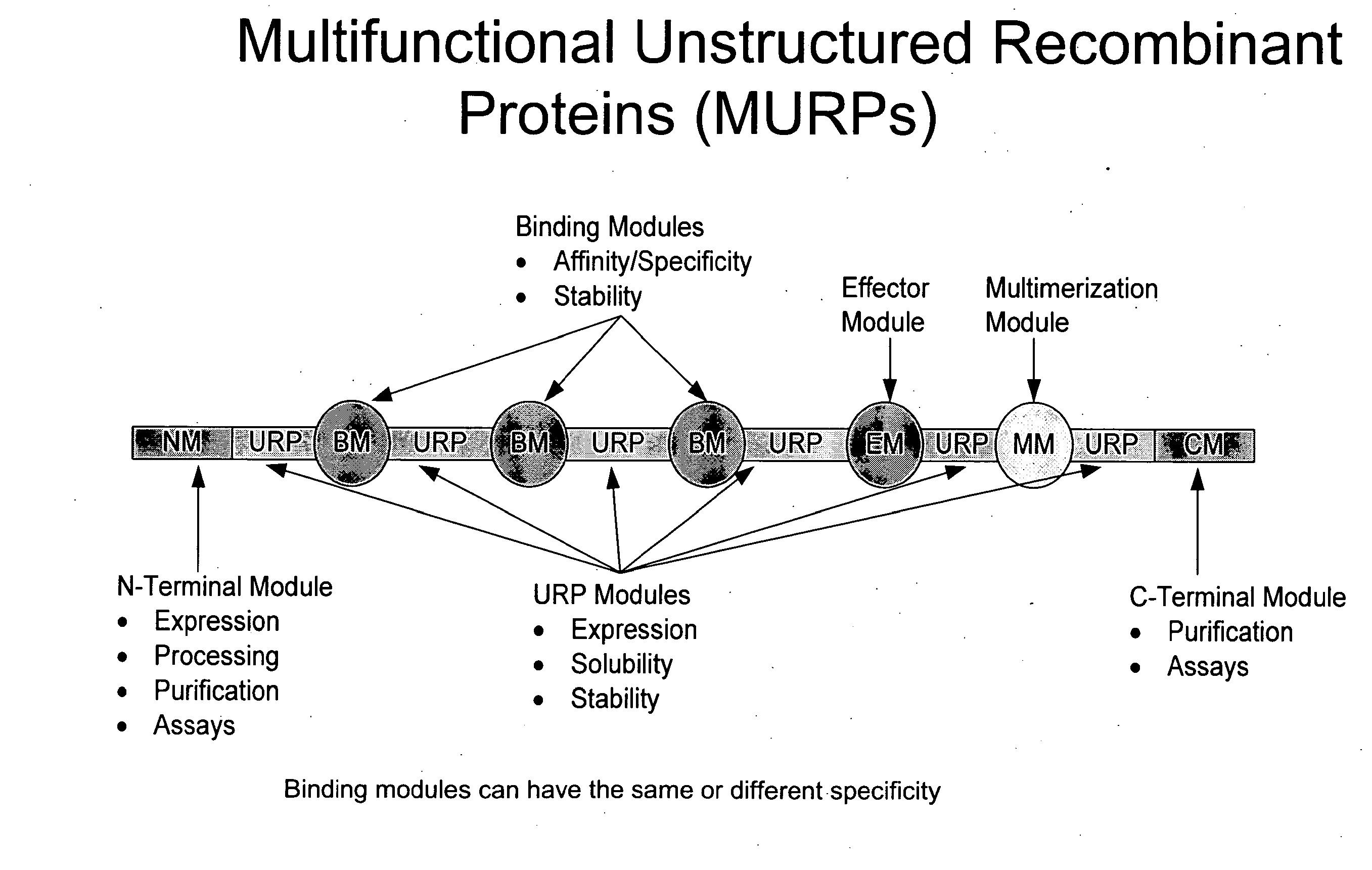
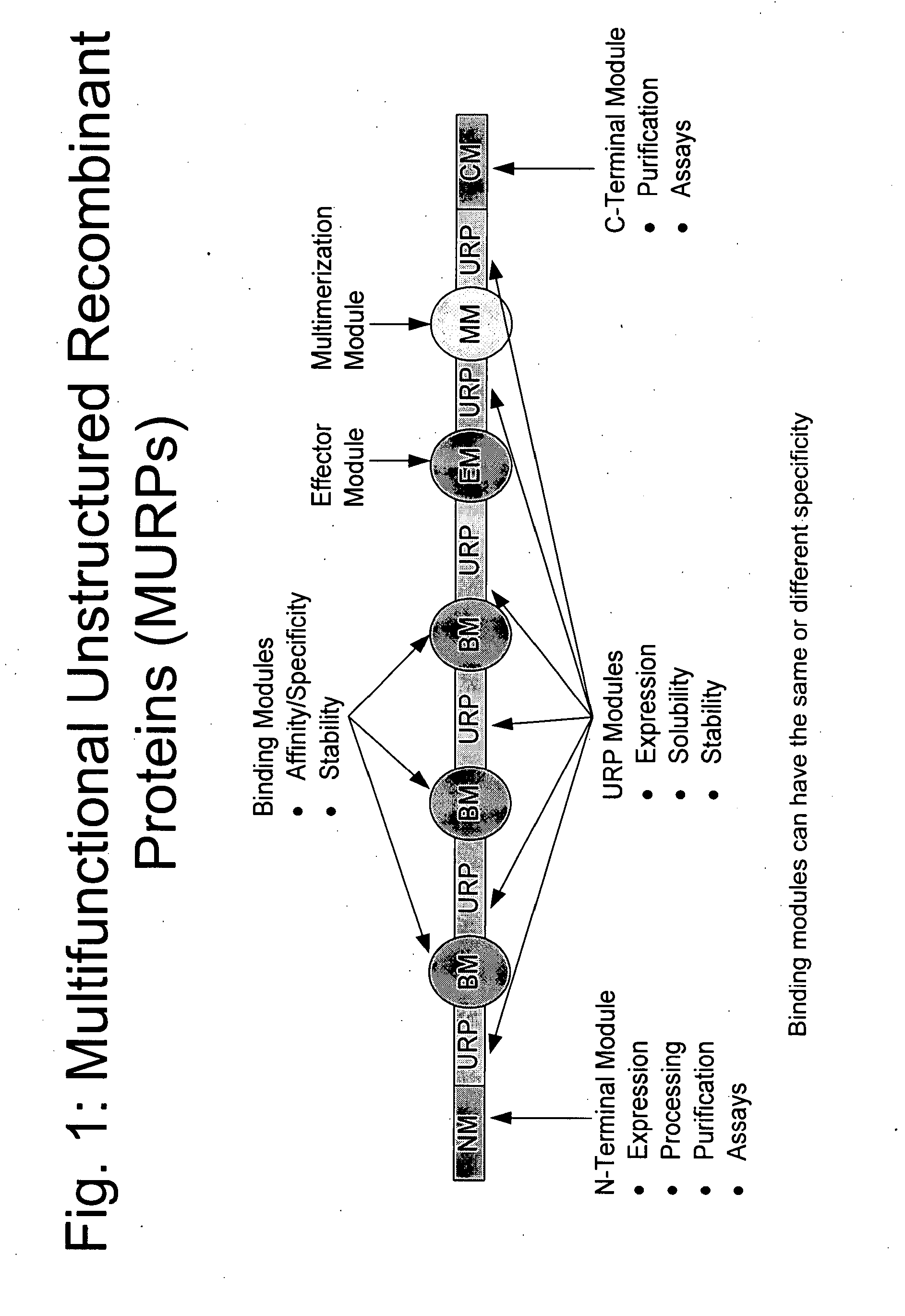
Methods for production of unstructured recombinant polymers and uses thereof
ActiveUS20080286808A1Extended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsToxinPolymer
Owner:AMUNIX PHARMA INC
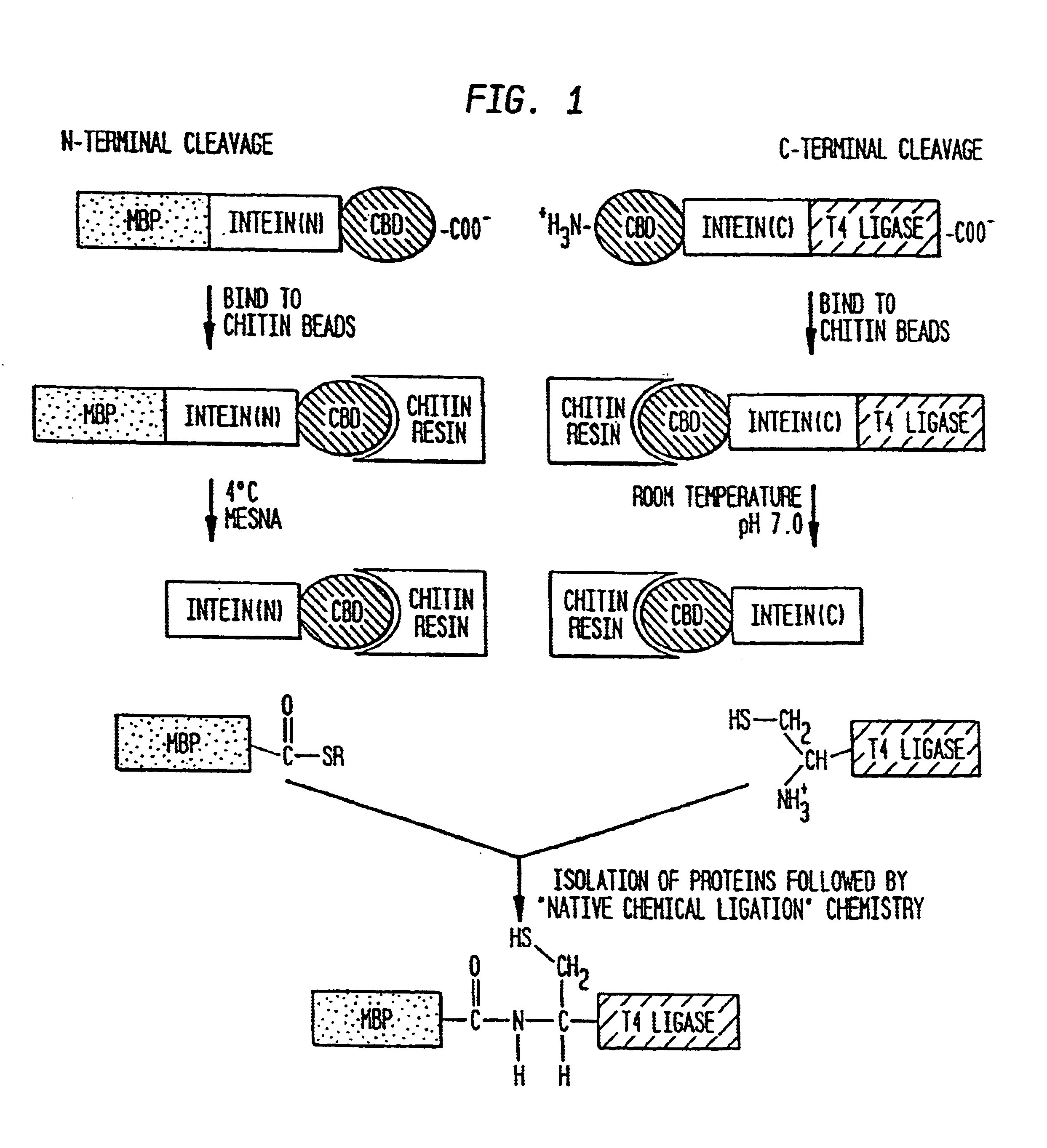
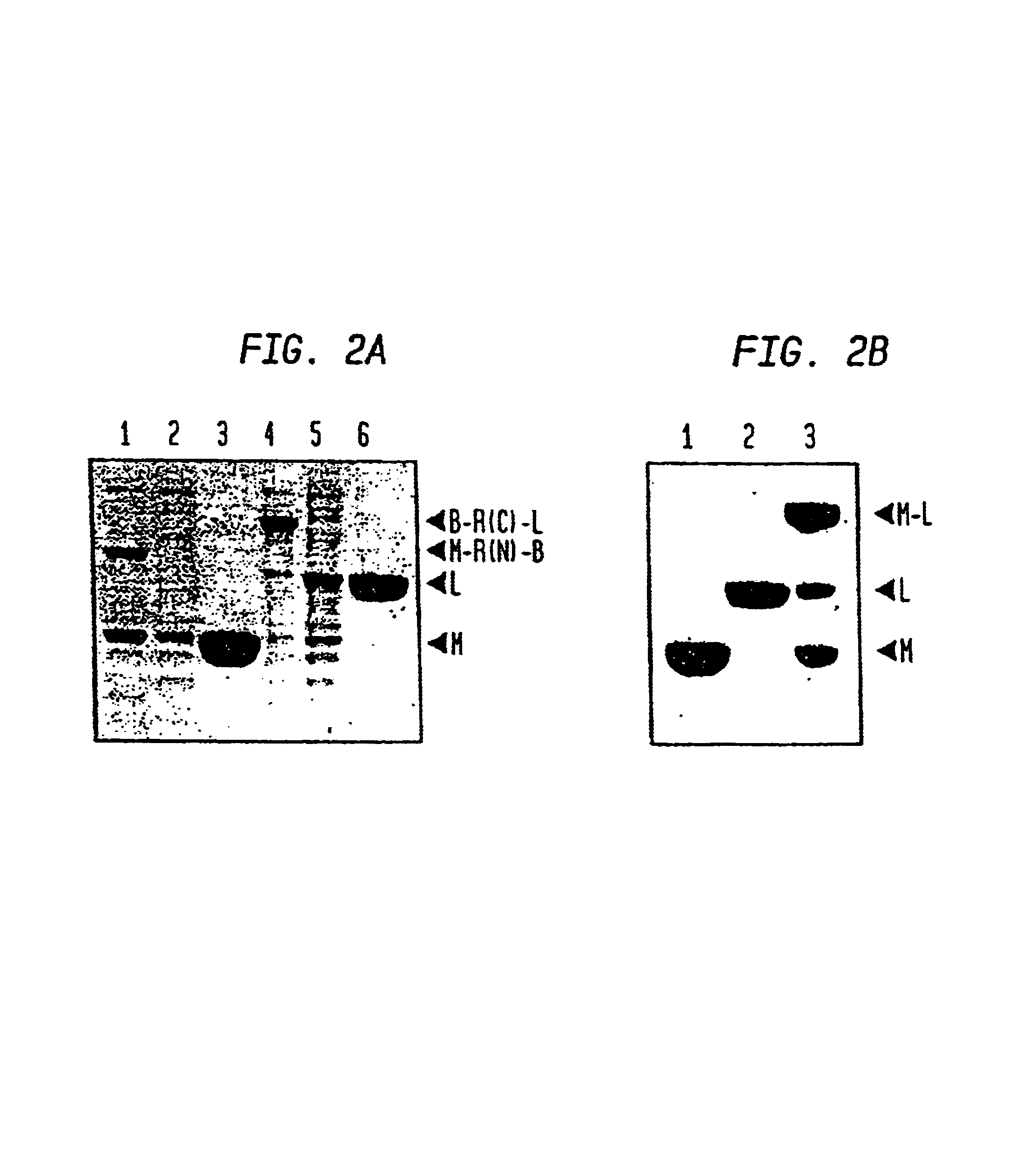
Intein-mediated protein ligation of expressed proteins
InactiveUS6849428B1Eliminate needBacteriaFusion with post-translational modification motifProtein targetIntein
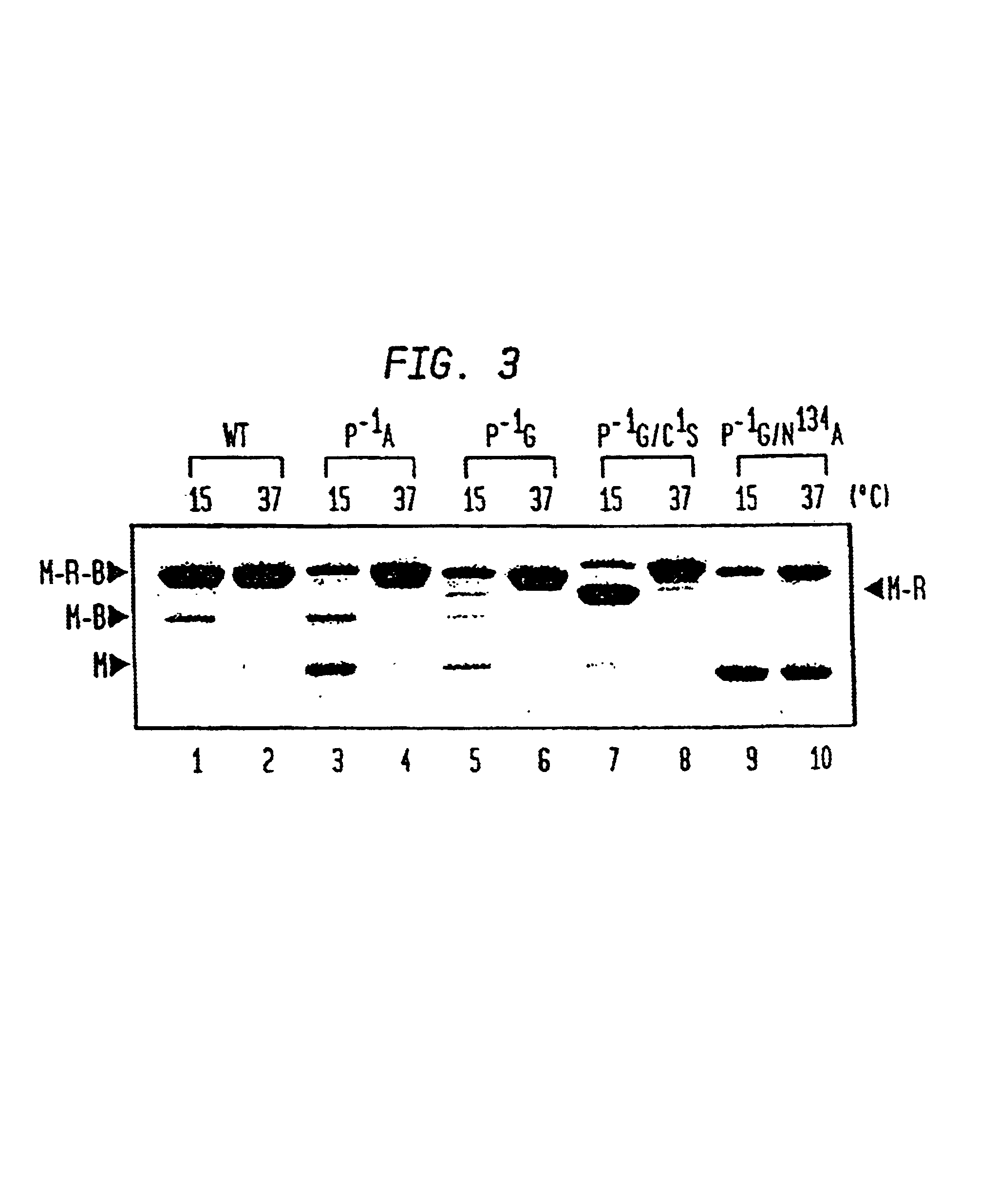
A method for the ligation of expressed proteins which utilizes inteins, for example the RIR1 intein from Methanobacterium thermotrophicum, is provided. Constructs of the Mth RIR1 intein in which either the C-terminal asparagine or N-terminal cysteine of the intein are replaced with alanine enable the facile isolation of a protein with a specified N-terminal, for example, cysteine for use in the fusion of two or more expressed proteins. The method involves the steps of generating a C-terminal thioester-tagged target protein and a second target protein having a specified N-terminal via inteins, such as the modified Mth RIR1 intein, and ligating these proteins. A similar method for producing a cyclic or polymerized protein is provided. Modified inteins engineered to cleave at their C-terminus or N-terminus, respectively, and DNA and plasmids encoding these modified inteins are also provided.
