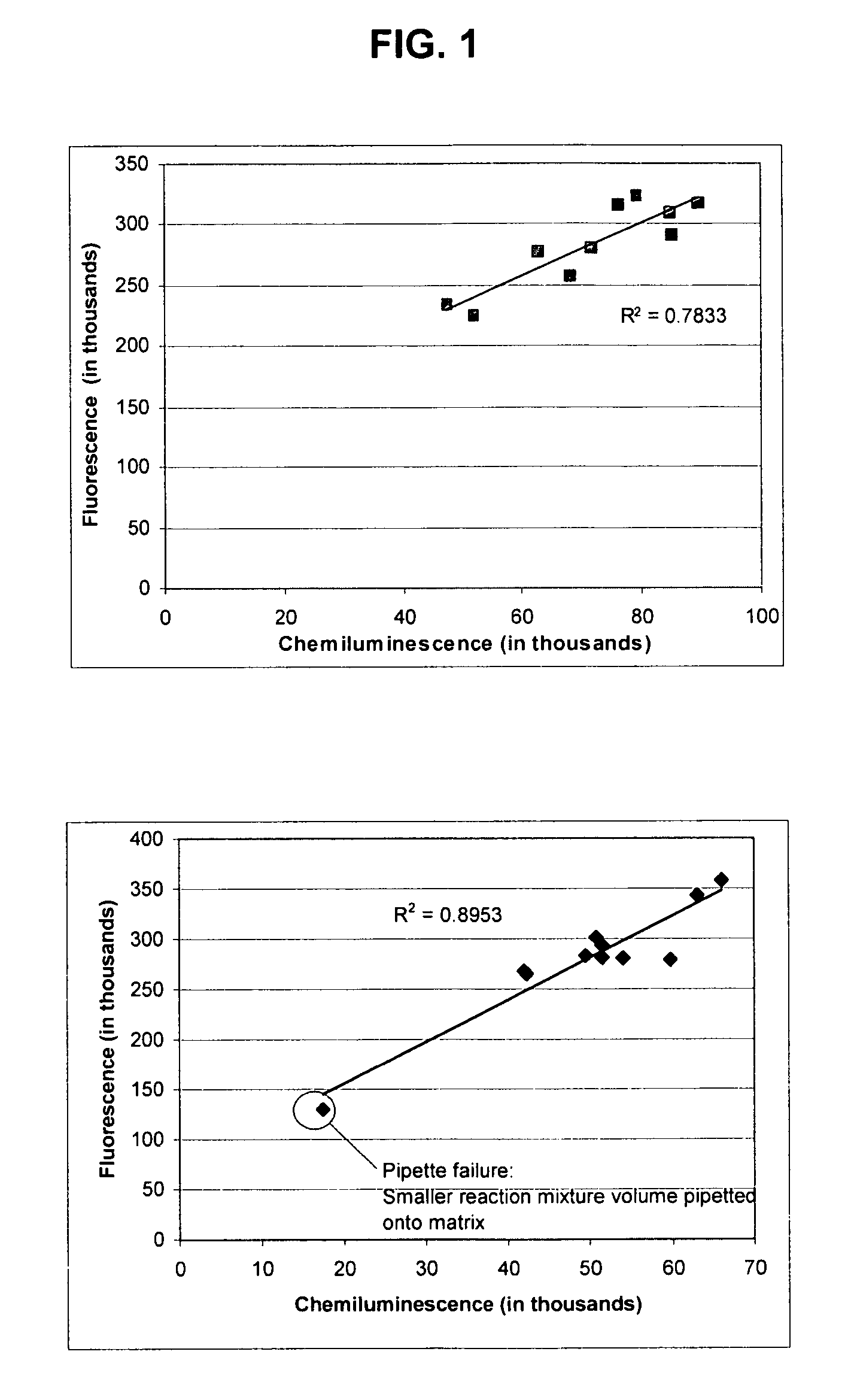
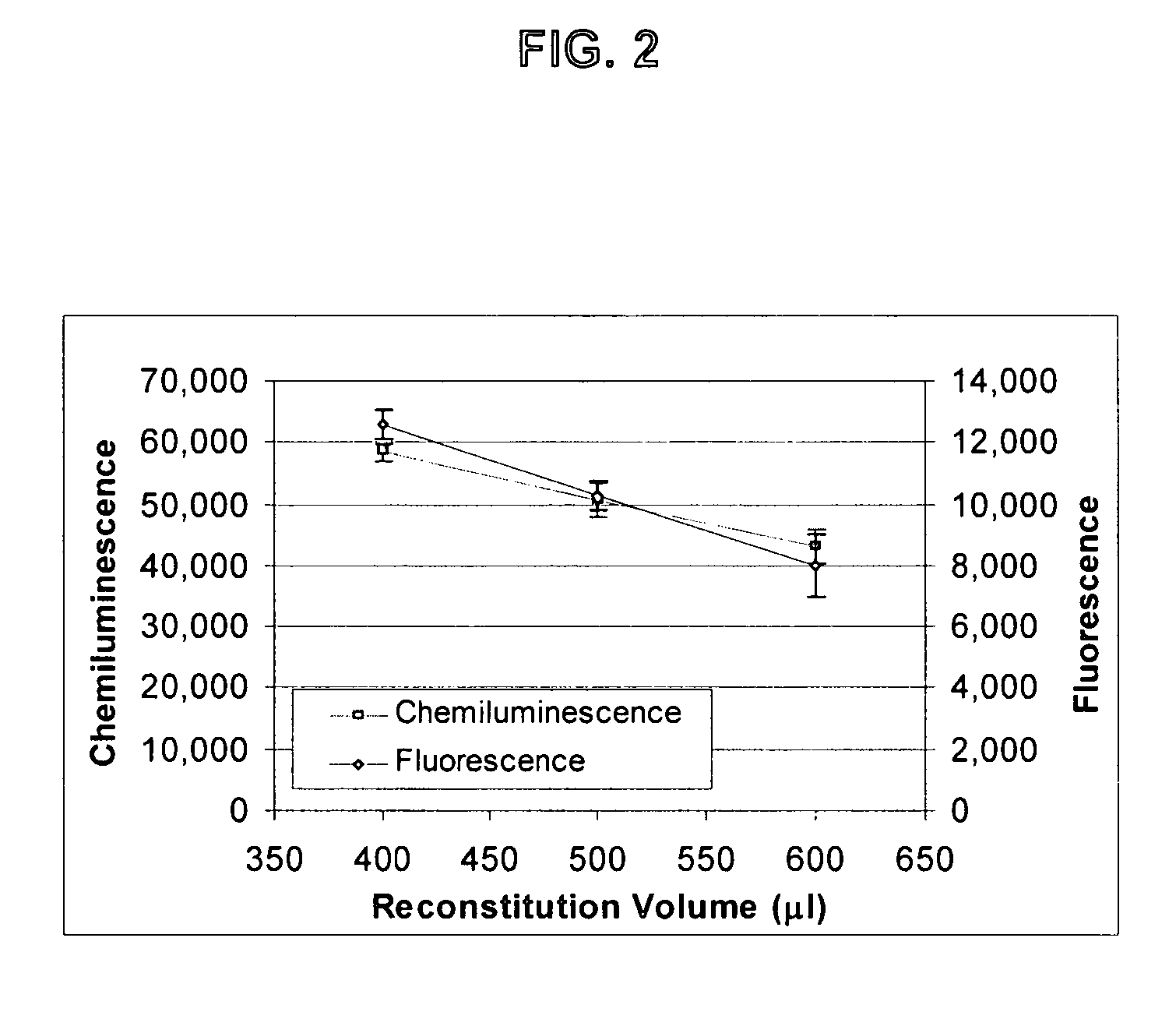
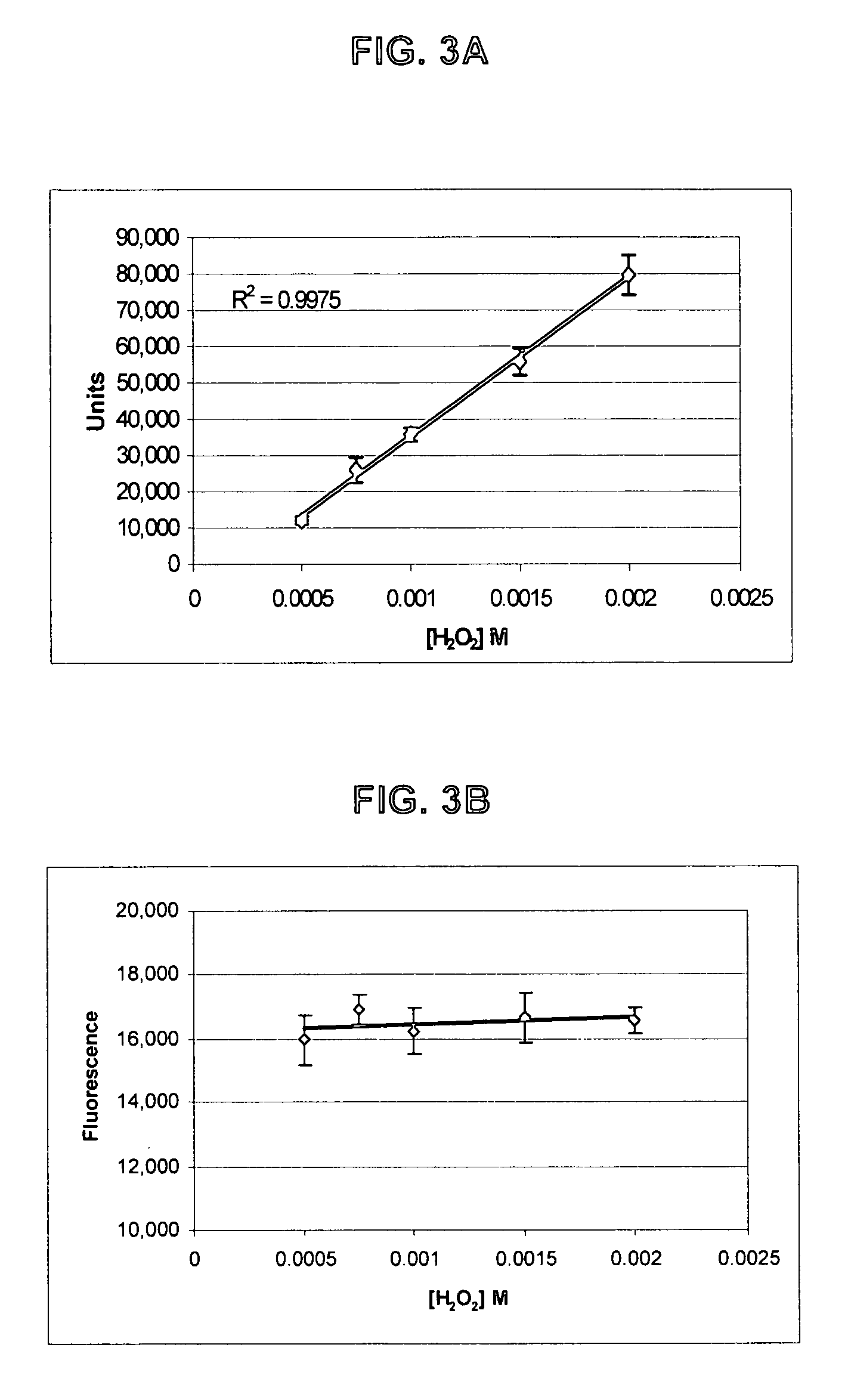
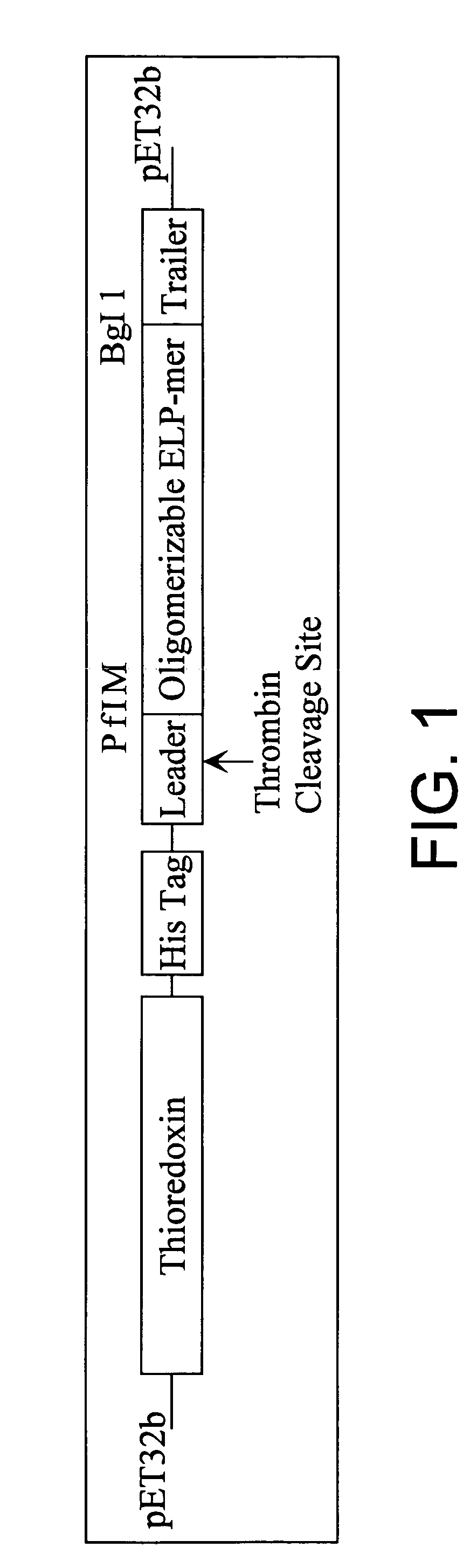
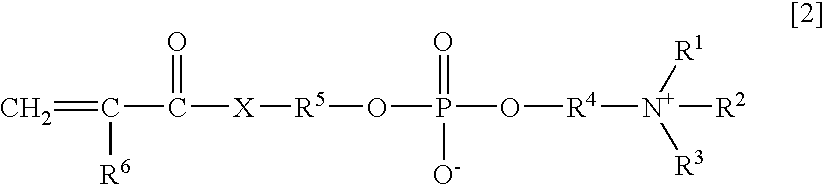
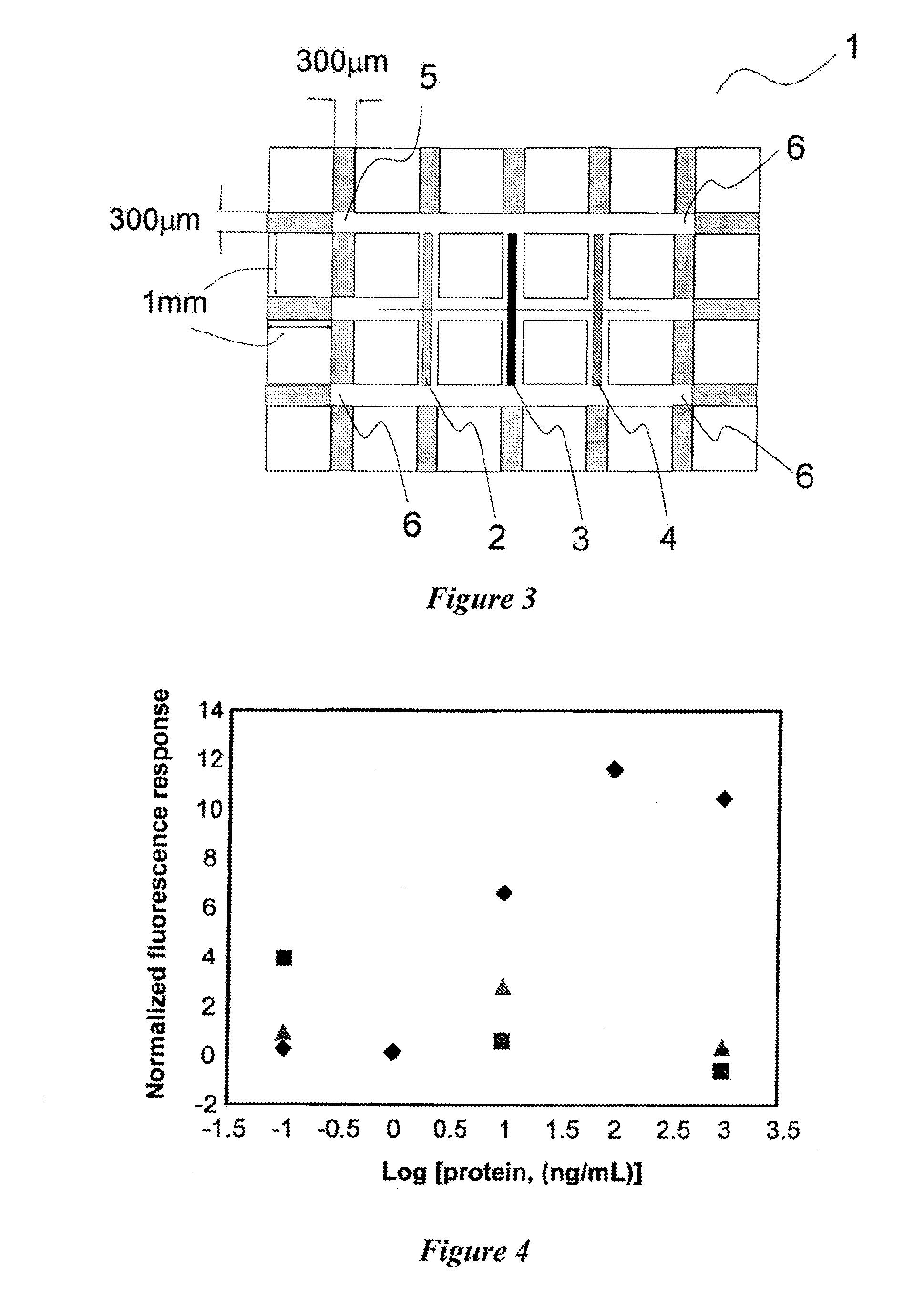
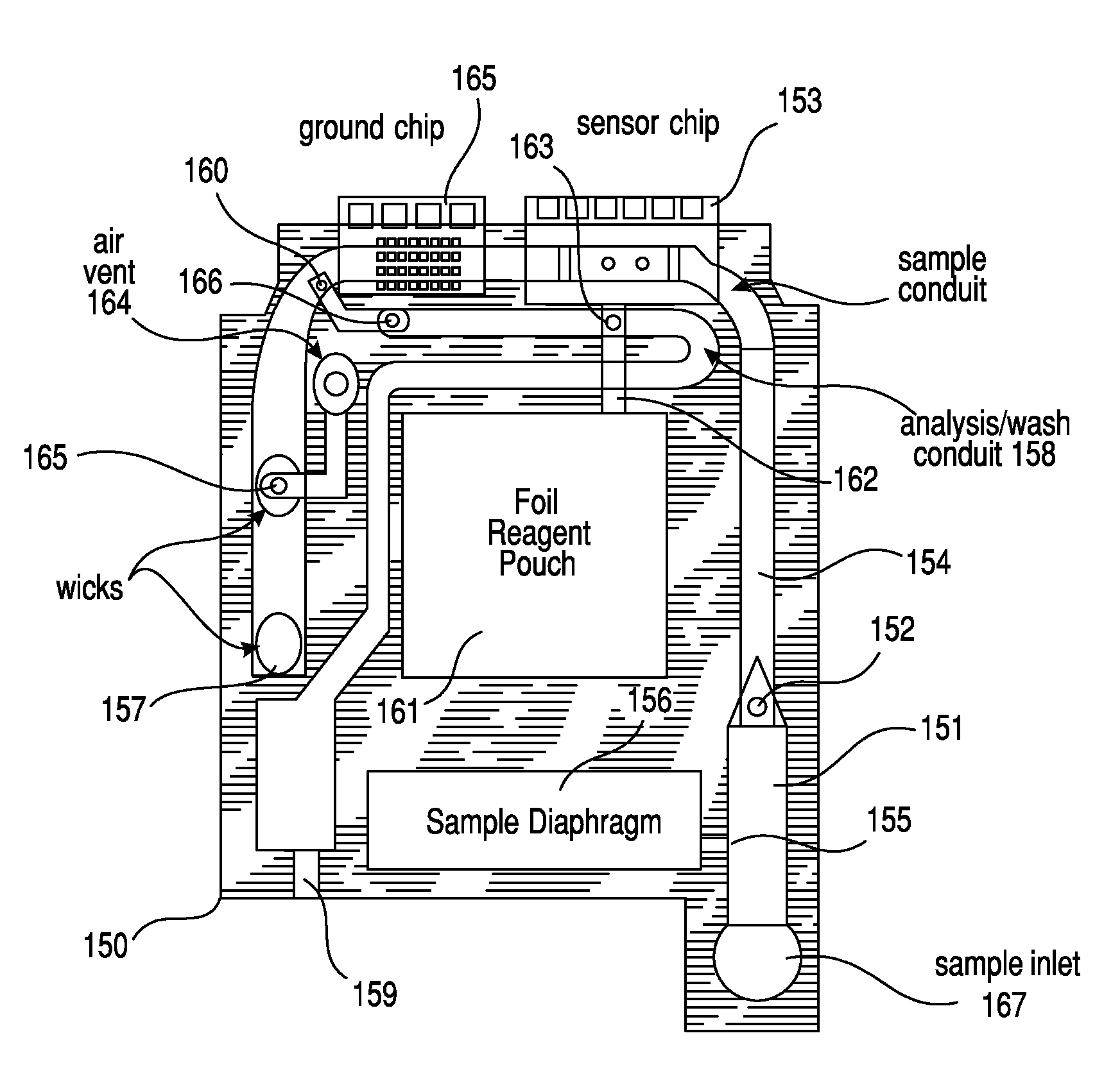
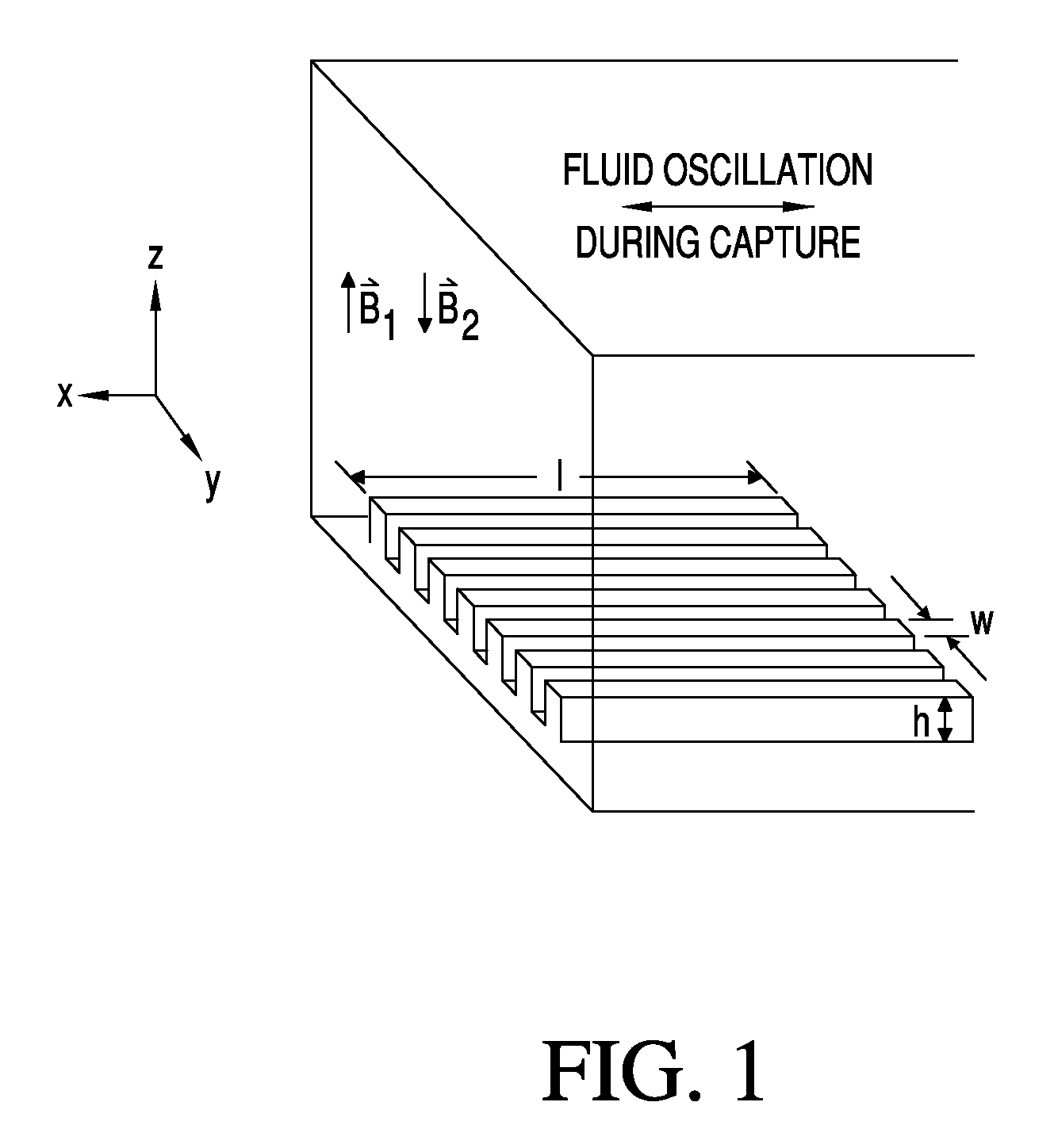
Patents
Literature
746 results about "Immunoassay method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
