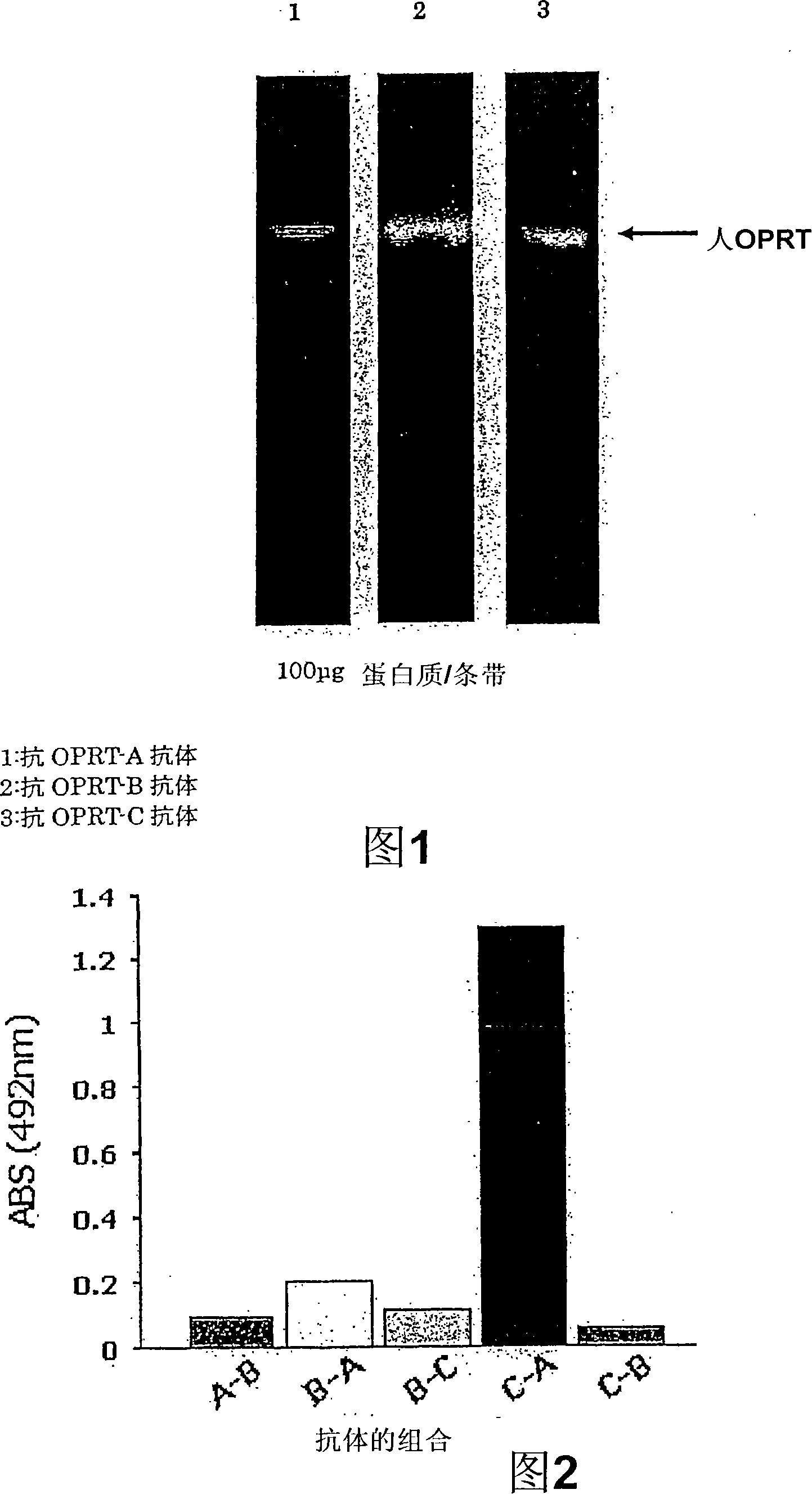
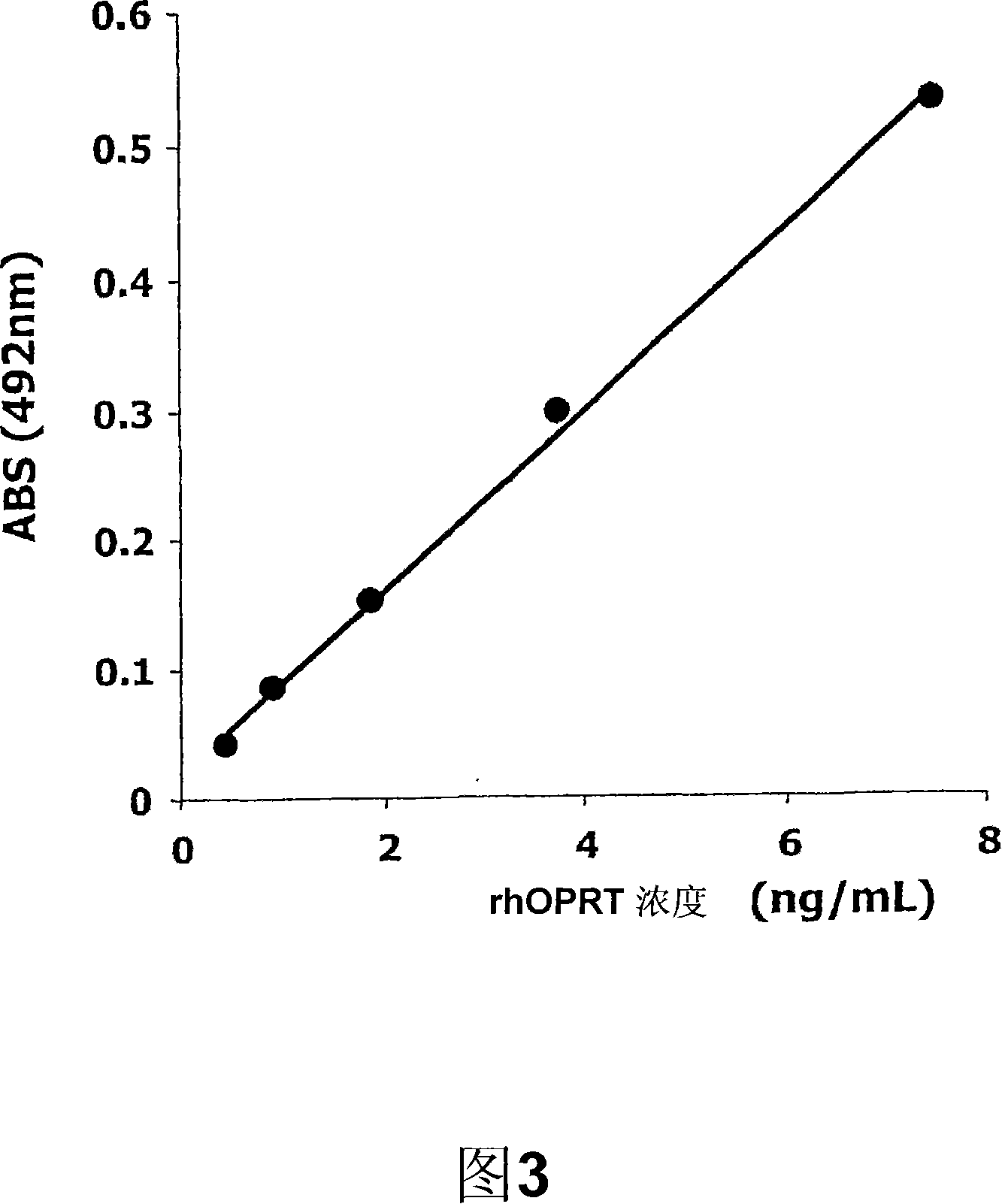
Assay method for human orotate phosphoribosyltransferase protein
A technology of orotate phosphoribosyl and determination method, applied in anti-enzyme immunoglobulin, microbial determination/inspection, biochemical equipment and methods, etc., can solve the problems of complicated operation, unable to fully reflect protein quality and enzyme activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] Hereinafter, the present invention will be described in more detail with reference to examples, but the present invention is not limited to these examples.
Embodiment 1
[0055] Embodiment 1 (preparation of antibody)
[0056] According to the following operation, anti-OPRT polyclonal antibody was obtained.
[0057] (1) Peptide synthesis
[0058] Synthesize a peptide having the amino acid sequence of 86th to 108th from the N-terminus of human OPRT, a peptide having the sequence of amino acids 428th to 446th from the N-terminus of human OPRT, and a peptide having an amino acid sequence of 428th to 446th from the N-terminus of human OPRT The peptides of the 454th to 474th amino acid sequences were obtained as artificial peptides consisting of the following amino acid sequences.
[0059] Peptide having the amino acid sequence at position 86-108 from the N-terminal of human OPRT (antigen name: OPRT-A)