Owner:NEW ENGLAND BIOLABS
Methods for protein labeling based on acyl carrier protein
InactiveUS7666612B2Cell receptors/surface-antigens/surface-determinantsSugar derivativesCoenzyme A biosynthesisCarrier protein
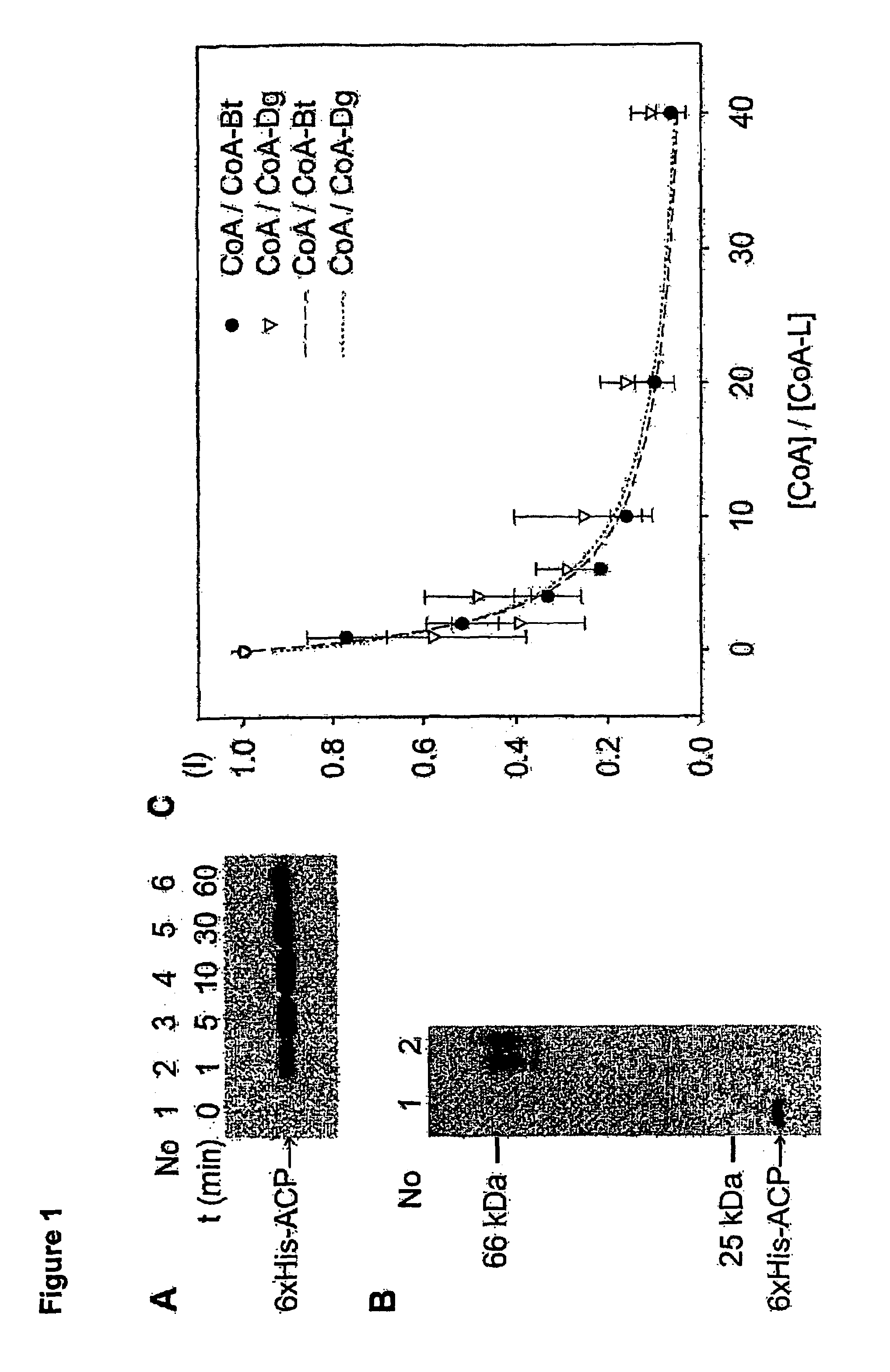
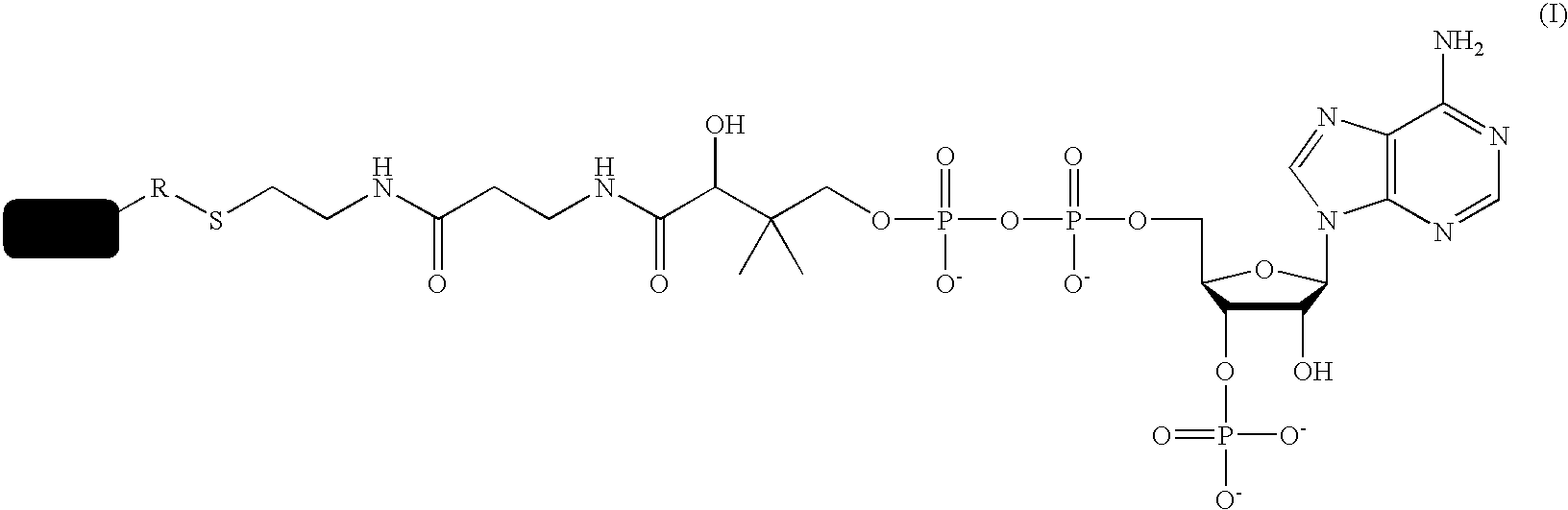
A method for labeling acyl carrier protein (ACP) fusion proteins with a wide variety of different labels is disclosed. The method relies on the transfer of a label from a coenzyme A type substrate to an ACP fusion protein using a holo-acyl carrier protein synthase (ACPS) or a homologue thereof. The method allows detecting and manipulating the fusion protein, both in vitro and in vivo, by attaching molecules to the fusion proteins that introduce a new physical or chemical property to the fusion protein. Examples of such labels are, among others, spectroscopic probes or reporter molecules, affinity tags, molecules generating reactive radicals, cross-linkers, ligands mediating protein-protein interactions or molecules suitable for the immobilization of the fusion protein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Bispecific antibodies
ActiveUS20050136050A1Less complexReduce in quantityAntipyreticAntibody mimetics/scaffoldsBispecific antibodyImmune effector cell
The present invention discloses bispecific antibodies comprising two antibody variable domains on a single polypeptide chain, wherein a first portion of the bispecific antibody is capable of recruiting the activity of a human immune effector cell by specifically binding to an effector antigen on the human immune effector cell, the first portion consisting of one antibody variable domain, and a second portion of the bispecific antibody specifically binding to a target antigen other than the effector antigen, the target antigen on a target cell other than the human immune effector cell, the second portion comprising one antibody variable domain.
Owner:AMGEN RES (MUNICH) GMBH
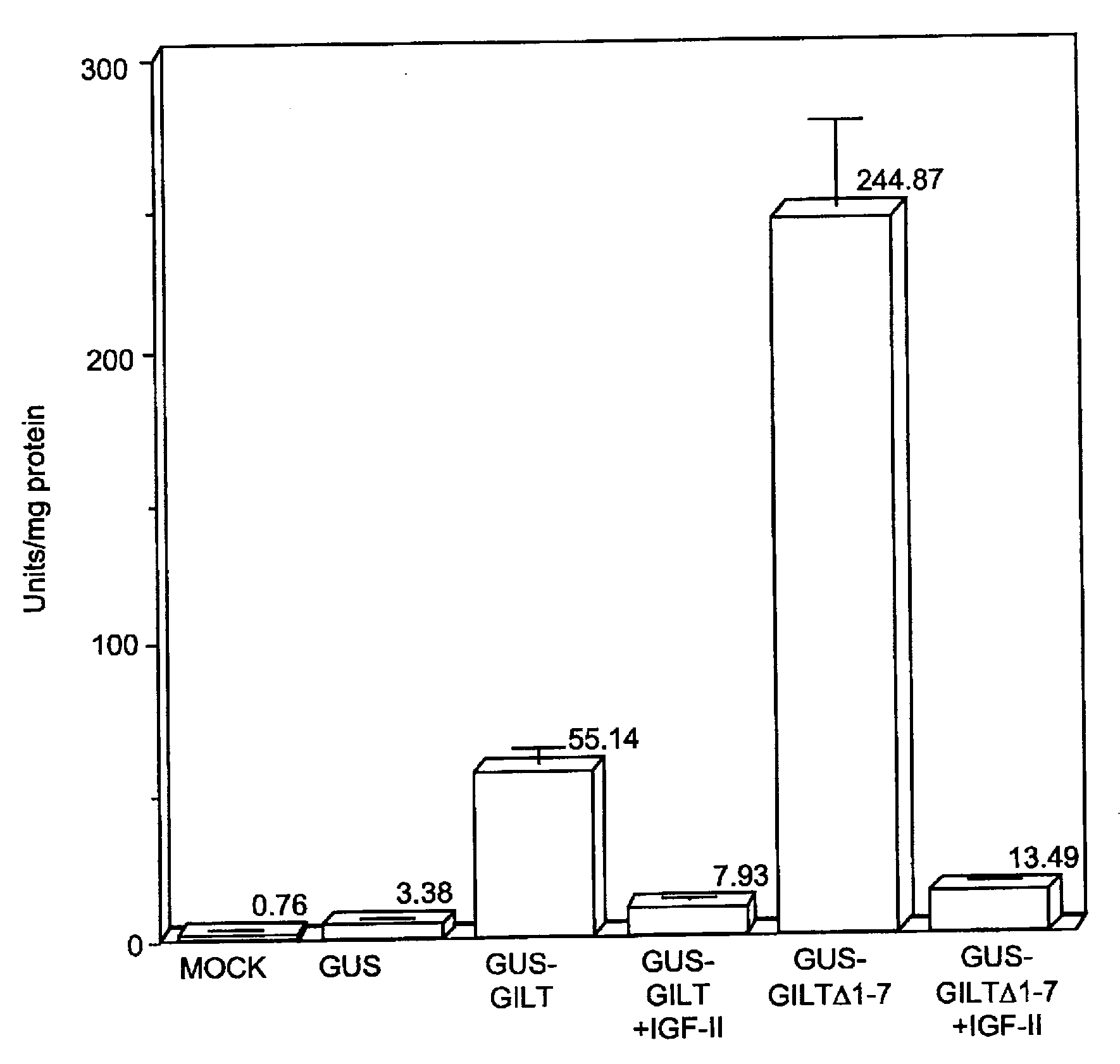
Subcellular targeting of therapeutic proteins
InactiveUS7396811B2Convenient treatmentSimple preparation processNervous disorderPeptide/protein ingredientsLysosomeTherapeutic protein
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Methods for production of unstructured recombinant polymers and uses thereof
Owner:AMUNIX PHARMA INC
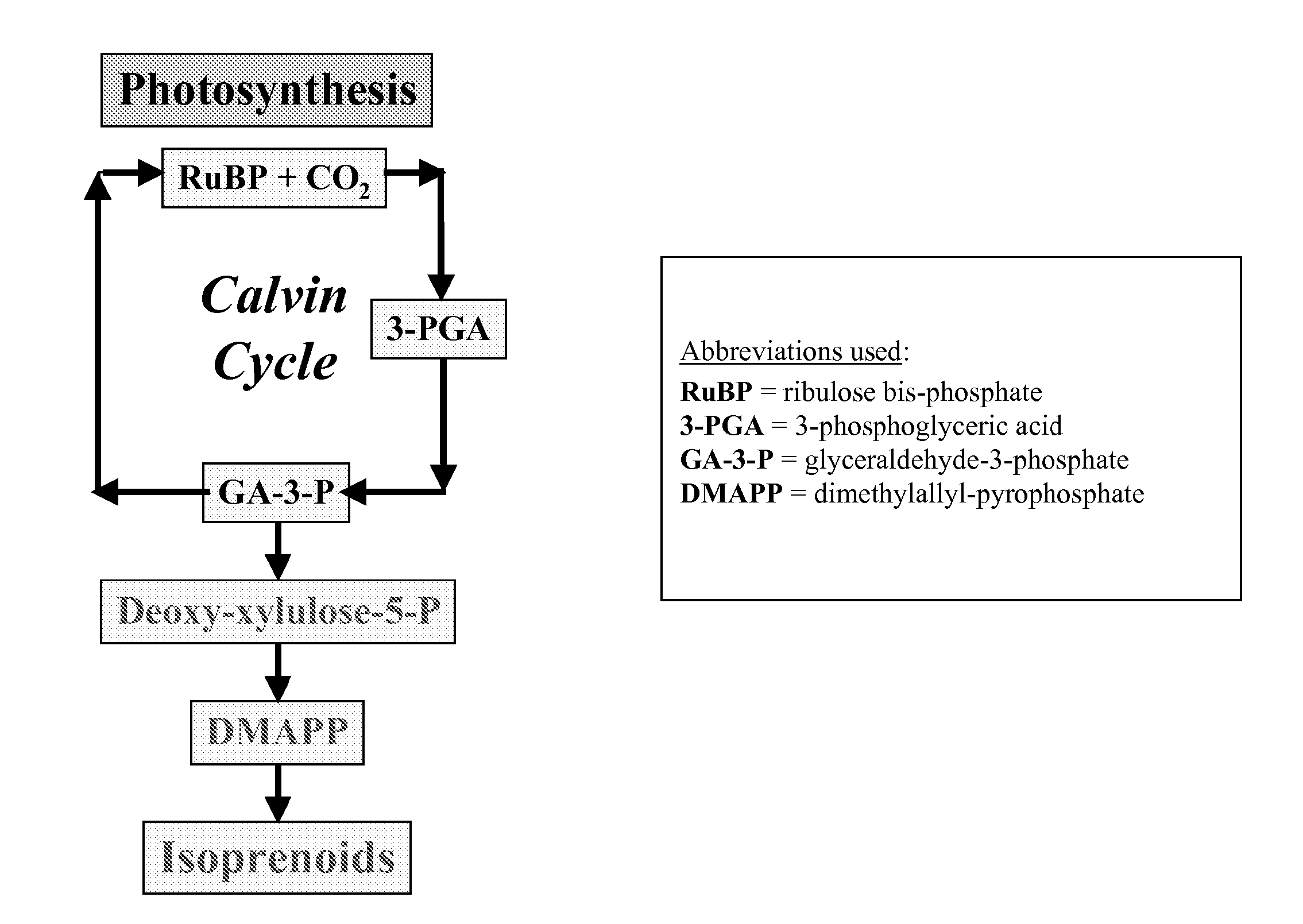
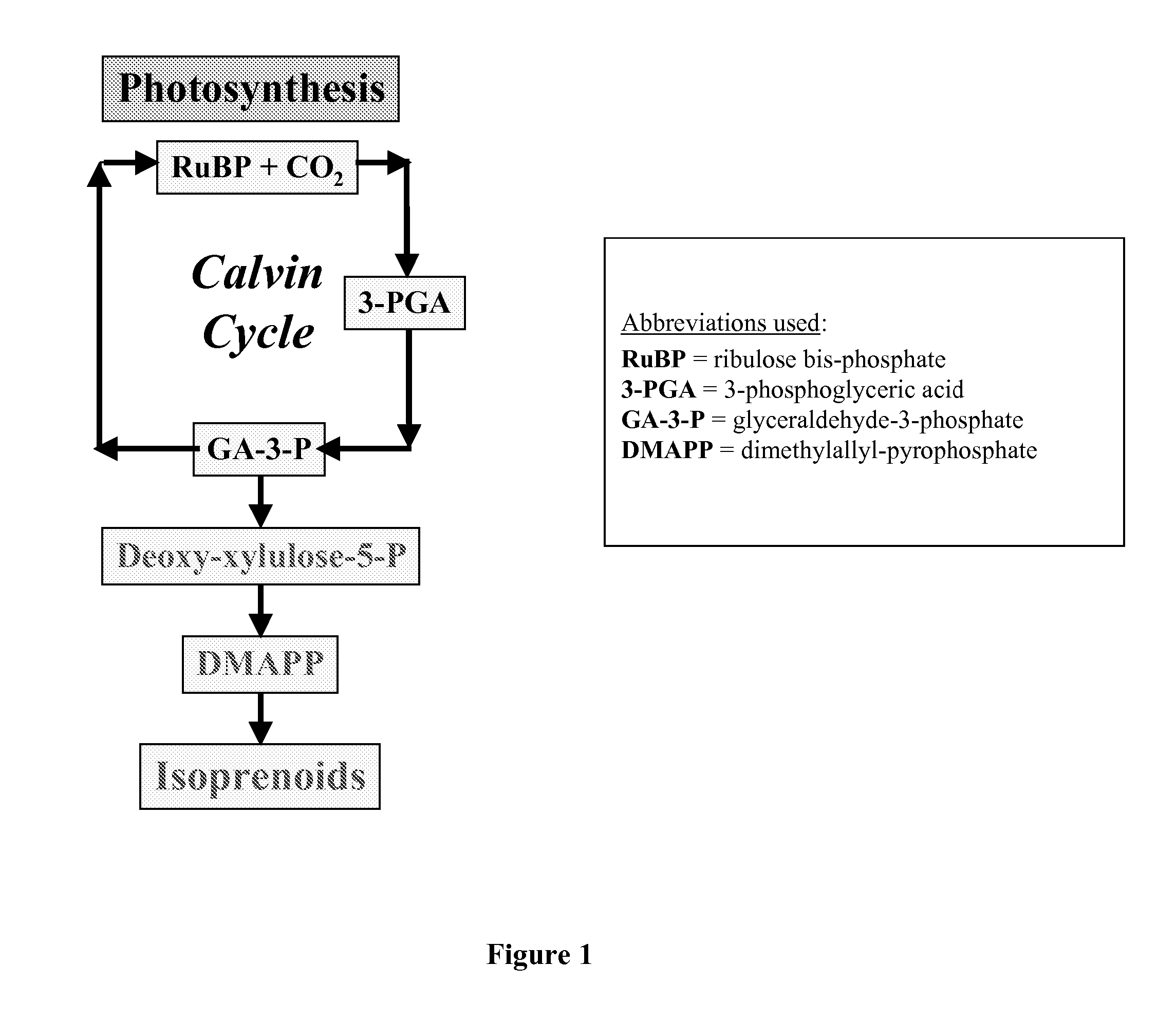
Short chain volatile hydrocarbon production using genetically engineered microalgae, cyanobacteria or bacteria
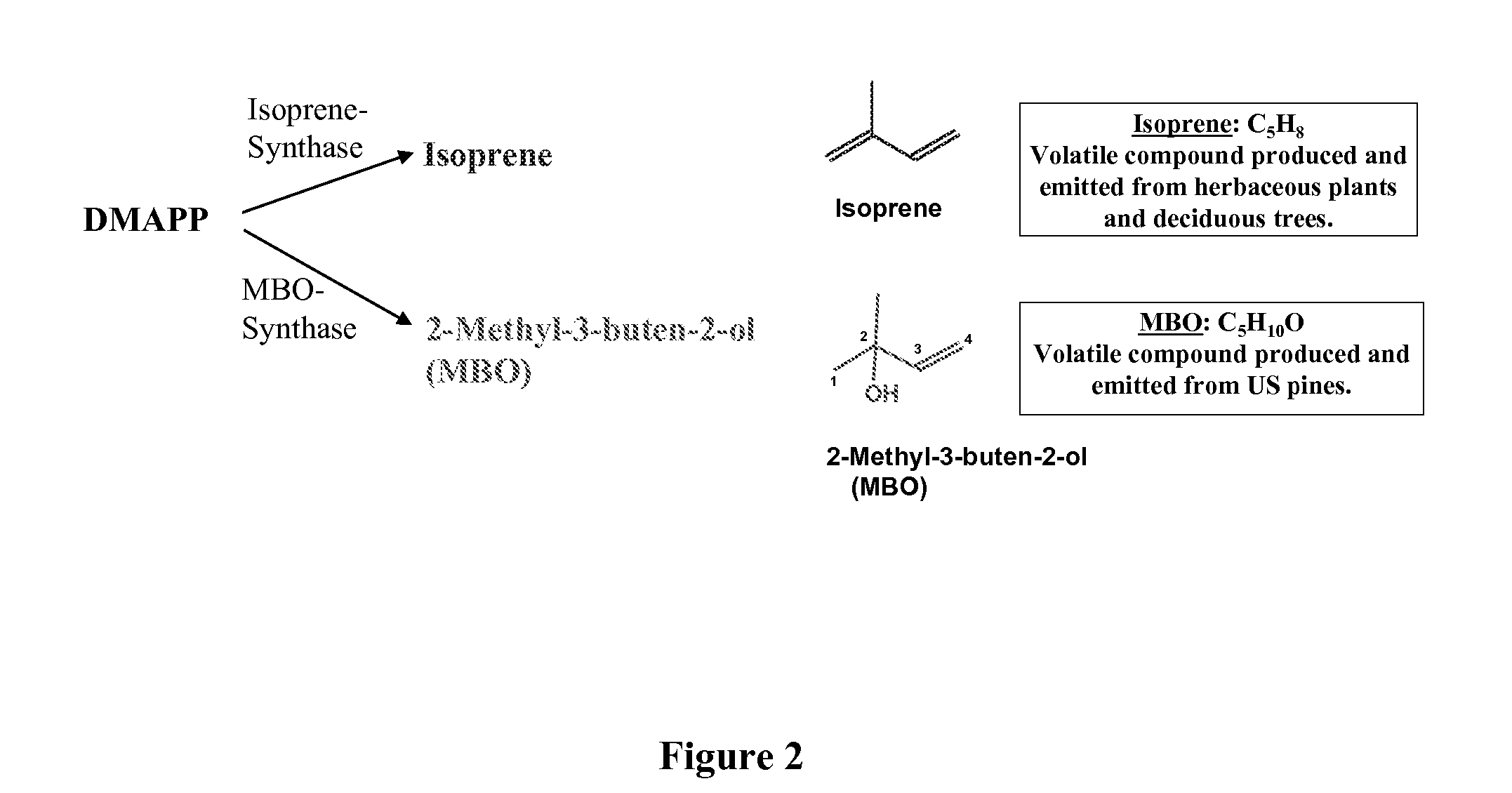
ActiveUS20080038805A1Promote reproductionLow costBacteriaUnicellular algaePhylum CyanobacteriaCyanobacteria
The present invention provides methods and compositions for producing isoprene hydrocarbons from microalgae, cyanobacteria, and photosynthetic and non-photosynthetic bacteria.