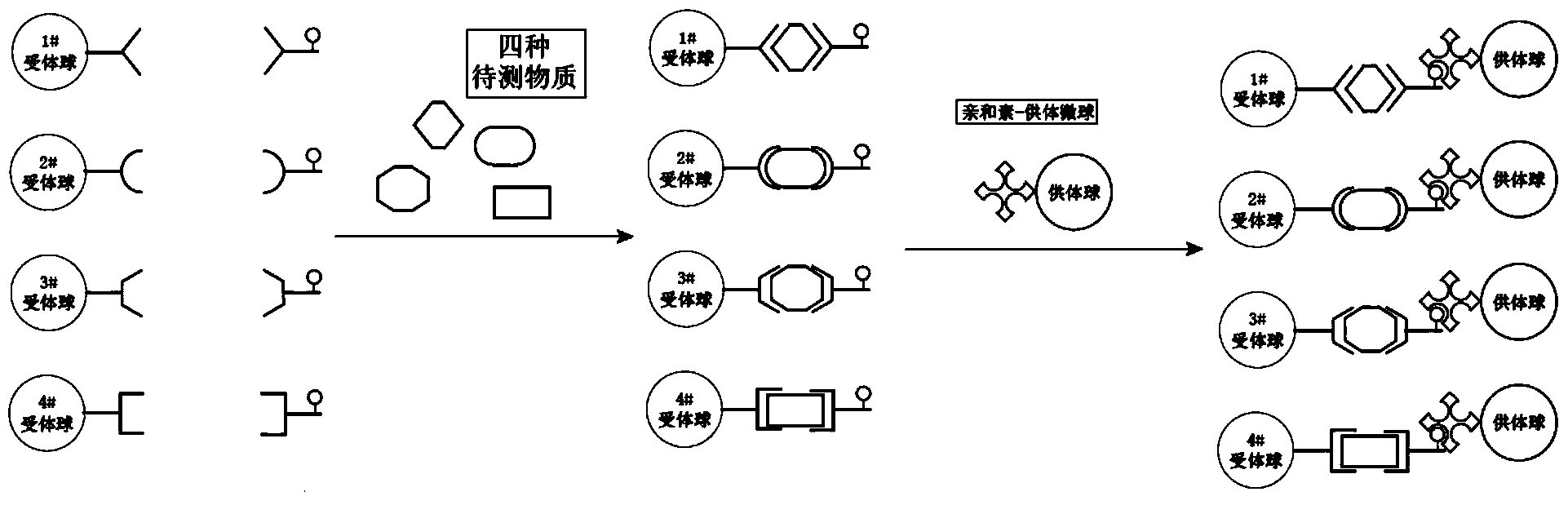
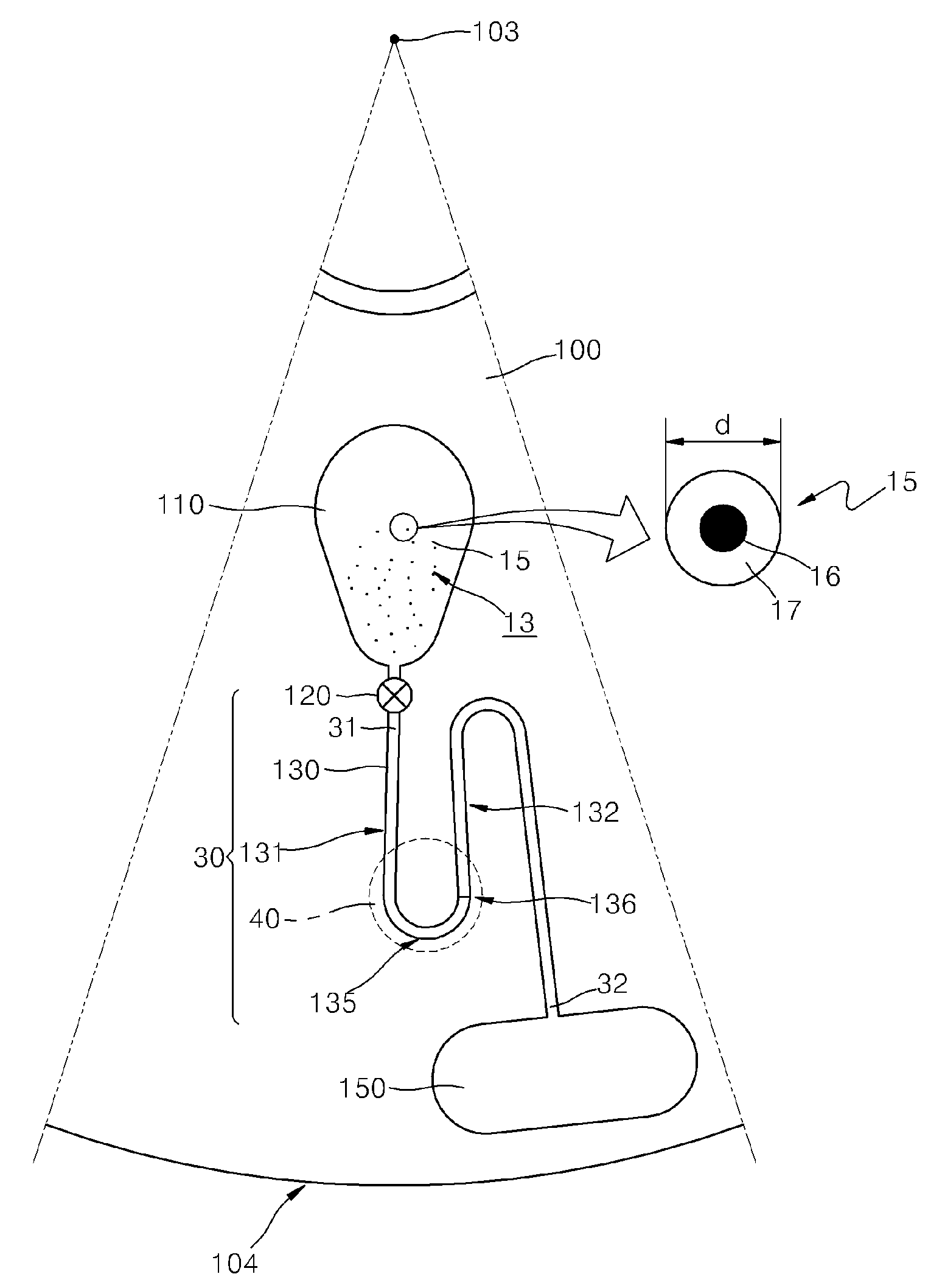
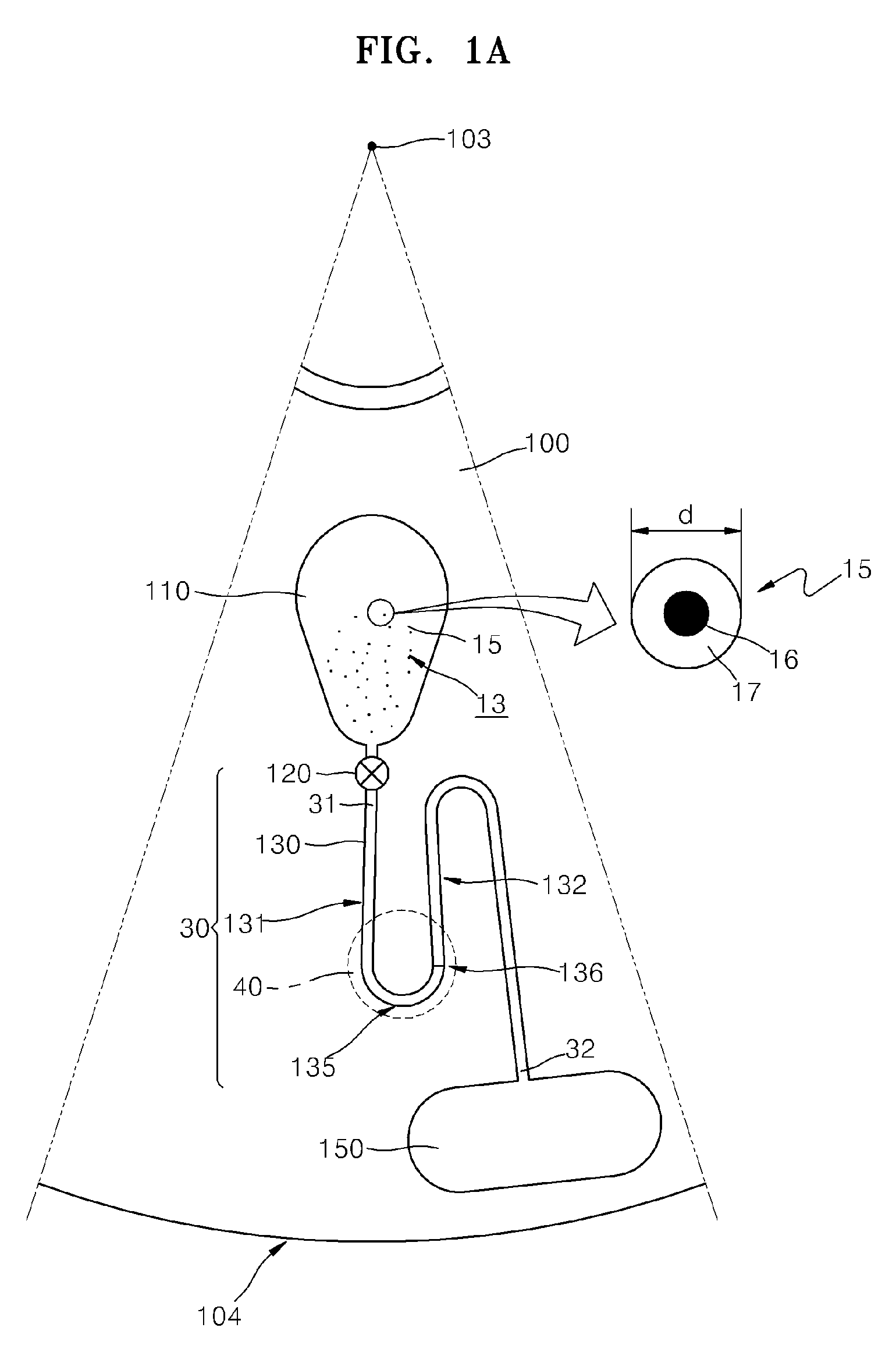
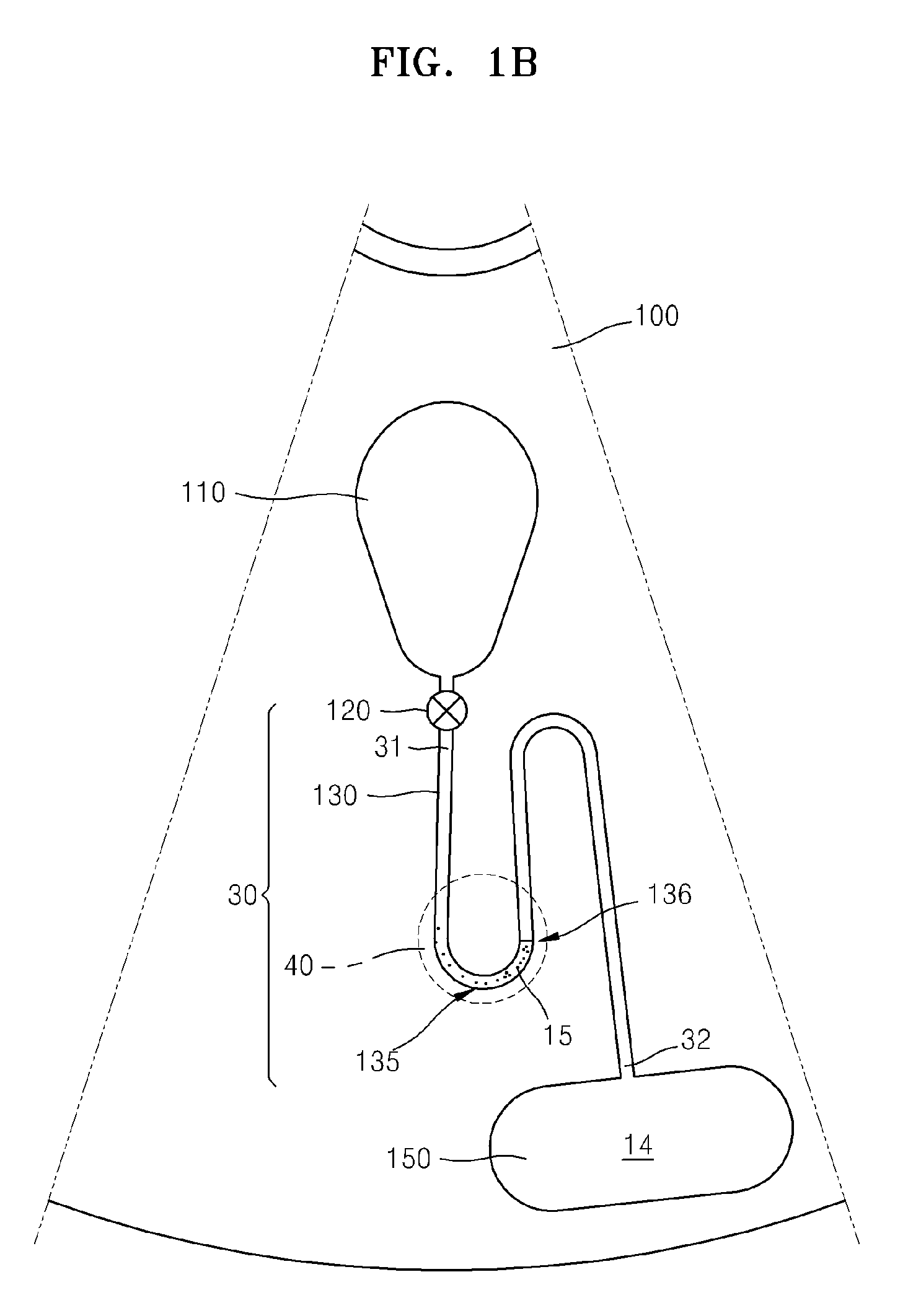
There are several different methods used in immunoassay tests. Immunoprecipitation. The simplest immunoassay method measures the quantity of precipitate, which forms after the reagent antibody (precipitin) has incubated with the sample and reacted with its respective antigen to form an insoluble aggregate.
Lateral flow assay and device using magnetic particles
A complex including magnetic particle bound to a metal colloid. The complex may be part of a reagent for use in a method for determining analytes. The reagent may include a binding partner specific for an analyte. The reagent may further include a first label that is distinguishable from a second label that is used to detect the analyte. The reagent is used in kits and methods for detecting analytes in samples. The methods include immunoassay methods, including method where the first label is used to calibrate the assay.
Owner:IDEXX LABORATORIES
Methods of using bioelastomers
InactiveUS7429458B2Material nanotechnologyEnergy modified materialsCombinatorial chemistryAnimal subject
Bioelastomers are disclosed for use in methods of binding compounds including immunoassay methods, in biosensors and methods or regenerating biosensors, and in methods for targeting the delivery of a compound to a particular location within an animal subjects. In general, the bioelastomer is conjugated to a binding compound, which is in turn used to bind a compound of interest. For targeted compound delivery, the bioelastomer is conjugated to the compound to be delivered.