[0060] Cys-Ser-Thr-Asn-Gln-Ile-Pro-Met-Leu-Ile-Arg-Arg-Lys-Glu-Thr-Lys-Asp-Tyr-Gly-Thr-Lys-Arg-Leu (SEQ ID NO. 1)
[0061] Peptide having an amino acid sequence from 428th to 446th from the N-terminus of human OPRT (antigen name: OPRT-B)
[0062]...
Embodiment 2
[0075] Example 2 (quantification of human OPRT by sandwich ELISA)
[0076] (1) rhOPRT obtained by culturing Escherichia coli was used as a standard. The preparation method is as follows: use PCR to clone the full-length cDNA of human OPRT, prepare a plasmid expressing the fusion protein of glutathione-S-transferase (GST) and rhOPRT, introduce the plasmid into E. In the presence of penicillin, culture was shaken overnight at 37° C. in 100 mL of LB medium (manufactured by Wako Pure Chemical Industries, Ltd.). 10 mL of this culture solution was transferred to an Erlenmeyer flask containing 1 L of LB medium, and further cultured at 37° C. for 4 hours. After collecting bacteria by centrifugation, suspend the bacteria in 100mL of bacteria collection buffer (50mM Tris, 8M Urea, 1mM PMSF, 5mM EDTA, 5mMDTT, pH 7.4), and slowly stir at 4°C for 30 minutes. After the suspension was sonicated at 4°C, it was centrifuged at 15,000×g and 4°C for 30 minutes. Then, the concentration of urea ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com