Owner:RGT UNIV OF CALIFORNIA
Modified soluble T cell receptor
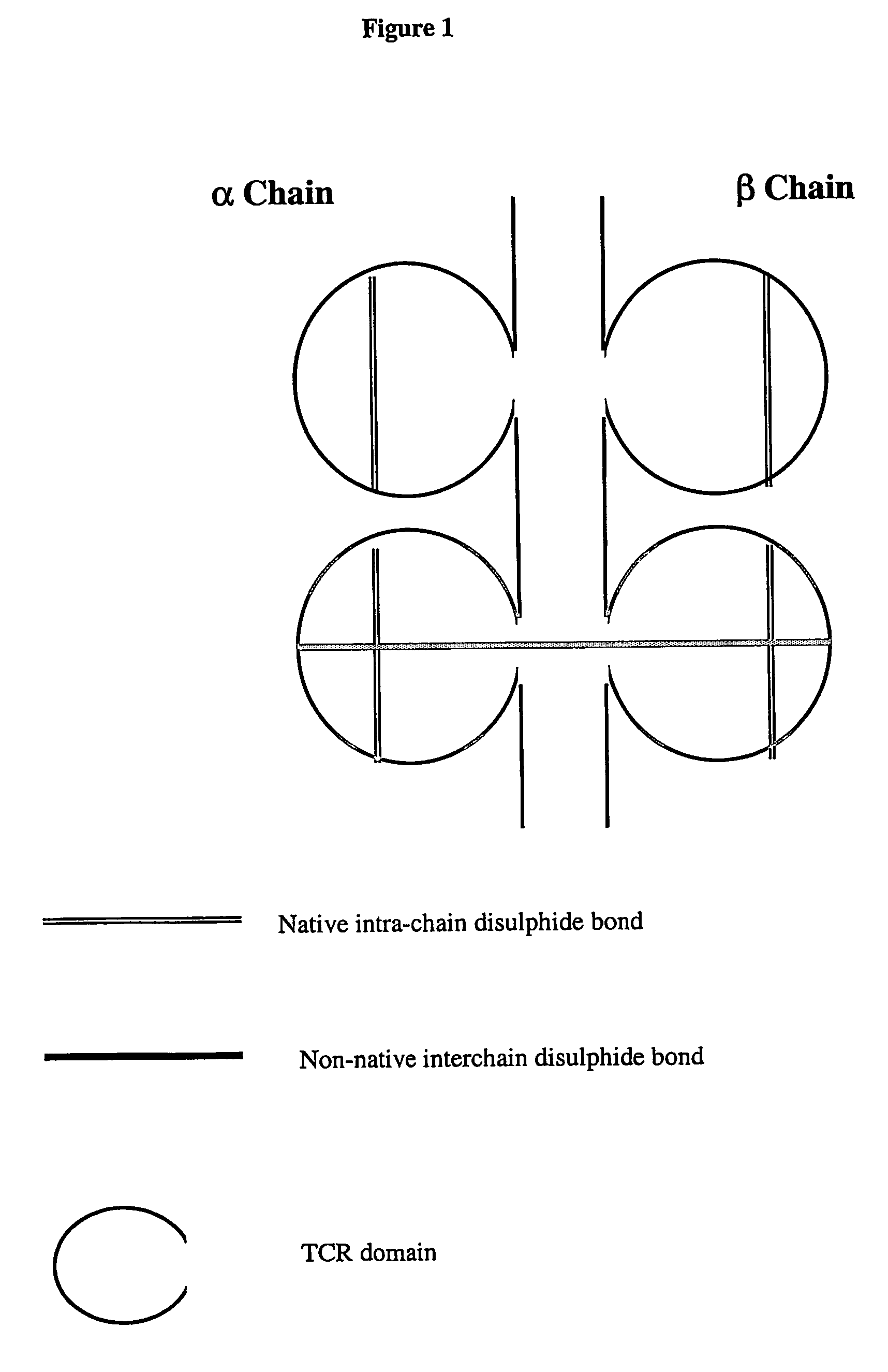
The present invention provides a soluble T cell receptor (sTCR), which comprises (i) all or part of a TCR $g(a) chain, except the transmembrane domain thereof, and (ii) all or part of a TCR $g(b) chain, except the transmembrane domain thereof. (i) and (ii) each comprise a functional variable domain and at least a part of the constant domain of the TCR chain, and are linked by a disulphide bond between constant domain residues which is not present in native TCR, characterized in that the sTCR recognizes a CD1-antigen complex, a bacterial superantigen or a peptide-MHC / superantigen complex.
Owner:IMMUNOCORE LTD +1
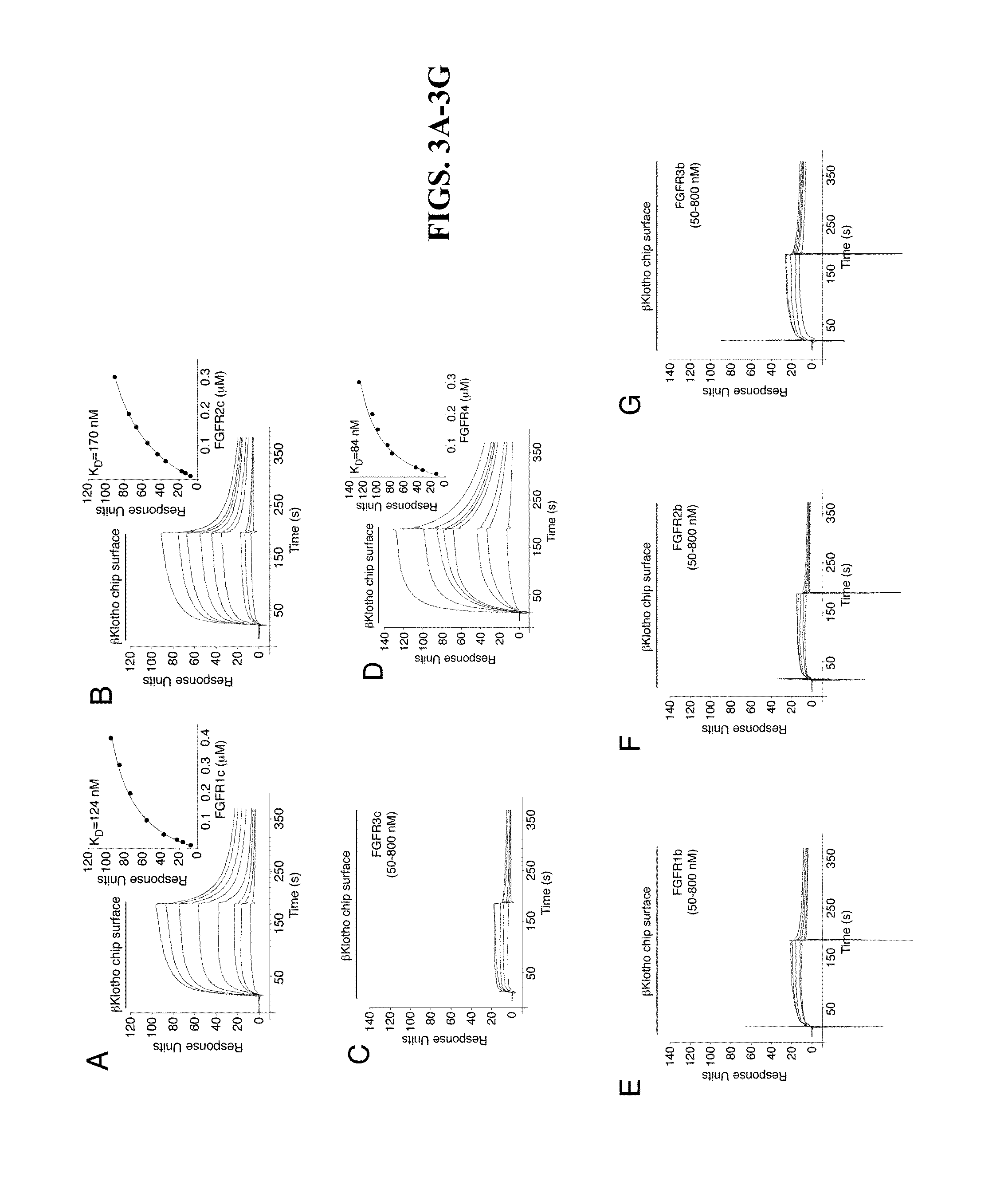
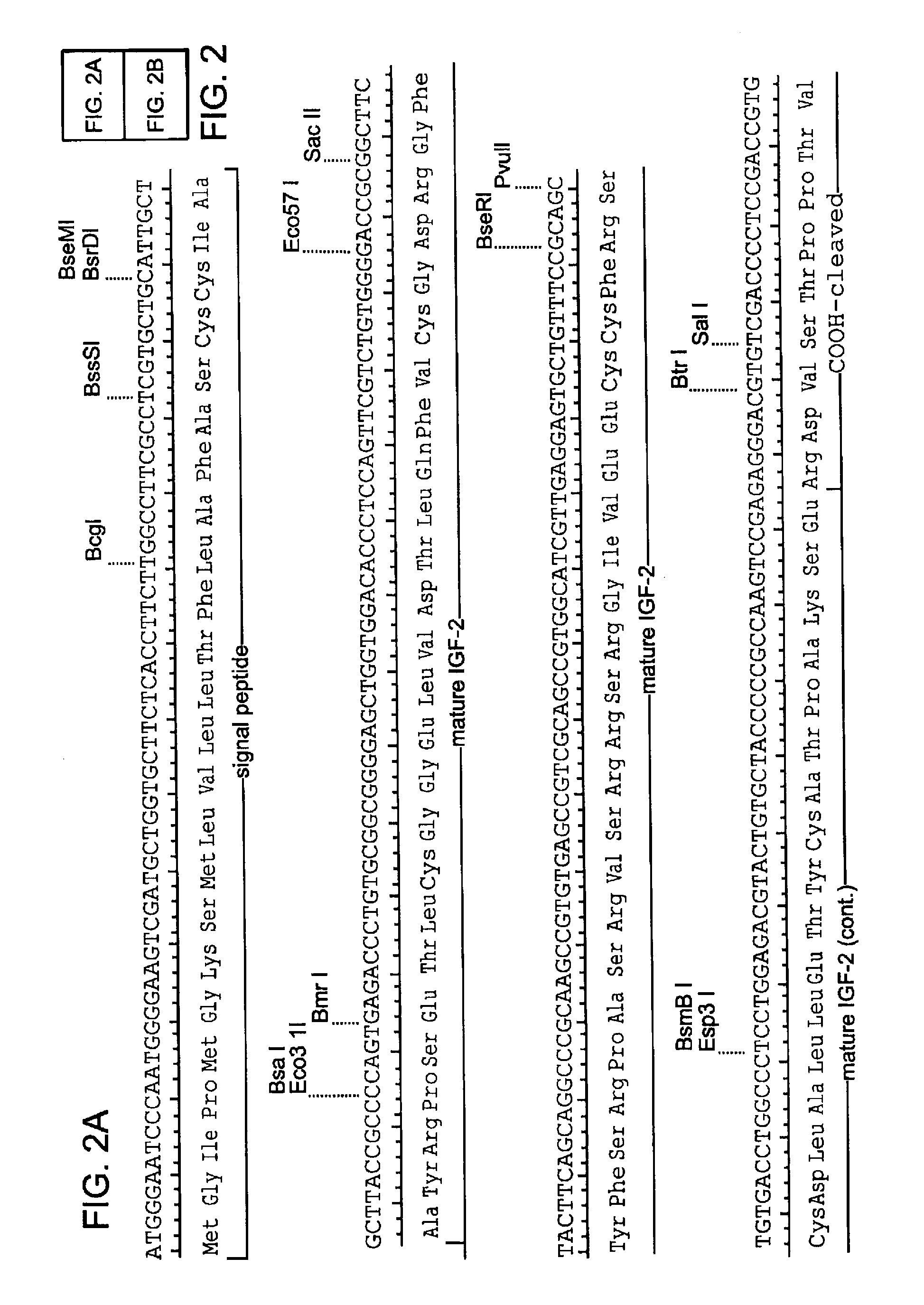
Chimeric fgf21 proteins with enhanced binding affinity for beta-klotho for the treatment of type ii diabetes, obesity, and related metabolic disorders
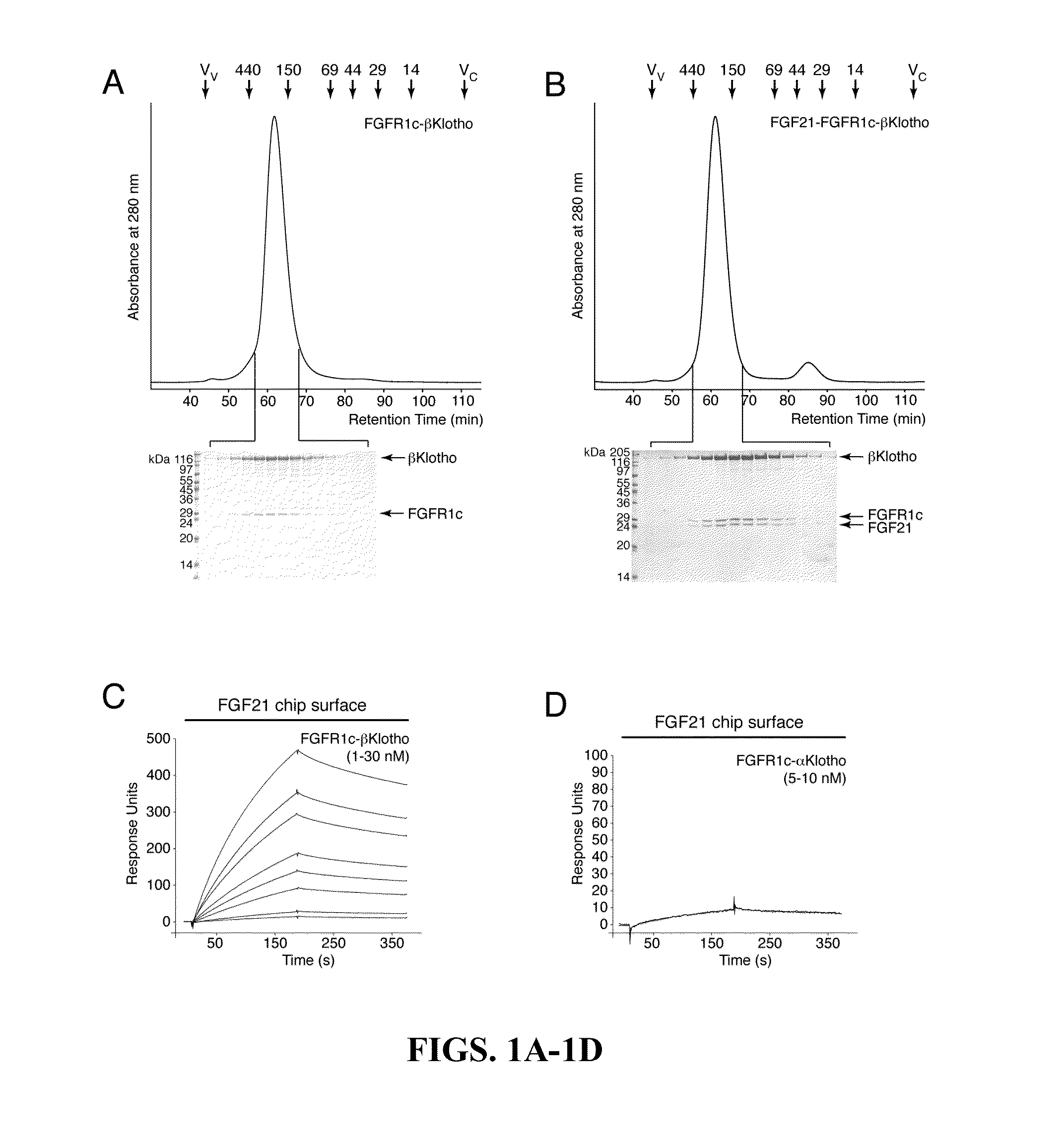
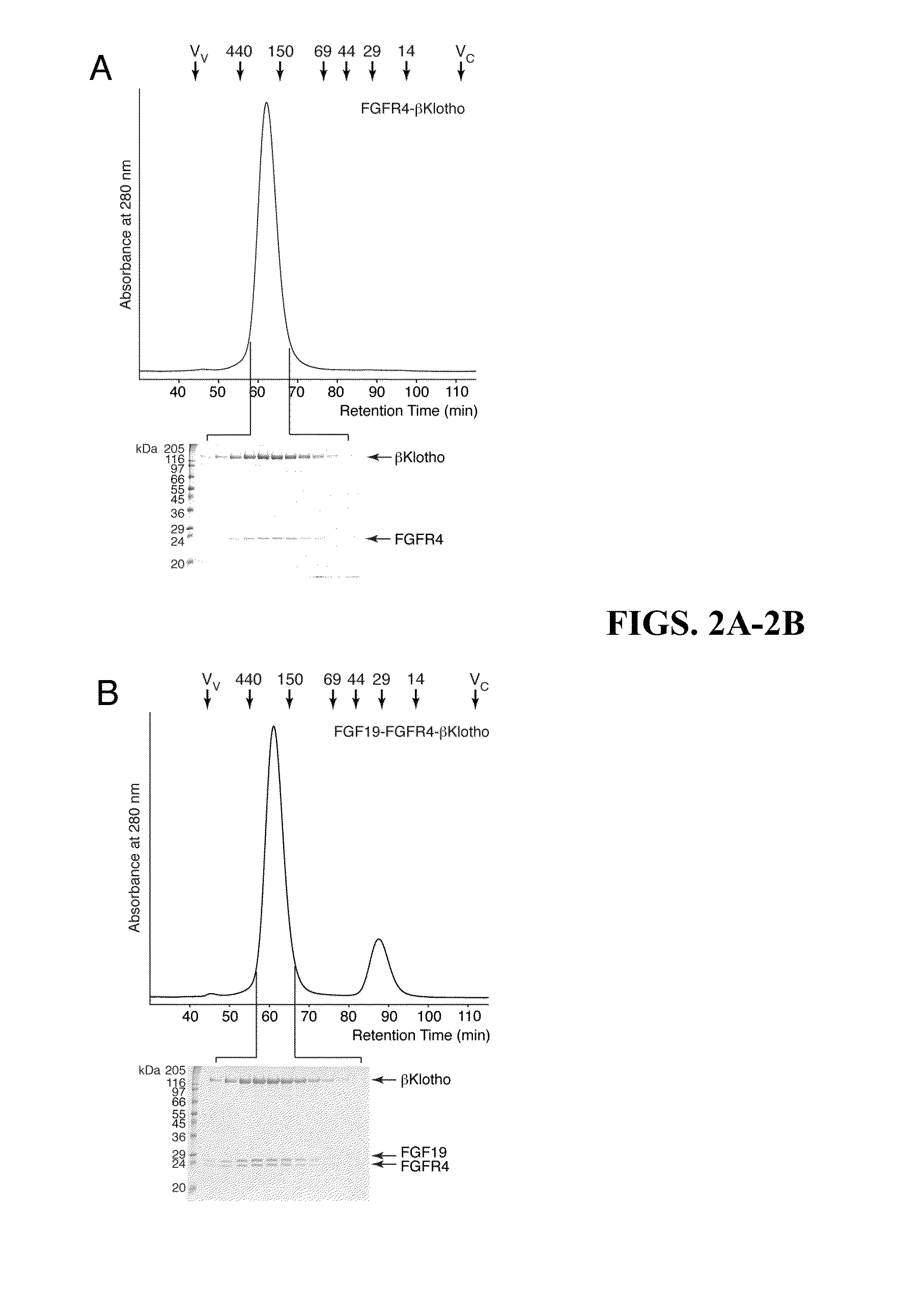
The present invention relates to chimeric proteins that include an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion of fibroblast growth factor 21 (“FGF21”) and the C-terminus includes a C-terminal portion of fibroblast growth factor 19 (“FGF19”). The present invention also relates to pharmaceutical compositions including chimeric proteins according to the present invention, as well as methods for treating a subject suffering from diabetes, obesity, or metabolic syndrome, methods of treating a subject in need of increased FGF21-βKlotho-FGF receptor complex formation, methods of causing increased FGF21 receptor agonist-βKlotho-FGF receptor complex formation, and methods of screening for compounds with enhanced binding affinity for the βKlotho-FGF receptor complex involving the use of chimeric proteins of the present invention.