Owner:DUKE UNIV
Apparatus and method for detecting microscopic living organisms using bacteriophage
InactiveUS20050003346A1Wide concentration rangeFast resultsMicrobiological testing/measurementMaterial analysisBacteroidesAntibiotic resistance
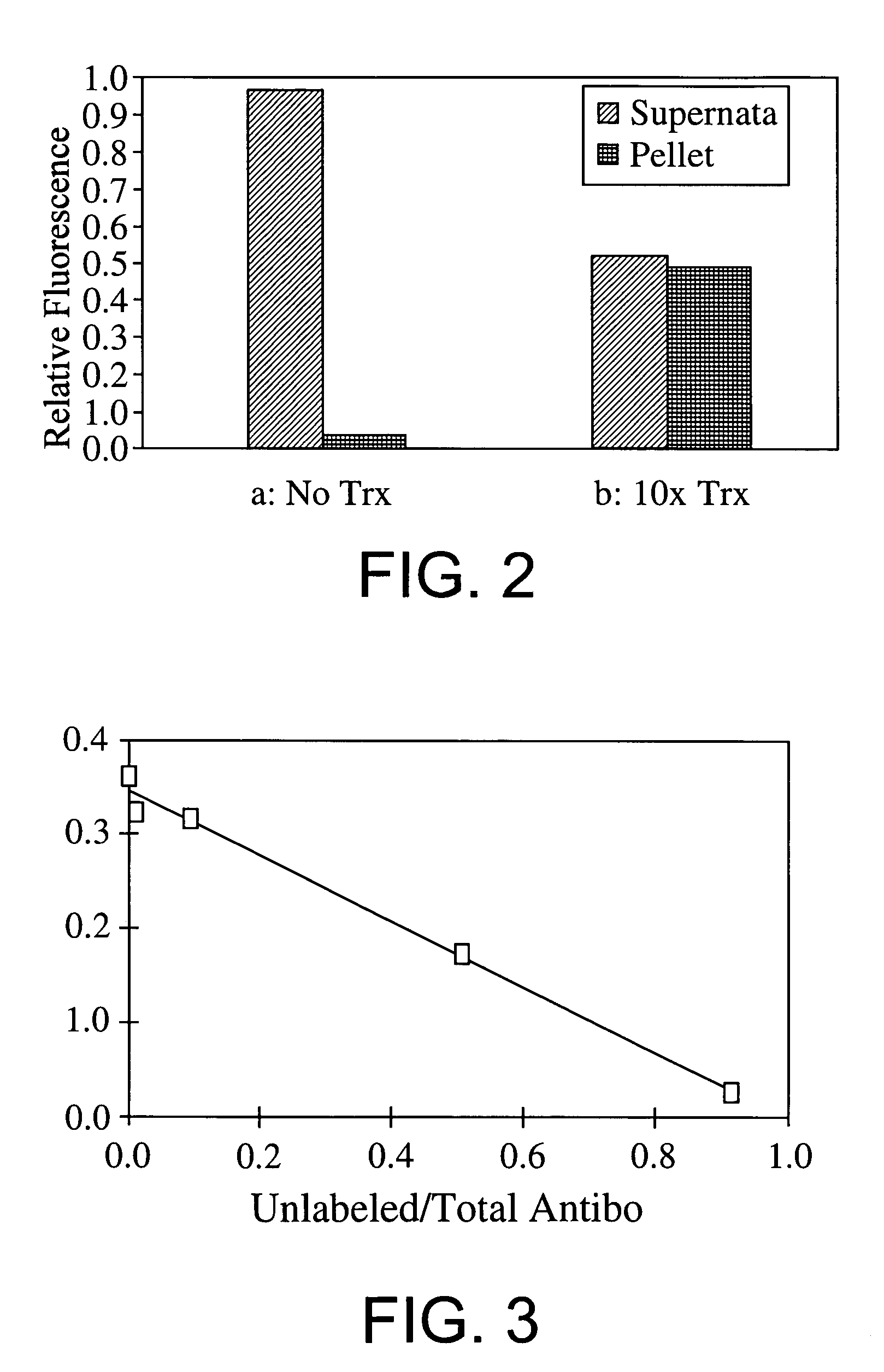
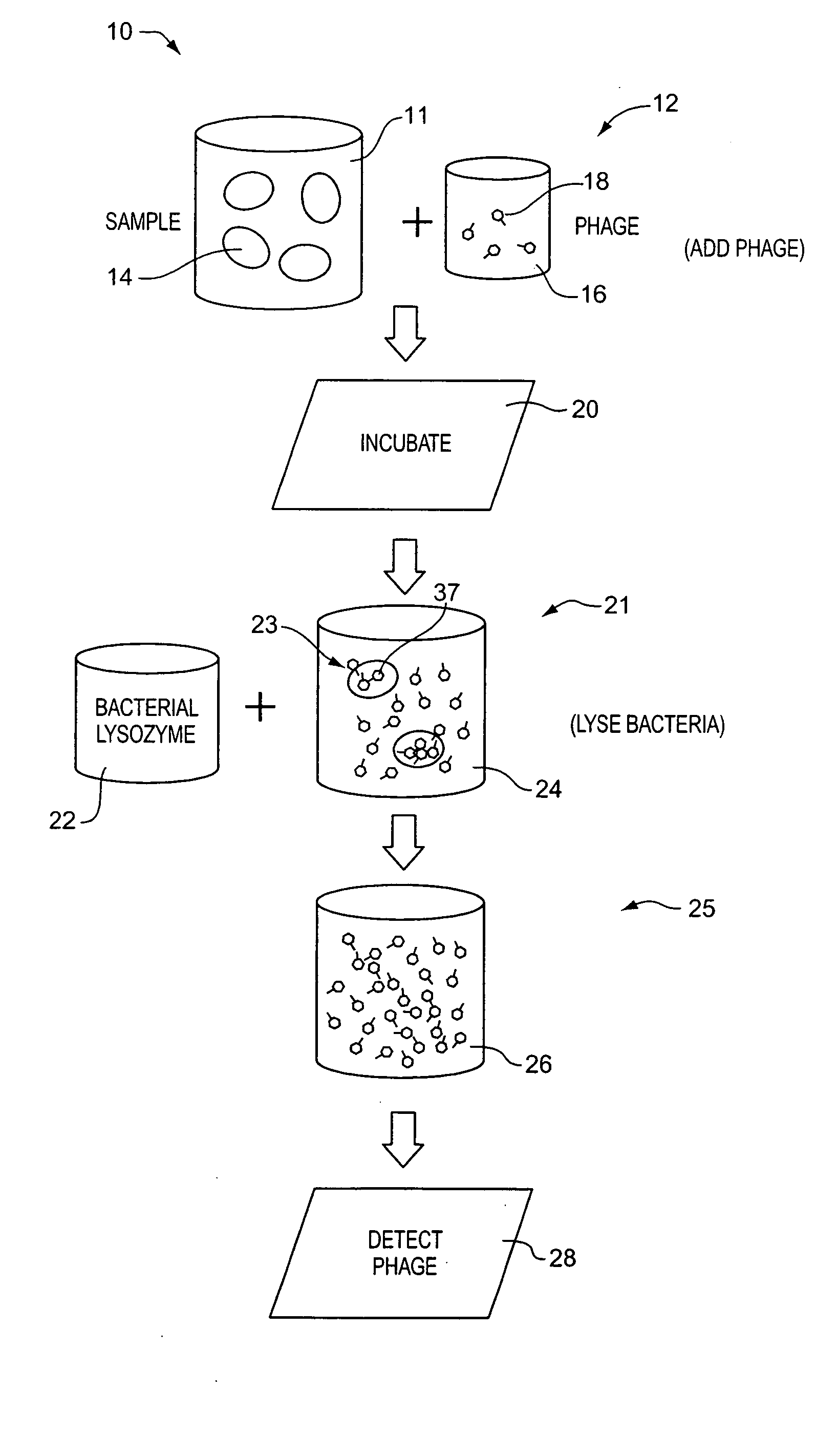
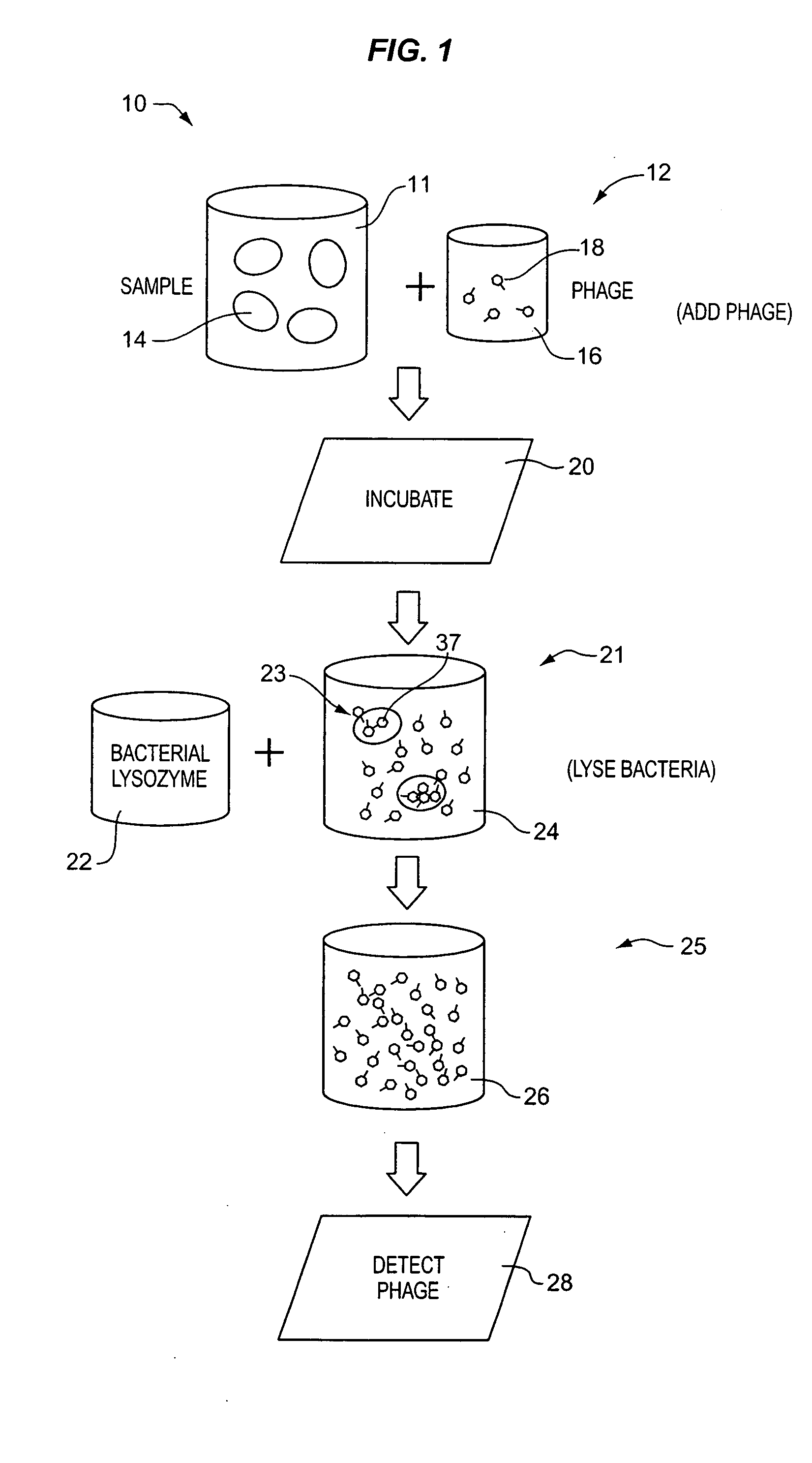
A method for detecting one or more target bacteria in a raw sample where: 1) bacteriophage(s) specific to each target bacterium are added to the raw sample, 2) the test sample is incubated, and 3) the test sample is tested for the presence of each phage in sufficient numbers to indicate the presence of the associated target bacteria in the raw sample. In one embodiment, each phage is initially added to the raw sample in concentrations