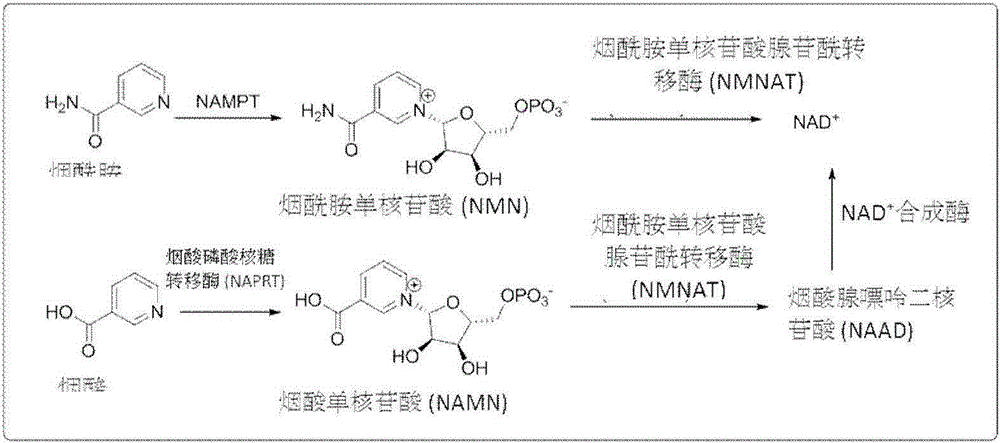
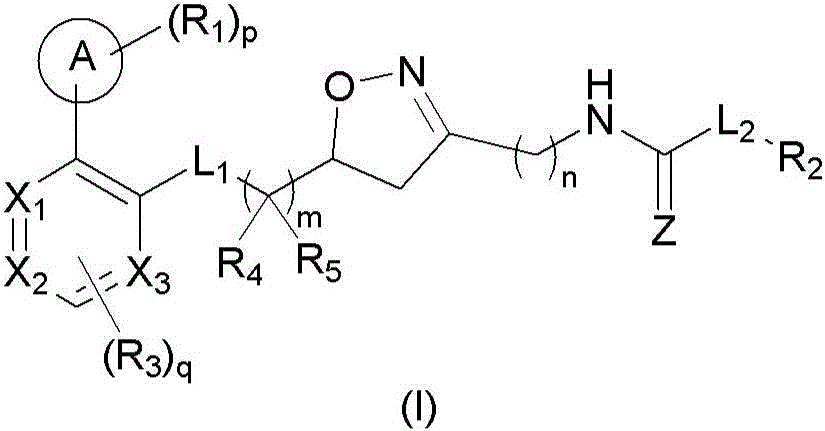
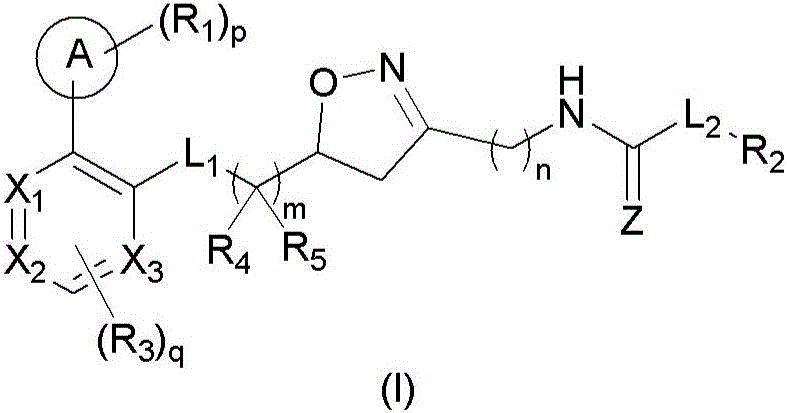
4,5-dihydroisoxazole derivatives as NAMPT inhibitors
A technology for compounds and medicinal salts, applied in the field of compounds for the treatment of nicotinamide phosphoribosyltransferase-related cancers and inflammatory diseases, can solve problems such as unsatisfied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1: N-((5-(((3-(1-methyl-1H-pyrazol-4-yl)pyridin-2-yl)oxy)methyl)-4,5-dihydroiso Oxazol-3-yl)methyl)imidazo[1,2-a]pyridine-6-carboxamide (±).