Owner:NEW YORK UNIV
Apolipoprotein analogues
InactiveUS20020156007A1Antibody mimetics/scaffoldsGenetic material ingredientsCubilinLipid formation
The invention relates to a pharmaceutical composition comprising an apolipoprotein construct, to an apolipoprotein construct, a nucleic acid sequence encoding the apolipoprotein construct, a vector comprising the nucleic acid sequence, a method for producing the apolipoprotein construct, and a method of treatment comprising administering the apolipoprotein construct. The presented data document that the constructs according to the invention are capable of binding lipids, are capable of binding cubilin, which is a strong Apo Al receptor, stronger than native Apo A-I and that the plasma half life of the constructs is at least tripled compared to native Apo A-I. Together these data document that the constructs according to the invention are strong candidates for treatment of cardiovascular diseases.
Owner:F HOFFMANN LA ROCHE & CO AG
Luciferase biosensor
ActiveUS20050153310A1Inhibitory activityCompound screeningApoptosis detectionLuciferaseEnzyme protein
Owner:PROMEGA
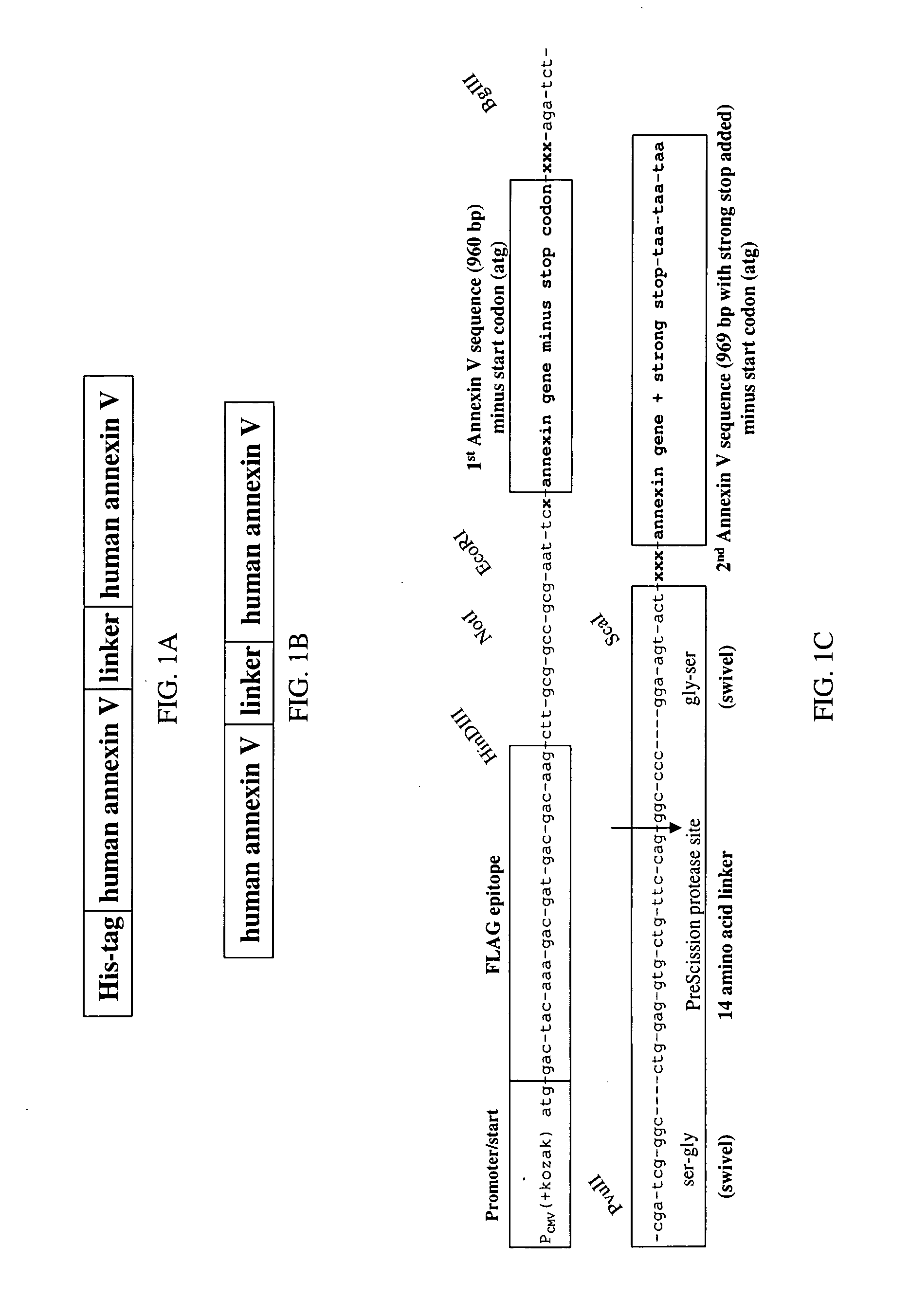
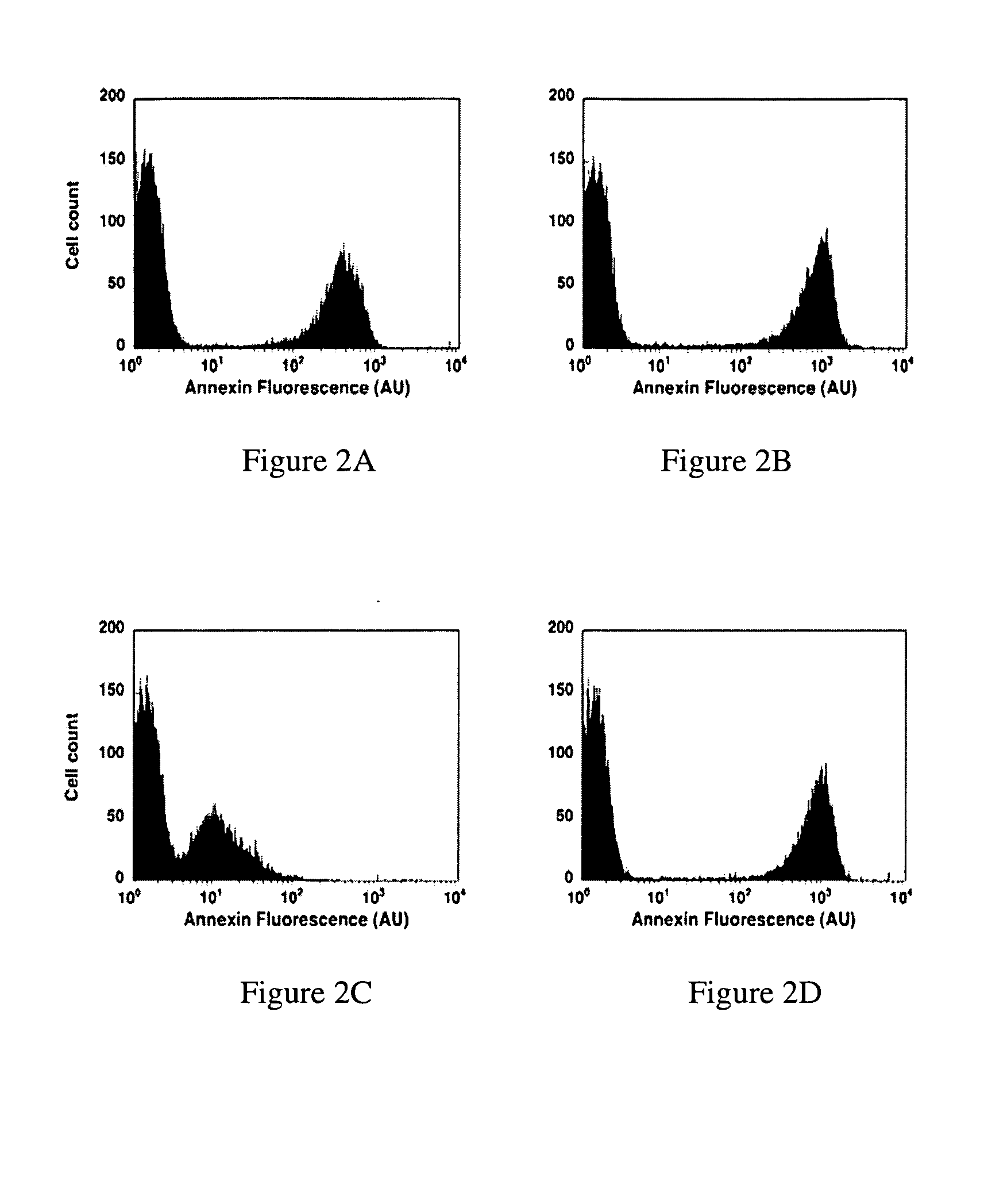
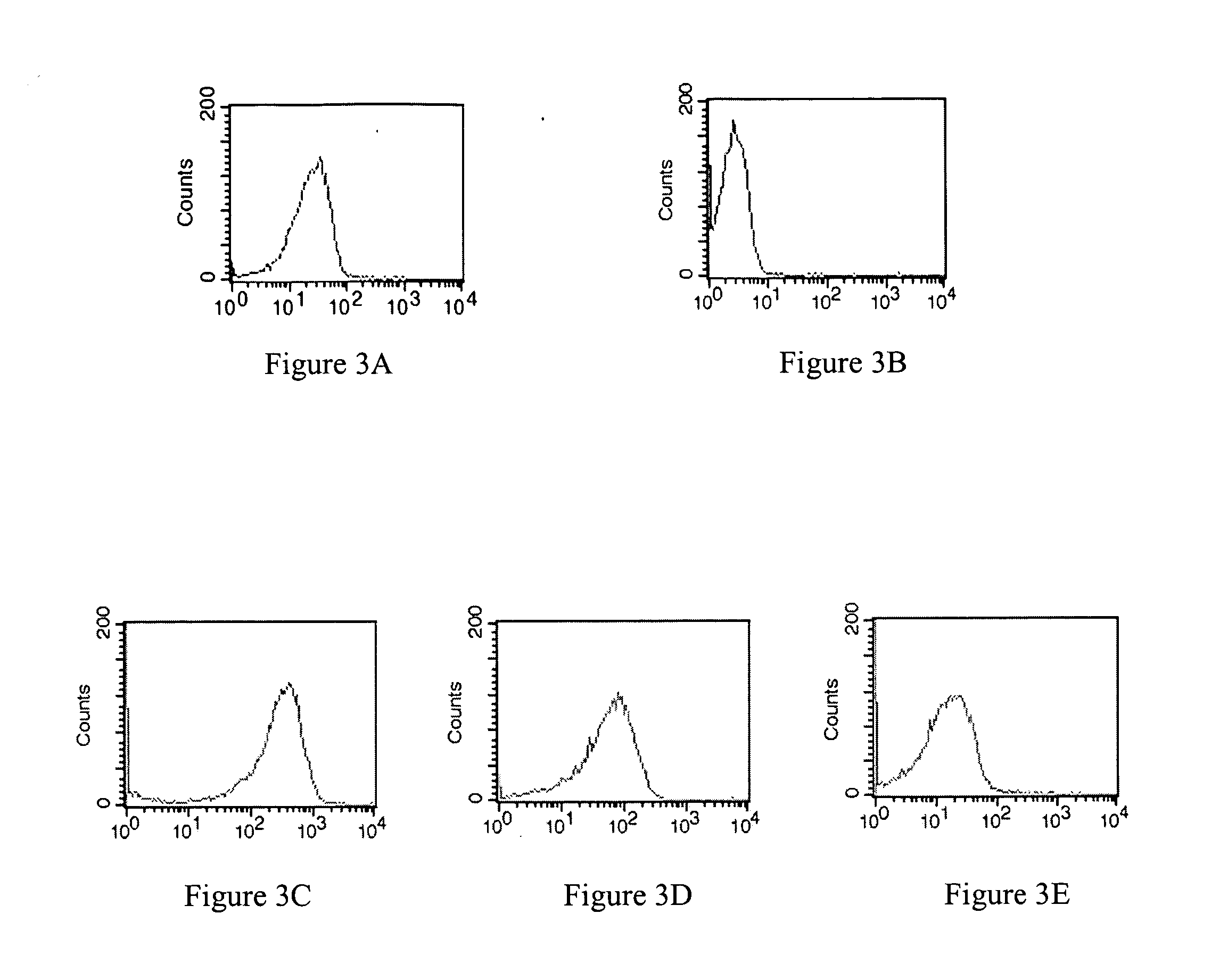
Modified annexin proteins and methods for preventing thrombosis
InactiveUS20050222030A1Inhibition of attachmentReducing endothelial cell damagePeptide/protein ingredientsAntibody mimetics/scaffoldsTreatment effectSufficient time
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:ALAVITA PHARMA
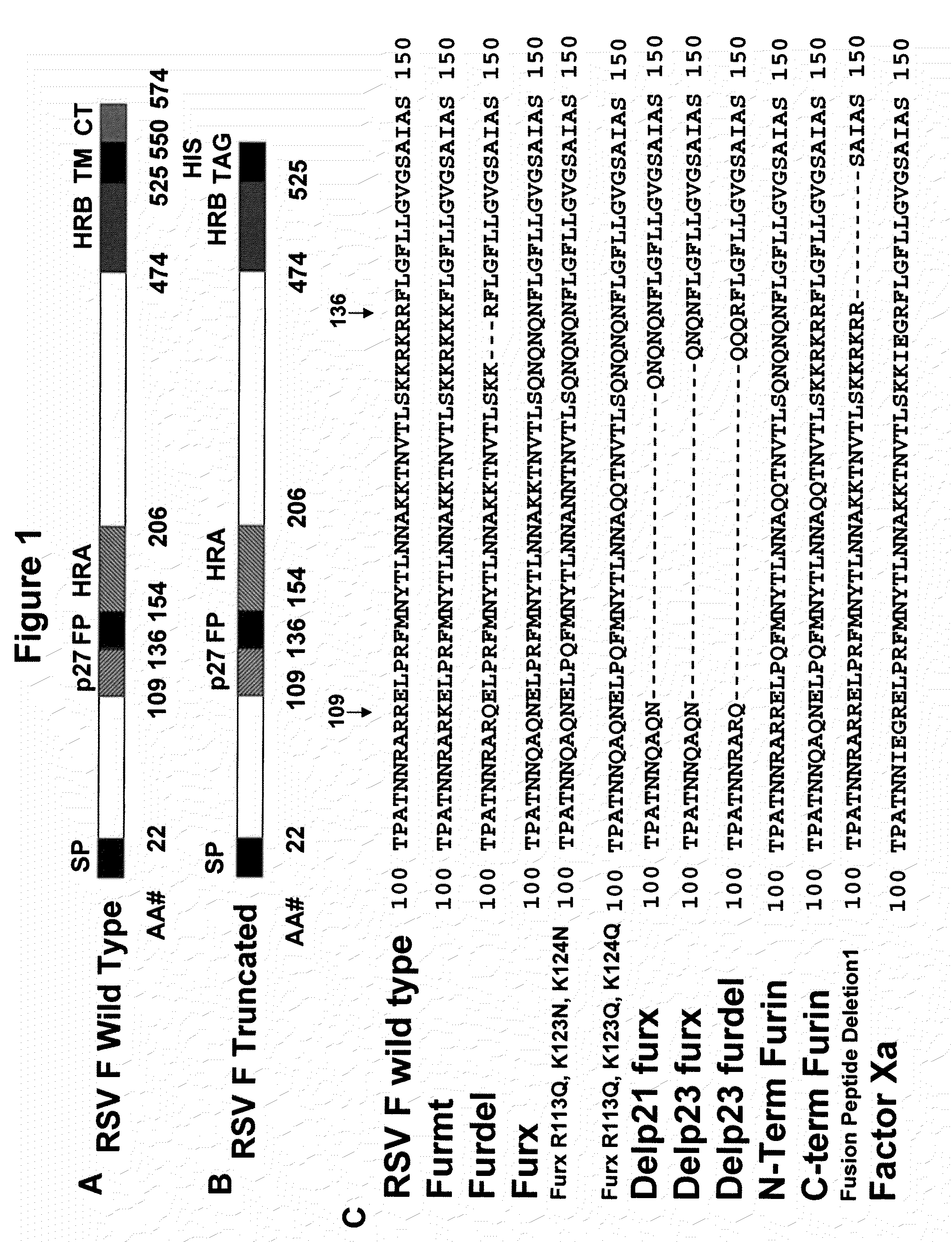
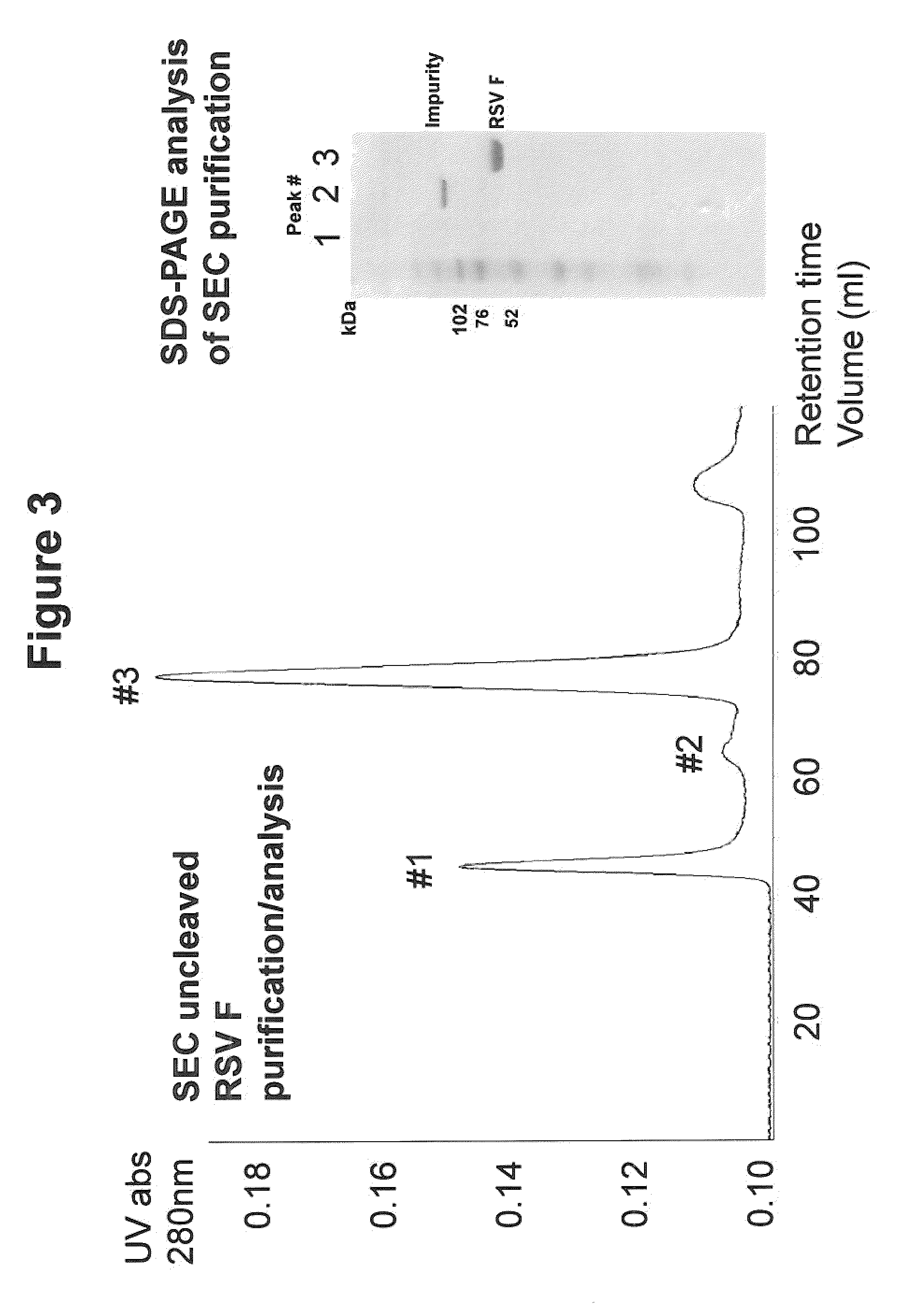
Rsv f protein compositions and methods for making same
InactiveUS20110305727A1Consistent stabilitySsRNA viruses negative-senseSsRNA viruses positive-senseF proteinImmunogenicity
The present invention relates to immunogenic compositions comprising RSV F protein, methods for preparing compositions that contain RSV F protein ecto-domain polypeptides, and to certain engineered RSV F proteins and nucleic acids that encode the engineered RSV F proteins. Compositions prepared using the methods can contain RSV F protein ecto-domain polypeptides in a predominant or single desired form and conformation. The invention also relates to methods for inducing an immune response to RSV F.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Chimeric antigens for eliciting an immune response
ActiveUS20050013828A1Effective presentationImprove efficiencyBiocideSsRNA viruses positive-senseHost immunityAntibody fragments
Disclosed herein are compositions and methods for eliciting immune responses against antigens. In particular embodiments, the compounds and methods elicit immune responses against antigens that are otherwise recognized by the host as “self” antigens. The immune response is enhanced by presenting the host immune system with a chimeric antigen comprising an immune response domain and a target binding domain, wherein the target binding domain comprises a xenotypic antibody fragment. By virtue of the target binding domain, antigen presenting cells take up, process, and present the chimeric antigen, eliciting both a humoral and cellular immune response.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
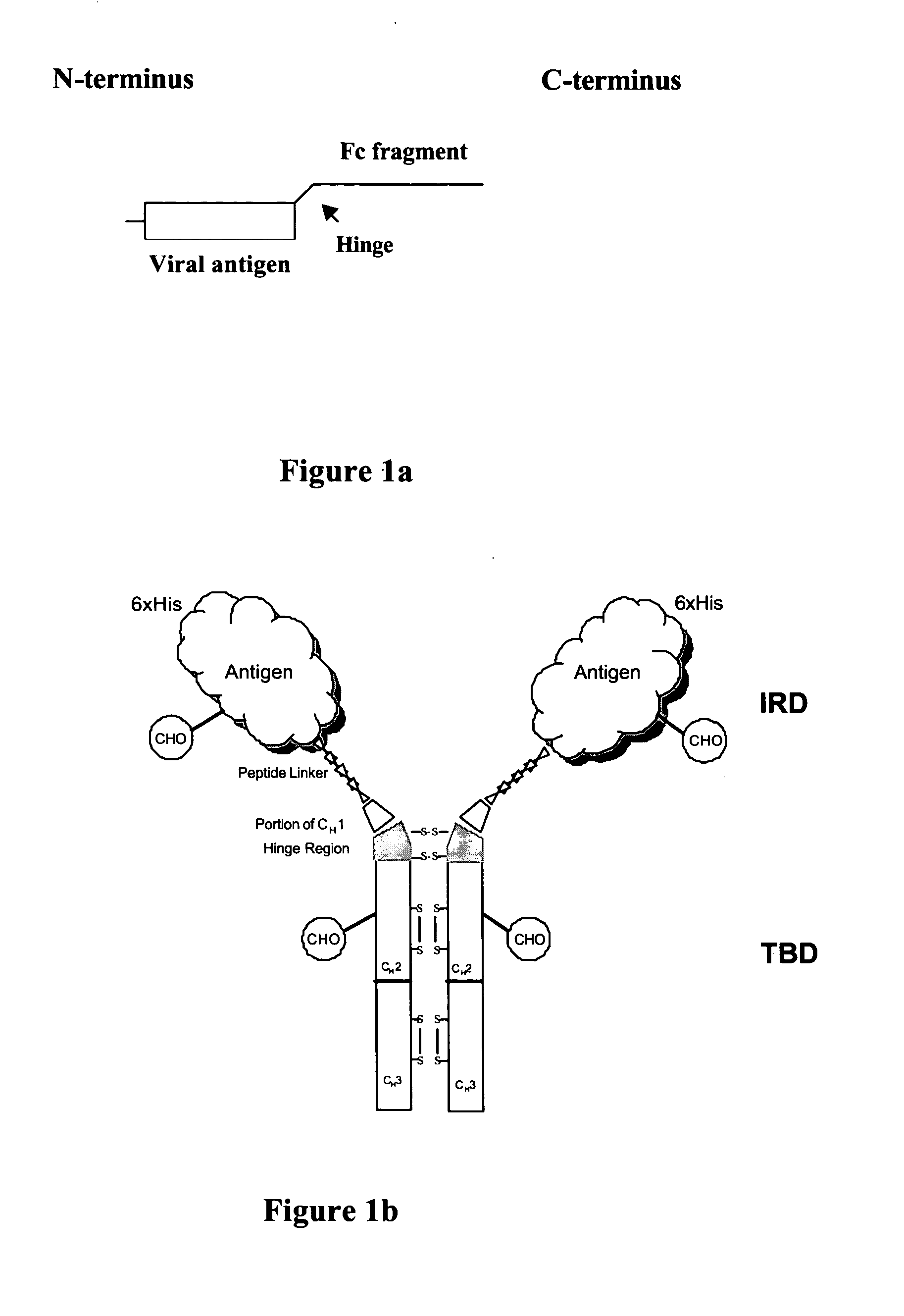
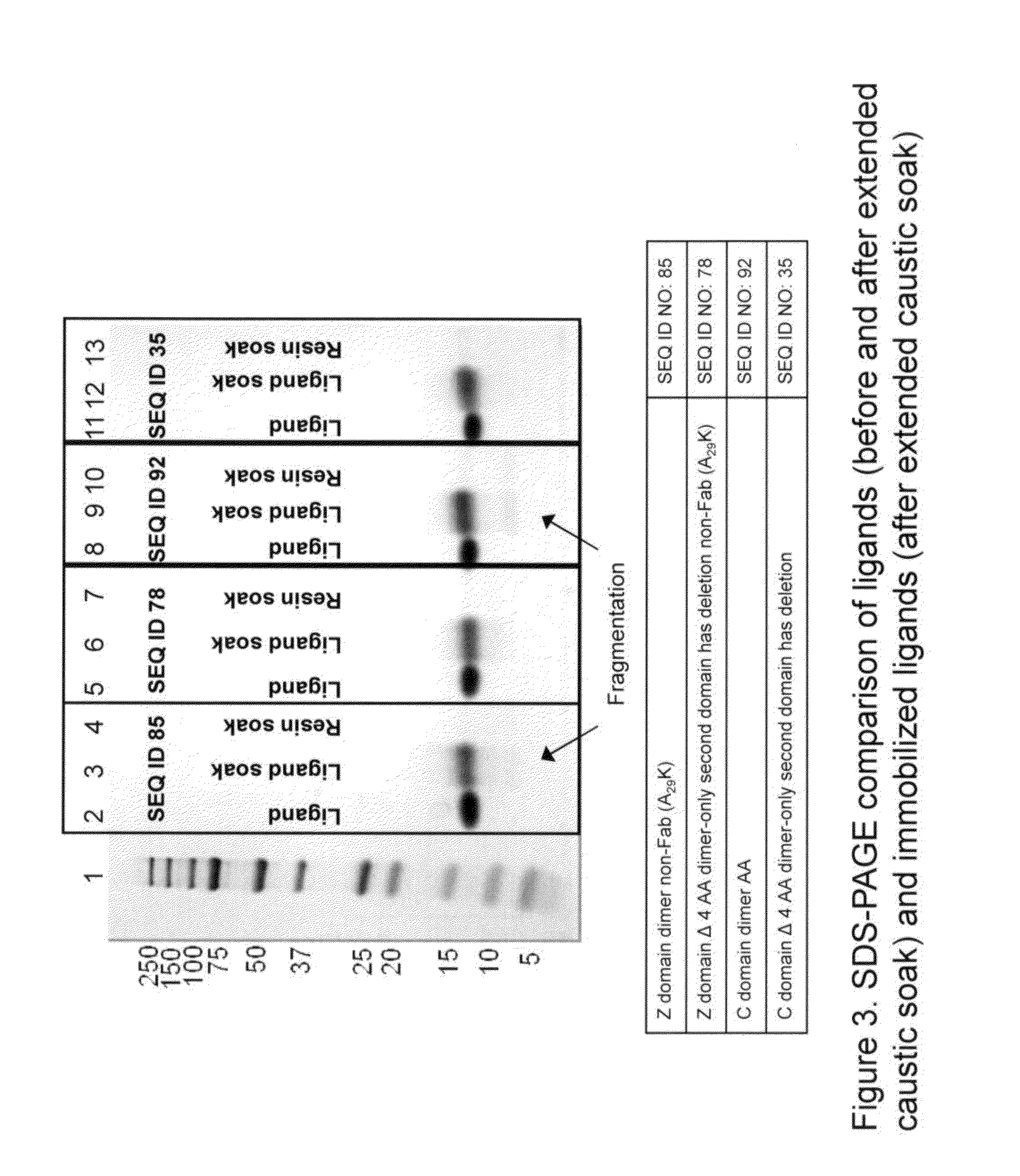
Chromatography matrices including novel staphylococcus aureus protein a based ligands
ActiveUS20130046056A1Reduce lossesLarge degree of fragmentationSolid sorbent liquid separationPeptide preparation methodsStaphylococcus aureusStaphylococcus aureus protein A
The present invention relates to chromatography matrices including ligands based on one or more domains of immunoglobulin-binding proteins such as, Staphylococcus aureus Protein A (SpA), as well as methods of using the same.
Owner:MILLIPORE CORP
Off-axis collimation optics
ActiveUS20120287511A1Large off-axis beam tilt angleConvenient lightingMechanical apparatusPoint-like light sourceEntocentric lensWide beam
A light funnel collimator has a central lens surface and a back reflecting surface, shaped to provide a wider back-ground beam and a narrower hotspot beam within but off-center of the wider beam. One of the beams is on-axis of the collimator, and the other beam is off-axis. The reflector is at least partly asymmetrical relative to the axis, and provides or contributes to the off-axis beam.