[0228] Step 1: 2-(allyloxy)-3-bromopyridine.
[0229]
[0230] To a solution of 3-bromo-2-fluoropyridine (1.0 g, 5.68 mmol) and allyl alcohol (1.96 mL, 5.0 mmol) in dry THF (10 mL) was added sodium hydride (0.55 g, 2.4 mmol, 60%) in portions . The reaction mixture was stirred at room temperature for 16 hours. Water (50 mL) was added to cool the reaction mixture, then extracted with ethyl acetate (2x100 mL). The combined organic layers were dried over sodium sulfate and evaporated under reduced pressure to obtain the title compound (1.4 g, 98%) as an oil. The crude product was carried on to the next step without further purification. LCMS: m / z 214[M+2] + ; 1 H NMR (300MHz, chloroform-d) δ8.13-8.00 (dd, J = 4.8, 1.8Hz, 1H), 7.88-7.72 (dd, J = 7.6, 1.7Hz, 1H), 6.88-6.68 (dd, J =7.6, 4.9Hz, 1H), 6.22-5.97 (ddt, J=1...
Embodiment 2
[0252] Example 2: N-((5-(((6'-fluoro-[3,3'-bipyridyl]-2-yl)oxy)methyl)-4,5-dihydroisoxazole-3 -yl)methyl)imidazo[1,2-a]pyridine-6-carboxamide (±).
[0253]
[0254] As described in step 8 of Example 1, (6-fluoropyridin-3-yl)boronic acid (0.207 g, 1.460 mmol) N-((5-(((3-bromopyridin-2-yl)oxy)methyl)-4,5-dihydroisoxazol-3-yl)methyl)imidazo [1,2-a]pyridine-6-carboxamide (±) (product of step 7 of Example 1, 0.420 g, 0.980 mmol). The resulting crude product was purified using preparative HPLC to obtain the title compound (0.191 g, 43% W / W) as a white solid. LCMS: m / z 447.1[M+H] + ; HPLC: 94.33%; 1 H NMR (400MHz, chloroform-d) δ9.06-8.90 (d, J = 1.7Hz, 1H), 8.63-8.44 (d, J = 2.5Hz, 1H), 8.31-8.07 (m, 2H), 8.05- 7.88(td, J=8.0, 7.5, 2.6Hz, 1H), 7.82-7.60(m, 5H), 7.15-6.97(m, 2H), 5.15-4.98(m, 1H), 4.76-4.61(dd, J =12.0, 2.6Hz, 1H), 4.52-4.41(dd, J=16.9, 5.8Hz, 1H), 4.39-4.25(m, 2H), 3.35-3.19(m, 1H), 3.10-2.99(dd, J = 17.4, 6.6 Hz, 1H). Preparative HPLC method: Liquid chro...
Embodiment 3
[0255] Example 3: N-((5-(((6'-fluoro-[3,3'-bipyridyl]-4-yl)oxy)methyl)-4,5-dihydroisoxazole-3 -yl)methyl)imidazo[1,2-a]pyridine-6-carboxamide (±).
[0256] Step 1: 4-(allyloxy)-3-iodopyridine.
[0257]
[0258] At reflux temperature, 3-iodo-4-hydroxypyridine (0.500g, 2.250mmol) was mixed with allyl bromide (0.409g, 3.380mmol), potassium carbonate (0.780g, 5.630mmol) and potassium iodide in acetone (20mL) (0.016 g, 0.100 mmol) reacted for 2 hours. The reaction mixture was then cooled to room temperature and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane / ethyl acetate=90 / 10) to obtain the target compound (0.400 g, 67%) as a solid. LCMS: m / z261.9[M+1] + ; 1 H NMR (300MHz, DMSO-d 6)δ7.86-7.85(d, J=2.4Hz, 1H), 7.31-7.28(m, 1H), 6.41-6.38(d, J=7.2Hz, 1H), 5.97-5.86(m, 1H), 5.43 -5.40 (dd, J=9.6, 1.5 Hz, 1H), 5.29-5.25 (dd, J=10.8, 1.0 Hz, 1H), 4.40-4.38 (m, 2H).
[0259] Step 2: tert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com