Owner:SEOUL SEMICONDUCTOR
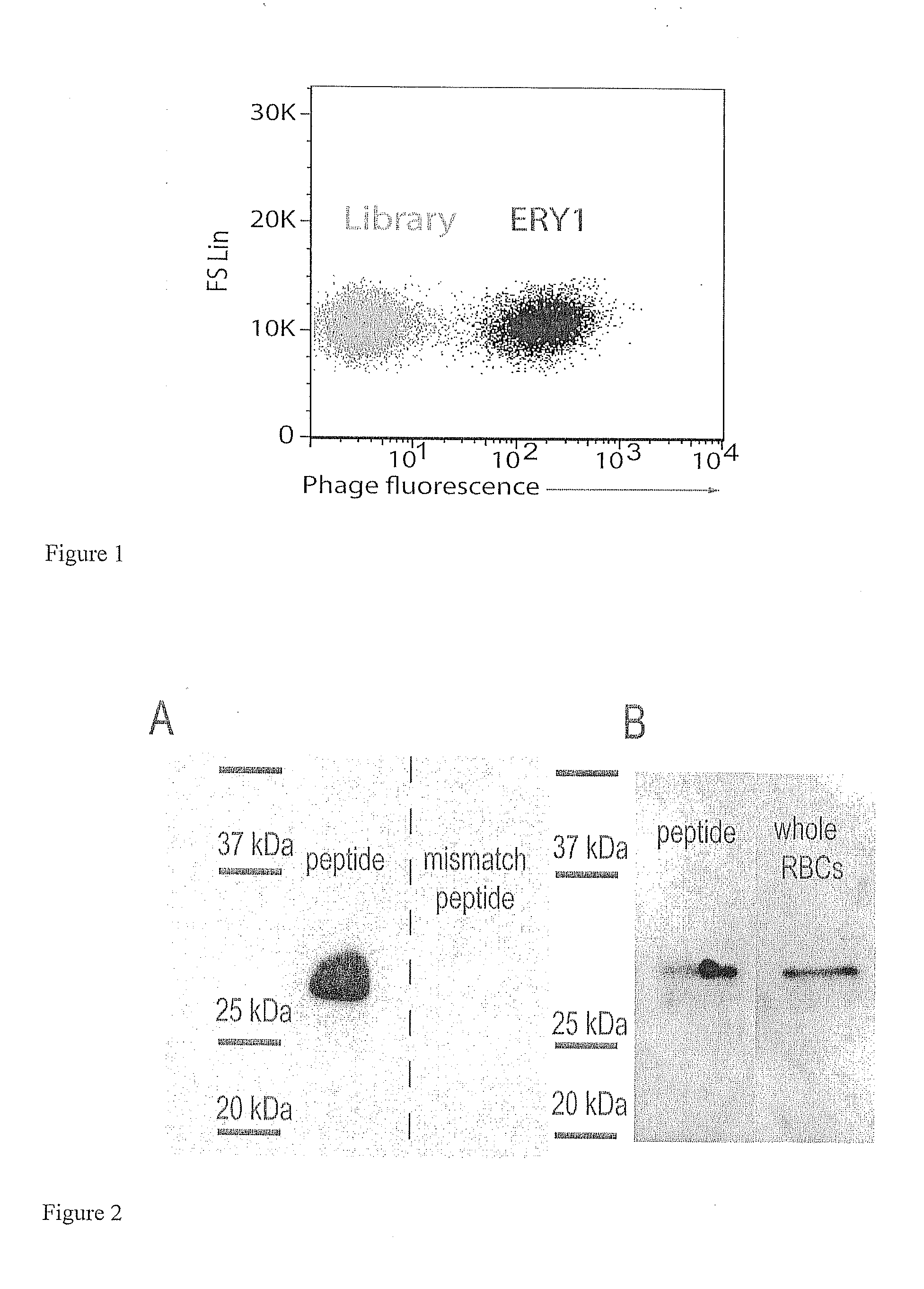
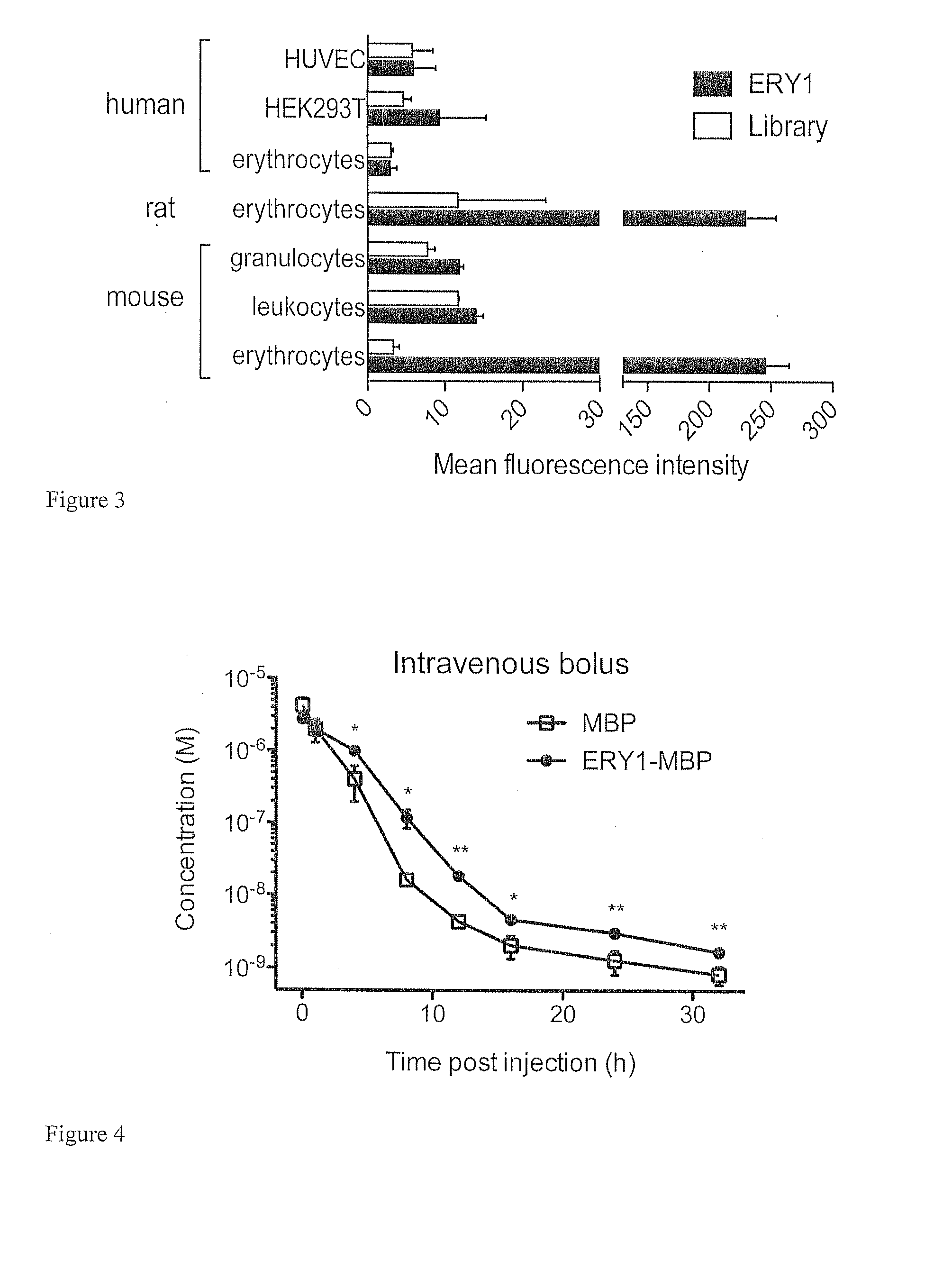
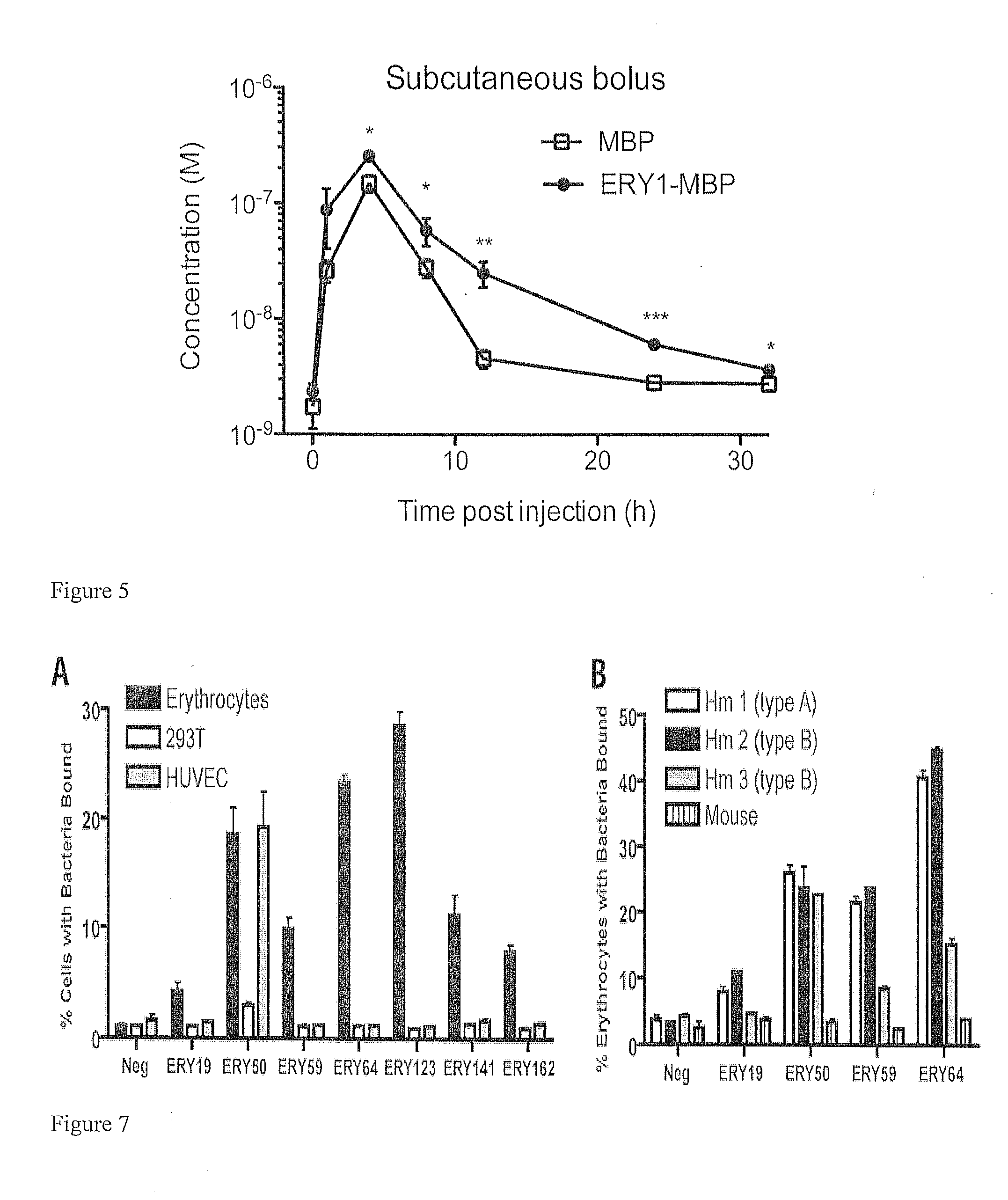
Erythrocyte-binding therapeutics
ActiveUS20120178139A1Blockage of blood supplySimple processHydrolasesPeptide/protein ingredientsErythrocyte bindingImmune tolerance
Peptides that specifically bind erythrocytes are described. These are provided as peptidic ligands having sequences that specifically bind, or as antibodies or fragments thereof that provide specific binding, to erythrocytes. The peptides may be prepared as molecular fusions with therapeutic agents, tolerizing antigens, or targeting peptides. Immunotolerance may be created by use of the fusions and choice of an antigen on a substance for which tolerance is desired.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
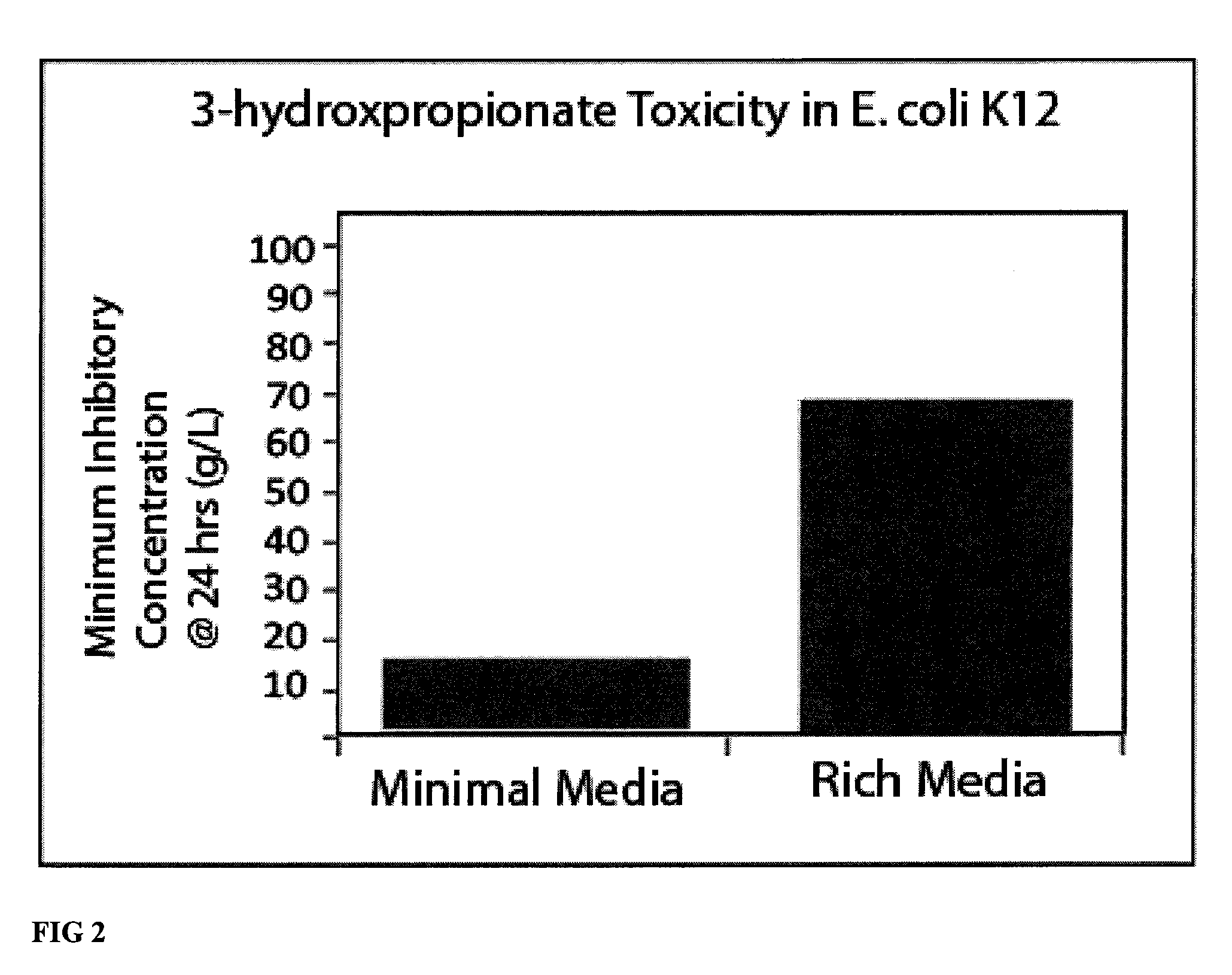
Compositions and methods for 3-hydroxypropionate bio-production from biomass
ActiveUS8048624B1BacteriaMicrobiological testing/measurement3-Hydroxypropionic acidDecarboxylase activity
Methods of obtaining mutant nucleic acid sequences that demonstrate elevated oxaloacetate α-decarboxylase activity are provided. Compositions, such as genetically modified microorganisms that comprise such mutant nucleic acid sequences, are described, as are methods to obtain the same.
Owner:OPX BIOTECH
Serum half-life extension using igbd
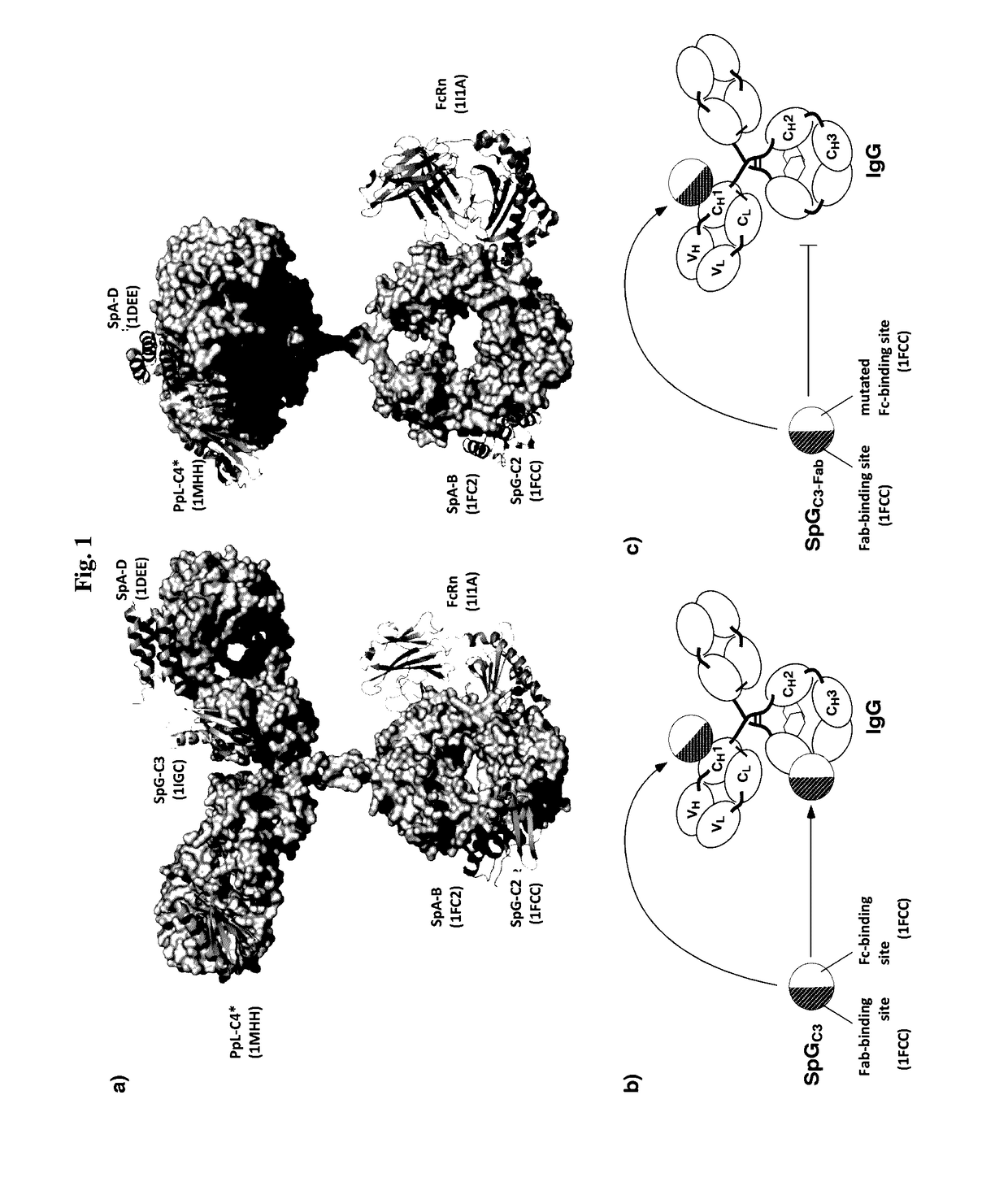
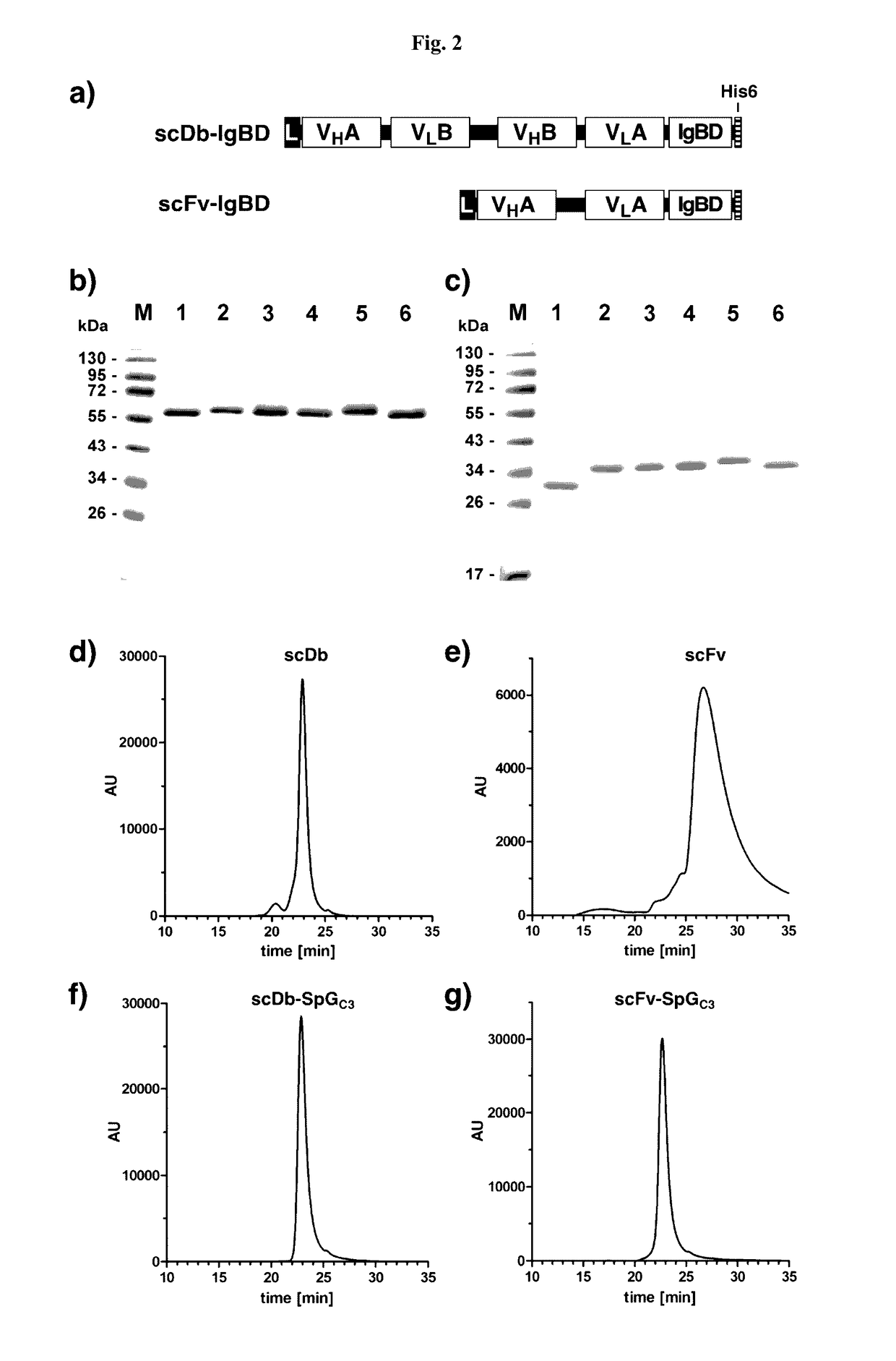
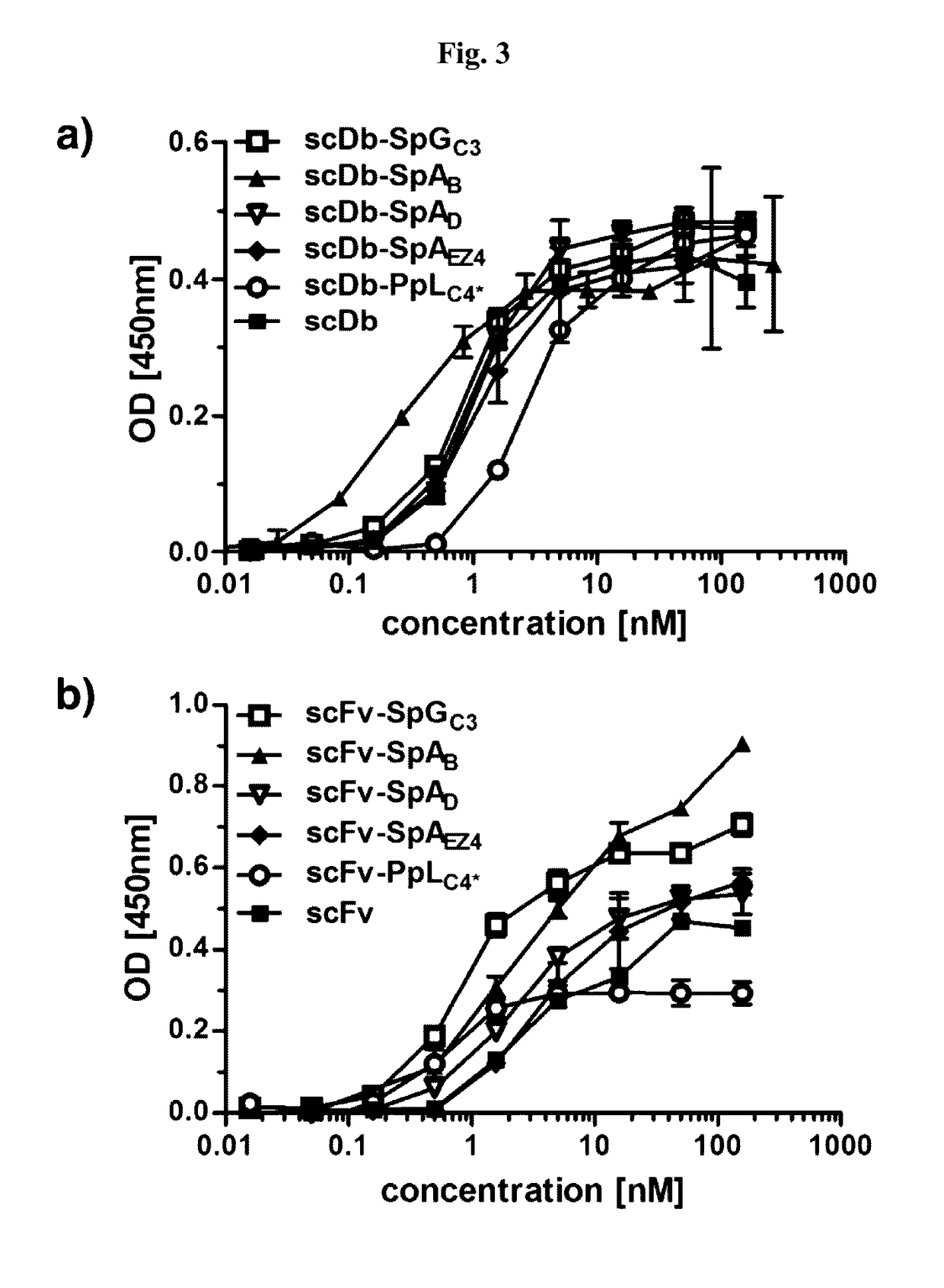
InactiveUS20170145062A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeHeavy chain
Owner:UNIV STUTTGART
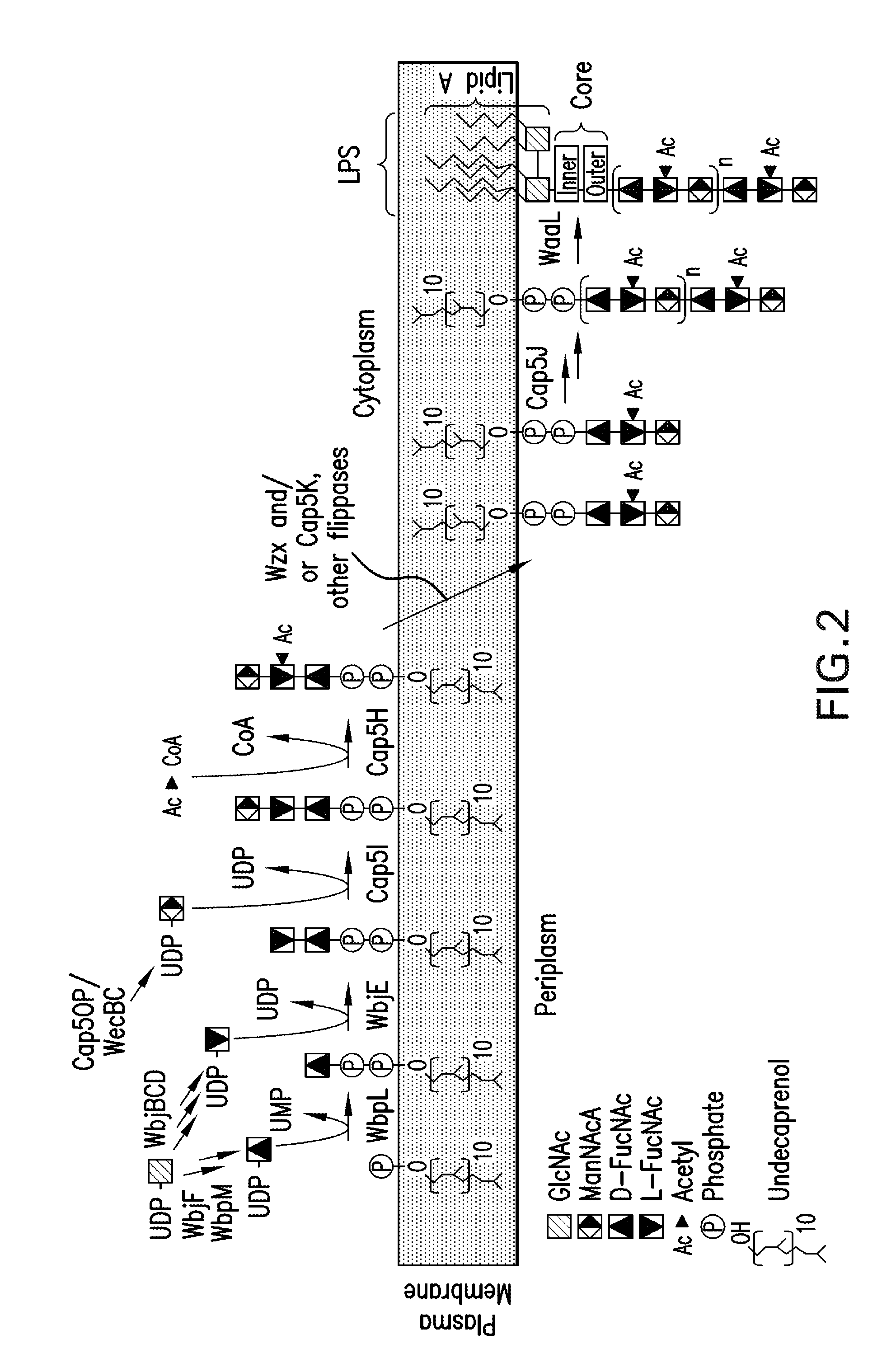
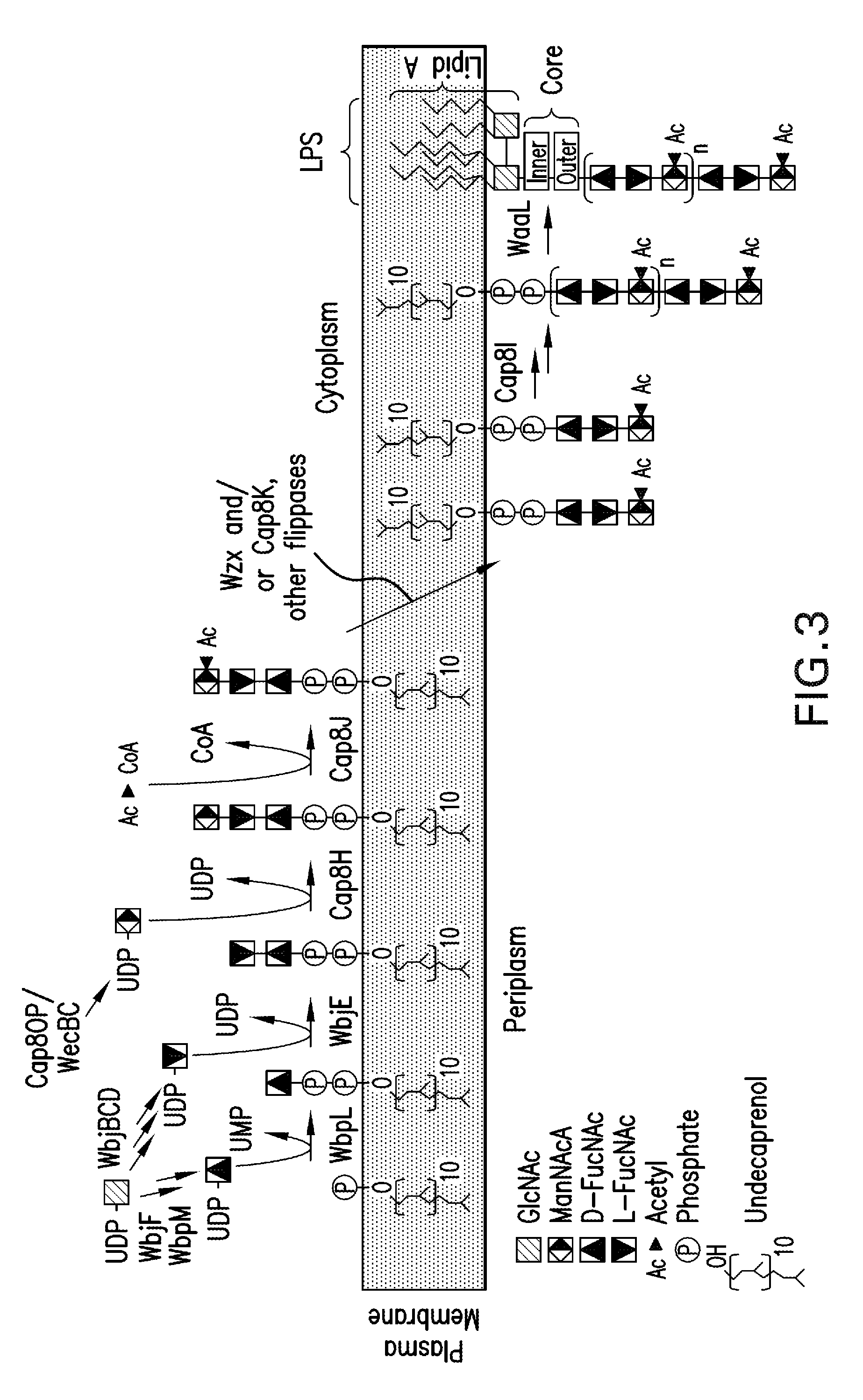
Capsular gram-positive bacteria bioconjugate vaccines
The present invention encompasses a novel S. aureus bioconjugate vaccine. More generally, the invention is directed to Gram-positive and other bioconjugate vaccines containing a protein carrier, at least one polysaccharide such as a capsular Gram-positive polysaccharide, and, optionally, an adjuvant or pharmaceutically acceptable carrier. The instant invention also includes methods of producing Gram-positive and other bioconjugate vaccines. An N-glycosylated protein is also provided that contains one or more polysaccharides such as Gram-positive polysaccharides. The invention is additionally directed to engineered prokaryotic organisms comprising nucleotide sequences encoding a glycosyltransferase of a first prokaryotic organism and a glycosyltransferase of a second prokaryotic organism. The invention further includes plasmids and prokaryotic cells transformed with plasmids encoding polysaccharides and enzymes which produce an N-glycosylated protein and / or bioconjugate vaccine. Further, the invention is directed to methods of inducing an immune response in a mammal comprising administering said bioconjugate vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
ERK ligands and polynucleotides encoding ERK ligands
ActiveUS20090186379A1Prevents ERK substrate phosphorylationPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideKinase
The invention relates to kinase inhibitor ligands and polyligands. In particular, the invention relates to ligands and polyligands that modulate ERK activity. The ligands and polyligands are utilized as research tools or as therapeutics. The invention includes linkage of the ligands and polyligands to a cellular localization signal, epitope tag and / or a reporter. The invention also includes polynucleotides encoding the ligands and polyligands.
Owner:PRECIGEN INC
Combinatorial protein library screening by periplasmic expression
InactiveUS20060029947A1Improve breathabilityPolypeptide with localisation/targeting motifBacteriaScreening techniquesSurface expression
The invention overcomes the deficiencies of the prior art by providing a rapid approach for isolating binding proteins capable of binding small molecules and peptides. In the technique, libraries of candidate binding proteins, such as antibody sequences, may be expressed in the periplasm of gram negative bacteria with at least one target ligand. In clones expressing recombinant polypeptides with affinity for the ligand, the ligand becomes bound and retained by the cell even after removal of the outer membrane, allowing the cell to be isolated from cells not expressing a binding polypeptide with affinity for the target ligand. The target ligand may be detected in numerous ways, including use of direct fluorescence or secondary antibodies that are fluorescently labeled, allowing use of efficient screening techniques such as fluorescence activated cell sorting (FACS). The approach is more rapid and robust than prior art methods and avoids problems associated with the outer surface-expression of ligand fusion proteins employed with phage display.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
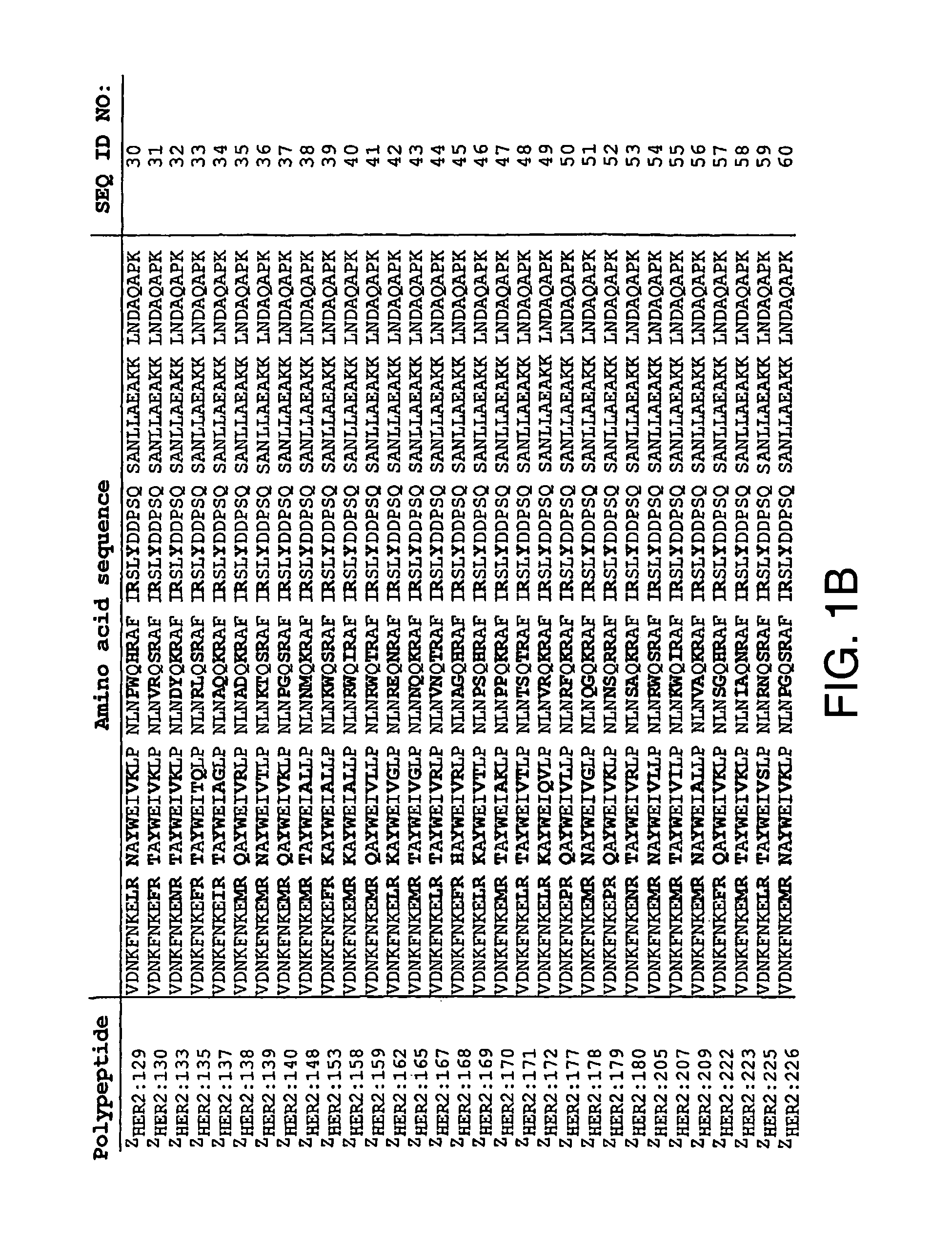
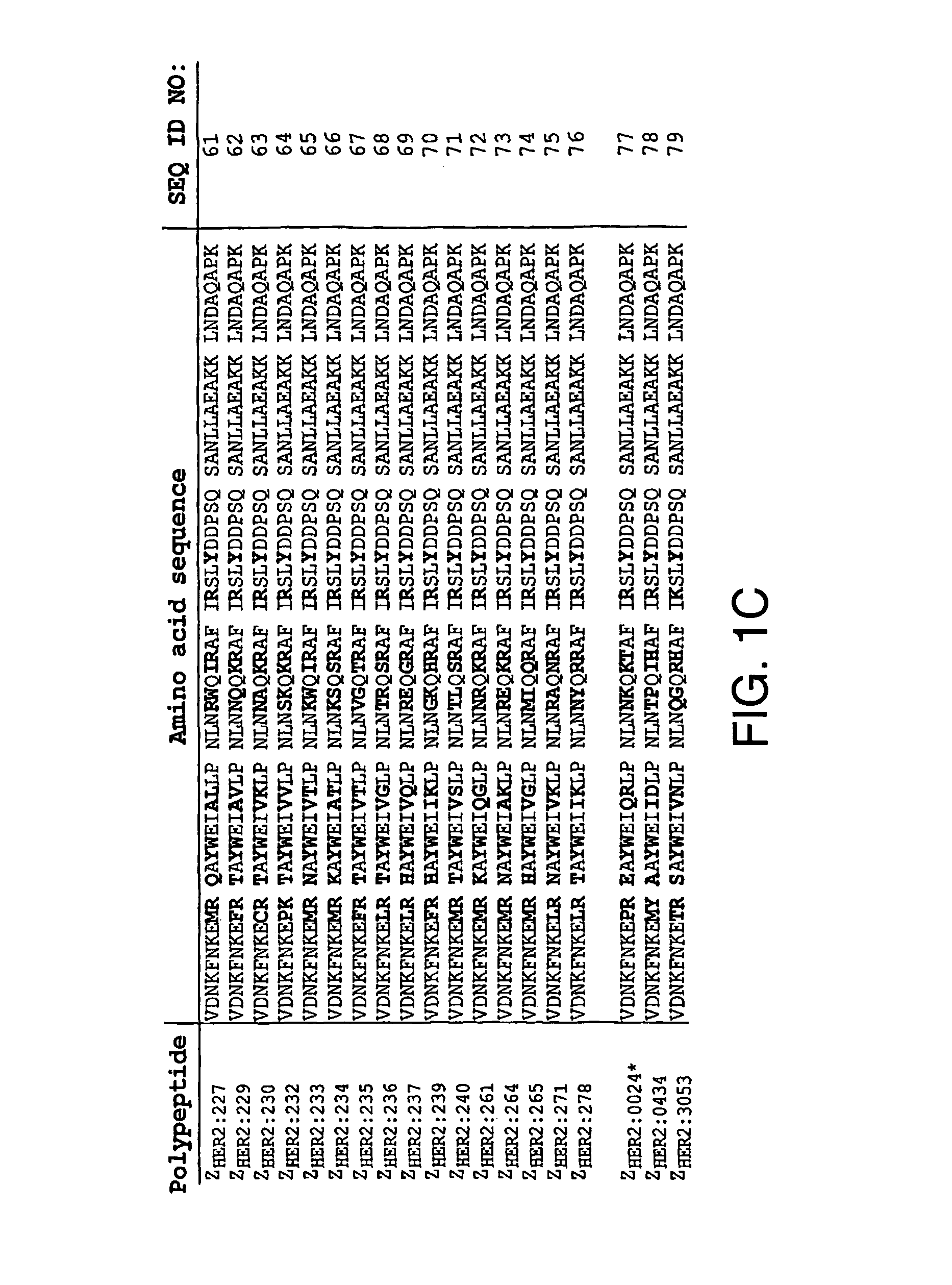
Polypeptides having binding affinity for HER2
ActiveUS7993650B2Easy to useImprove bindingPeptide/protein ingredientsImmunoglobulinsDirect substanceA domain
A polypeptide is provided, which has a binding affinity for HER2 and which is related to a domain of staphylococcal protein A (SPA) in that the sequence of the polypeptide corresponds to the sequence of the SPA domain having from 1 to about 20 substitution mutations. Nucleic acid encoding the polypeptide, as well as expression vector and host cell for expressing the nucleic acid, are also provided. Also provided is the use of such a polypeptide as a medicament, and as a targeting agent for directing substances conjugated thereto to cells overexpressing HER2. Methods, and kits for performing the methods, are also provided, which methods and kits rely on the binding of the polypeptide to HER2.
Owner:AFFIBODY TECH AB
Methods for production of unstructured recombinant polymers and uses thereof
InactiveUS20110151433A1Extended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsToxinPolymer
Owner:AMUNIX OPERATING INC
Serum half-life extension using igbd
The present invention relates to complexes comprising (i) an immunoglobulin (Ig) binding moiety and (ii) a pharmaceutically active moiety, wherein the Ig binding moiety specifically binds to the constant domain 1 of the heavy chain (CH1) of an Ig molecule and their use for therapy and prophylaxis.
Owner:UNIV STUTTGART
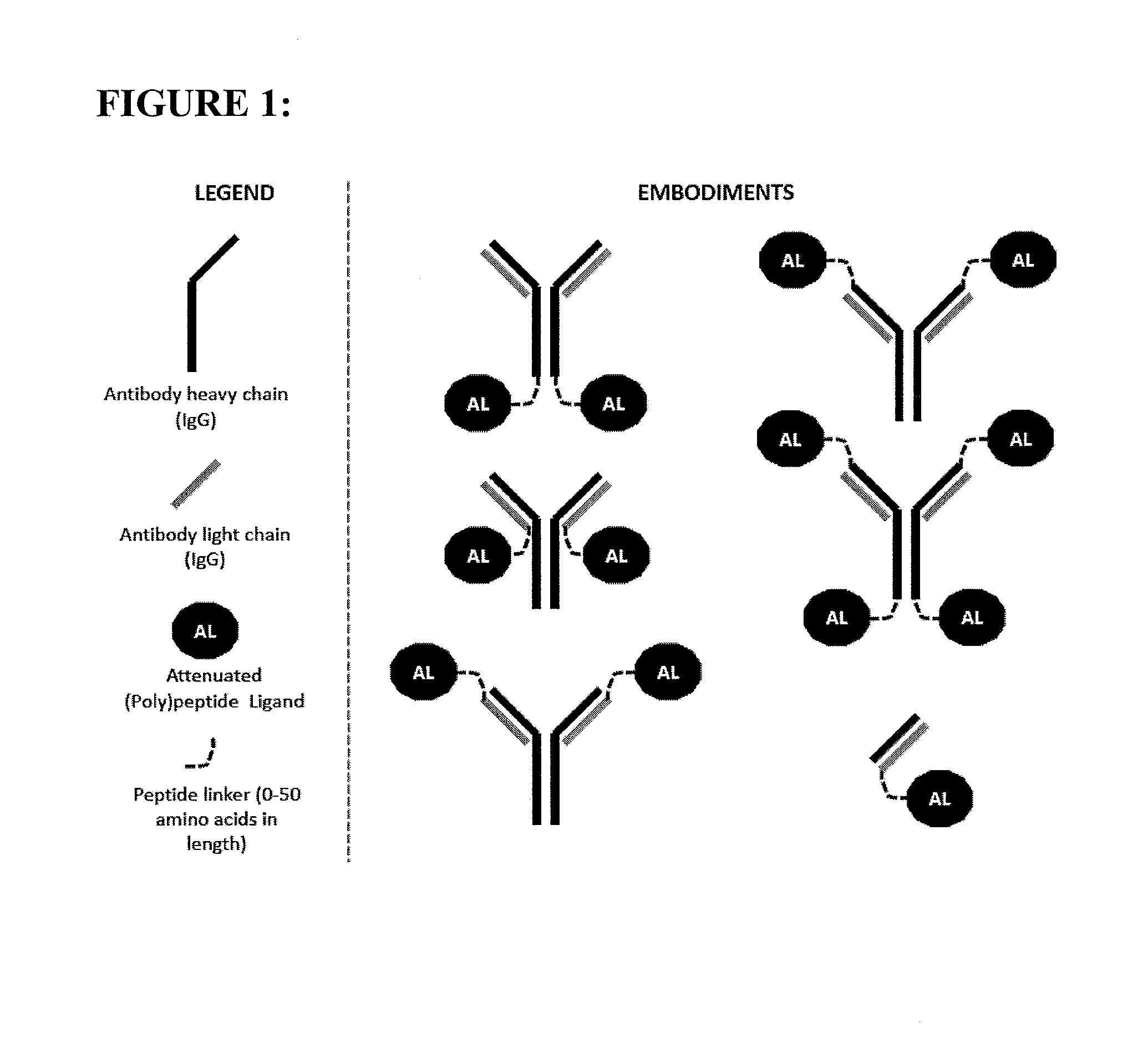
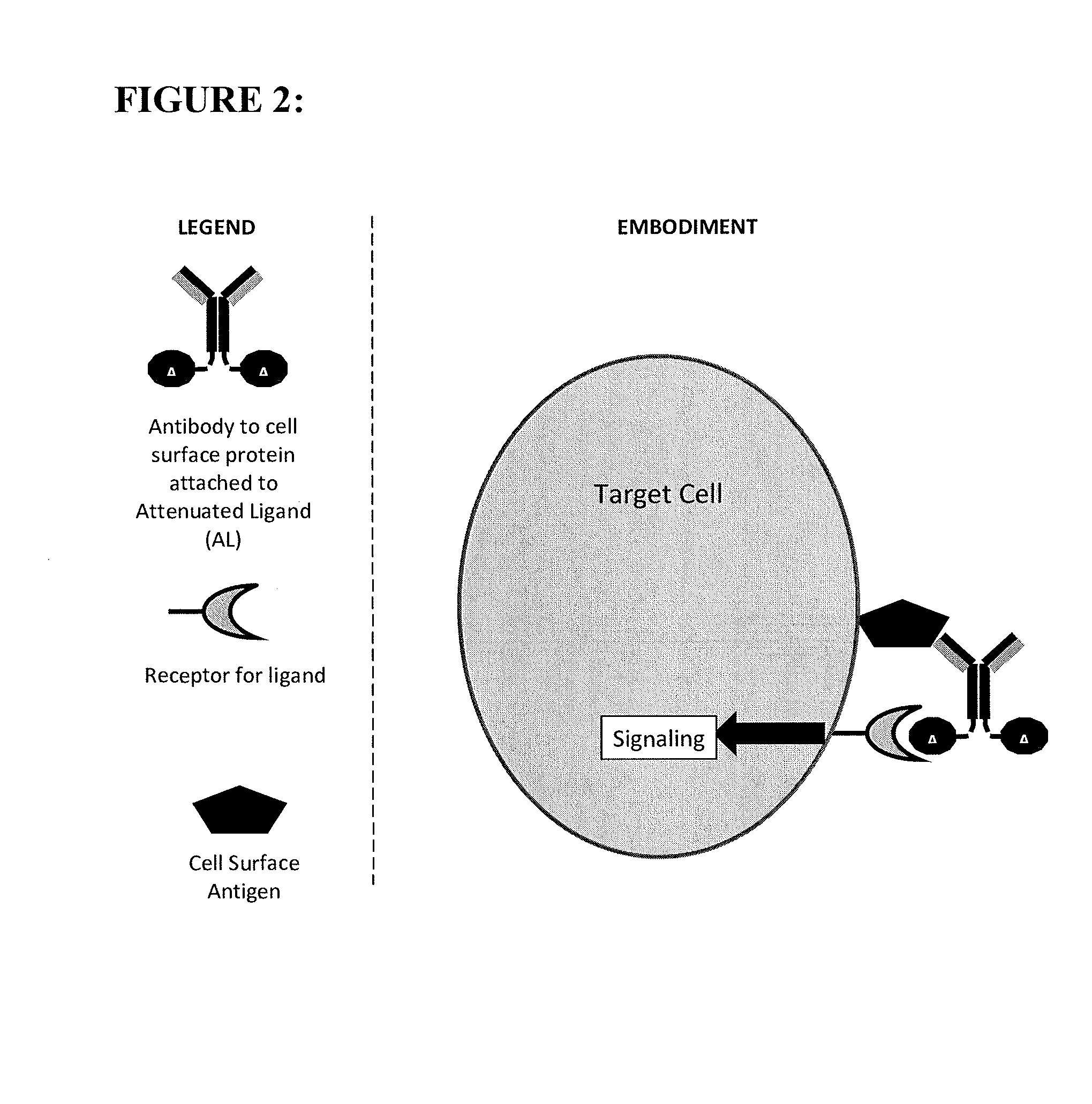
Polypeptide constructs and uses thereof
ActiveUS20140248238A1Strong activationLow toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAmino acid substitutionAntigen binding
The present invention provides a polypeptide construct comprising a peptide or polypeptide signaling ligand linked to an antibody or antigen binding portion thereof which binds to a cell surface-associated antigen, wherein the ligand comprises at least one amino acid substitution or deletion which reduces its potency on cells lacking expression of said antigen.
Owner:TEVA PHARMA AUSTRALIA PTY LTD
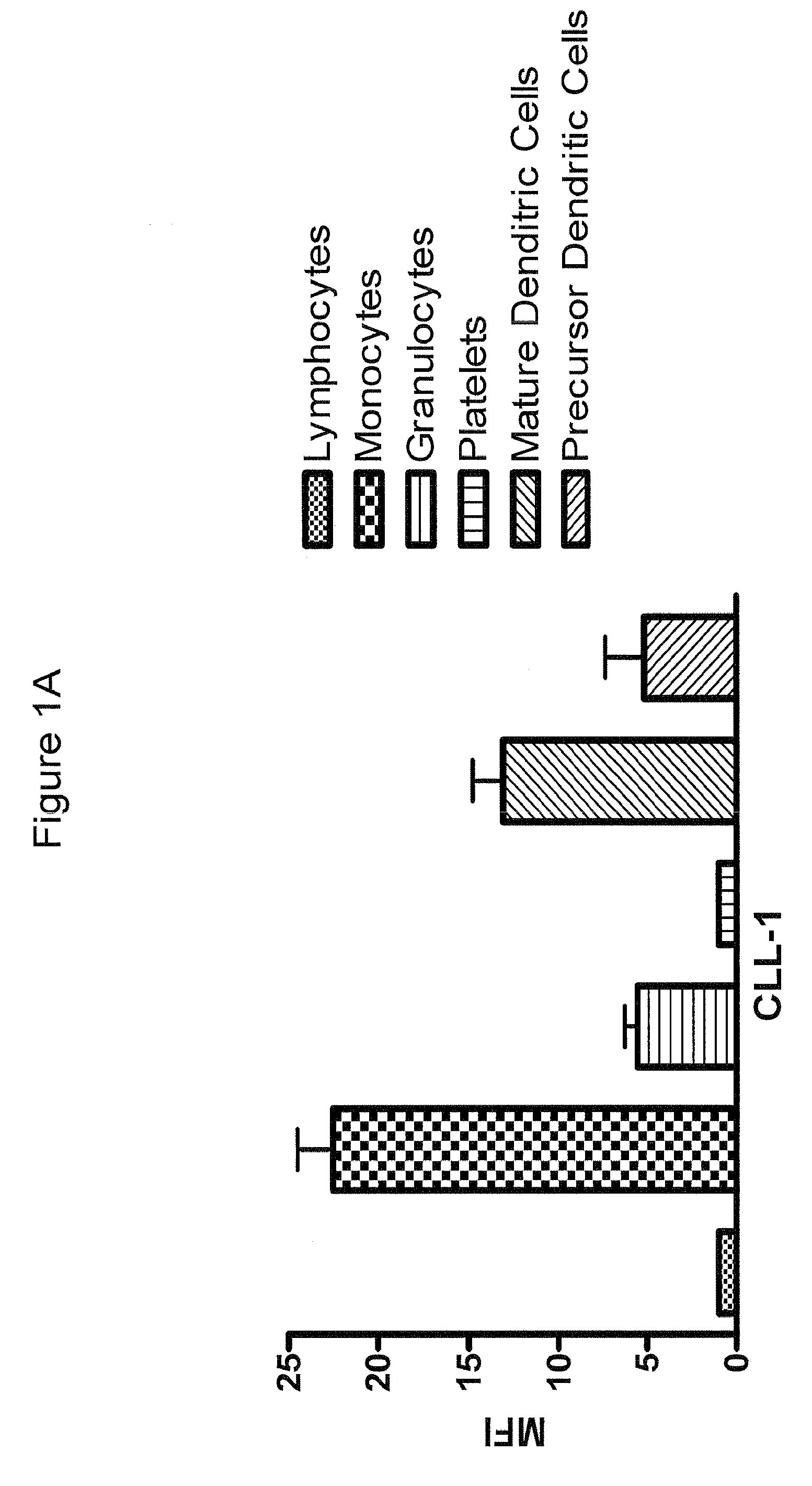
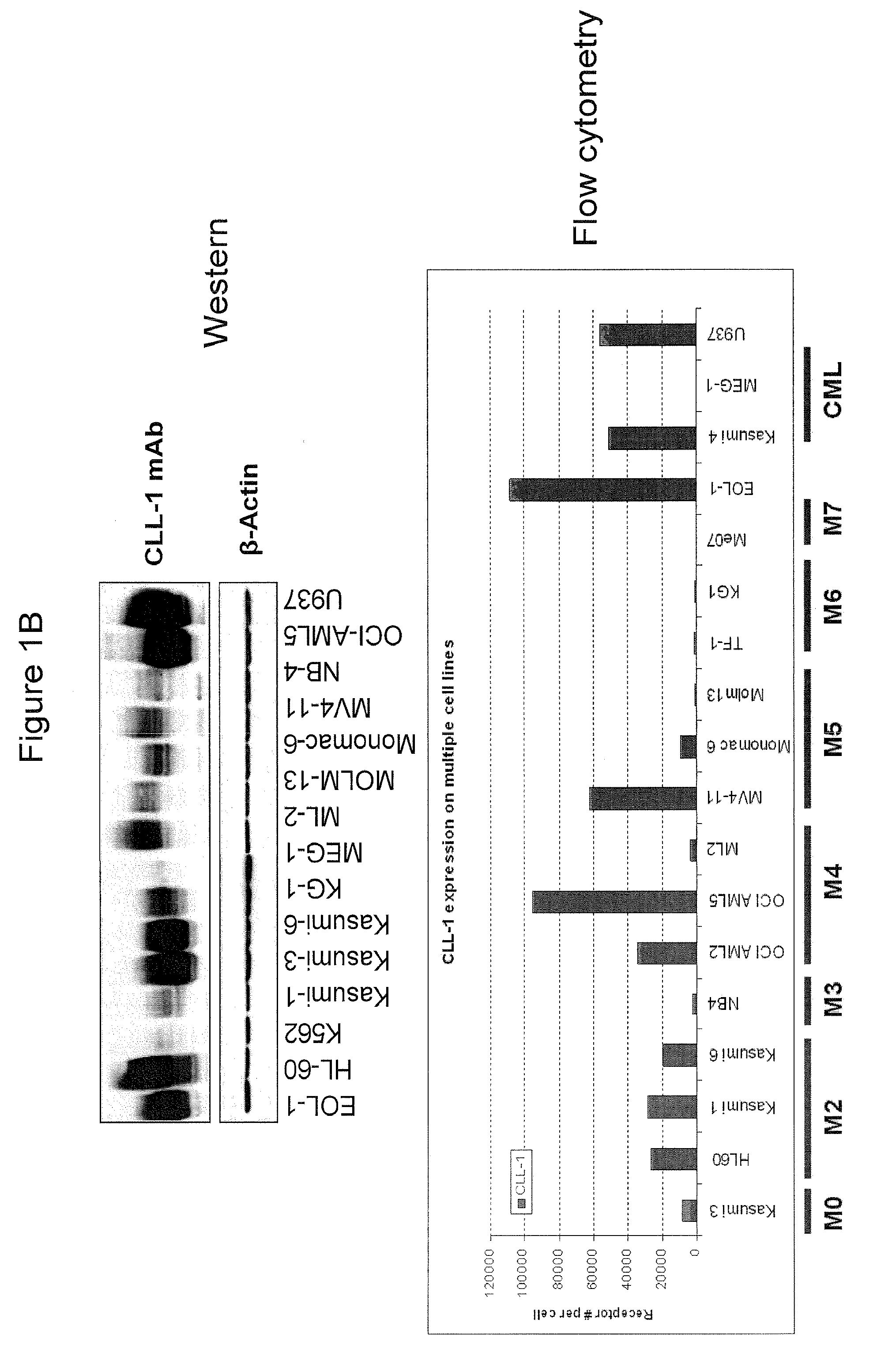
Antibodies to cll-1
Anti-CLL-1 antibodies and antigen-binding fragments thereof, as well as pharmaceutical compositions comprising such antibodies and antigen-binding fragments are described. Also described are methods of using such antibodies and antigen-binding regions to bind CLL-1 and treat diseases, such as hematologic malignancies, which are characterized by expression of CLL-1.
Owner:ARCA BIOPHARMA
Targeted therapeutic proteins
InactiveUS20090203575A1Convenient treatmentSimple preparation processPeptide/protein ingredientsAntibody mimetics/scaffoldsLysosomeTherapeutic protein
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com