below the detection limit of the final phage detection process. In another embodiment, the parent phages are tagged in such a way that they can be separated from the progeny phage prior to the detection process. Preferred phage detection processes are immunoassay methods utilizing antibodies that bind specifically to each phage. Antibodies can be used that bind to the protein capsid of the phage. Alternatively, the phage can by dissociated after the incubation process and the sample tested for the presence of individual capsid proteins or phage nucleic acids. The invention can be used to test target bacteria for antibiotic resistance.
Owner:MICROPHAGE +1
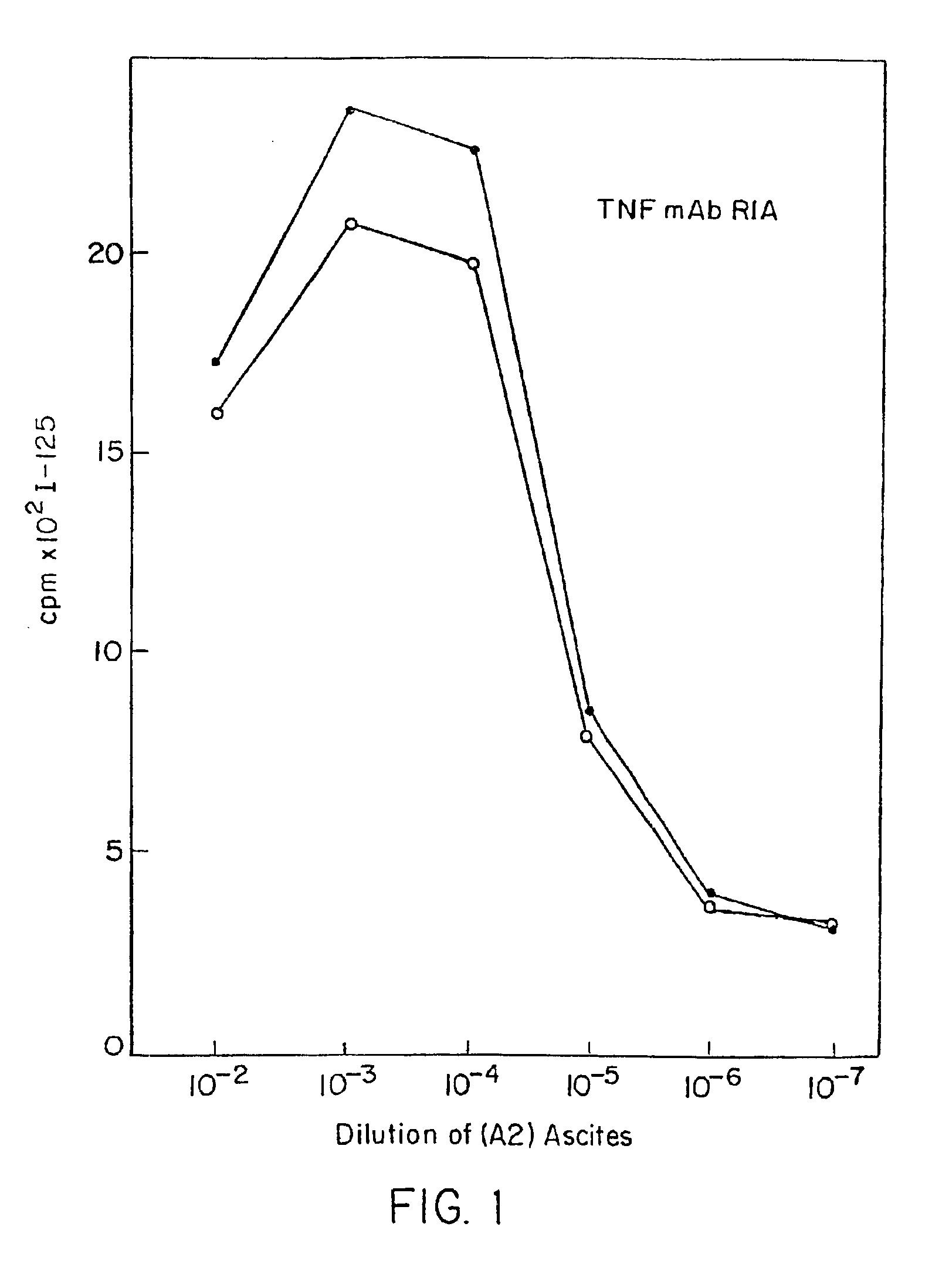
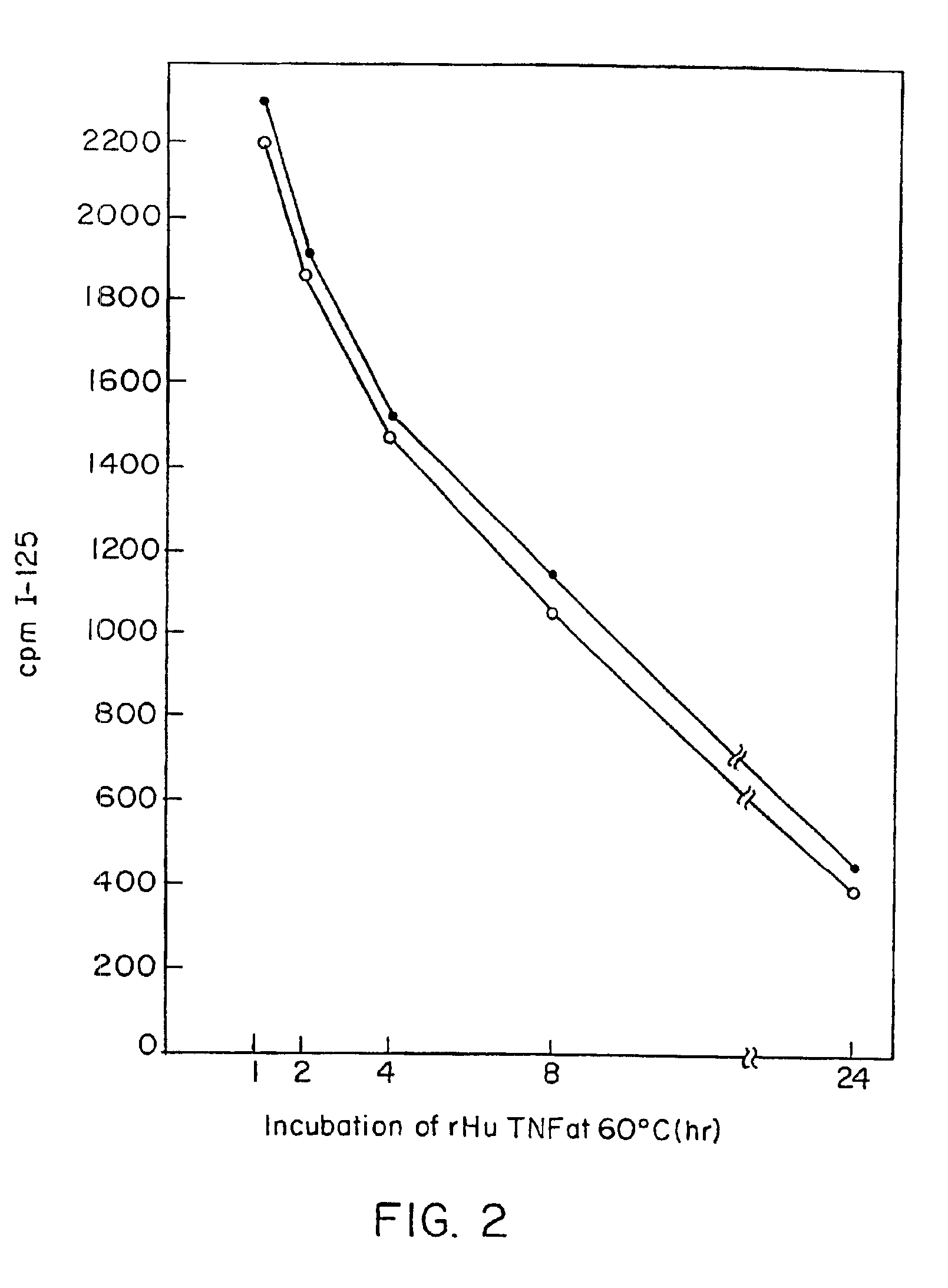
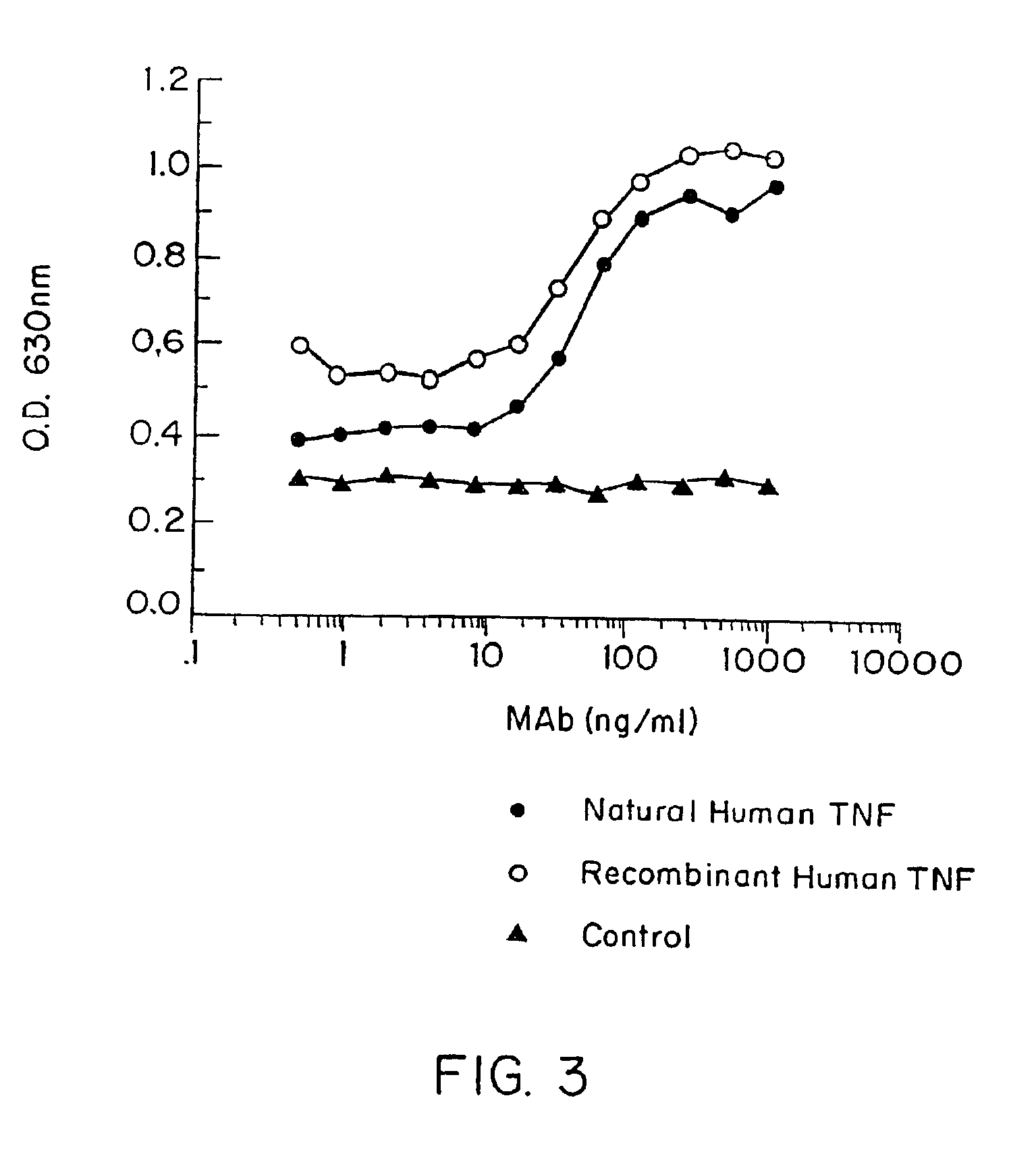
Anti-idiotypic anti-TNF antibodies and related immunoassay methods
Anti-TNF antibodies and anti-TNF peptides, specific for tumor necrosis factor (TNF) are useful for in vivo diagnosis and therapy of a number of TNF-mediated pathologies and conditions, as well as polynucleotides coding for anti-TNF murine and chimeric antibodies, peptides, methods of making and using the antibody or peptides in immunoassays and immuno-therapeutic approaches are provided, where the anti-TNF peptide is selected from a soluble portion of TNF receptor, an anti-TNF antibody or structural analog thereof.
Owner:NEW YORK UNIV +1
Dielectrophoretic separation and immunoassay methods on active electronic matrix devices
InactiveUS6887362B2Reduce the amount requiredDone quickly and efficientlyDielectrophoresisElectrostatic separatorsAssayMedical product
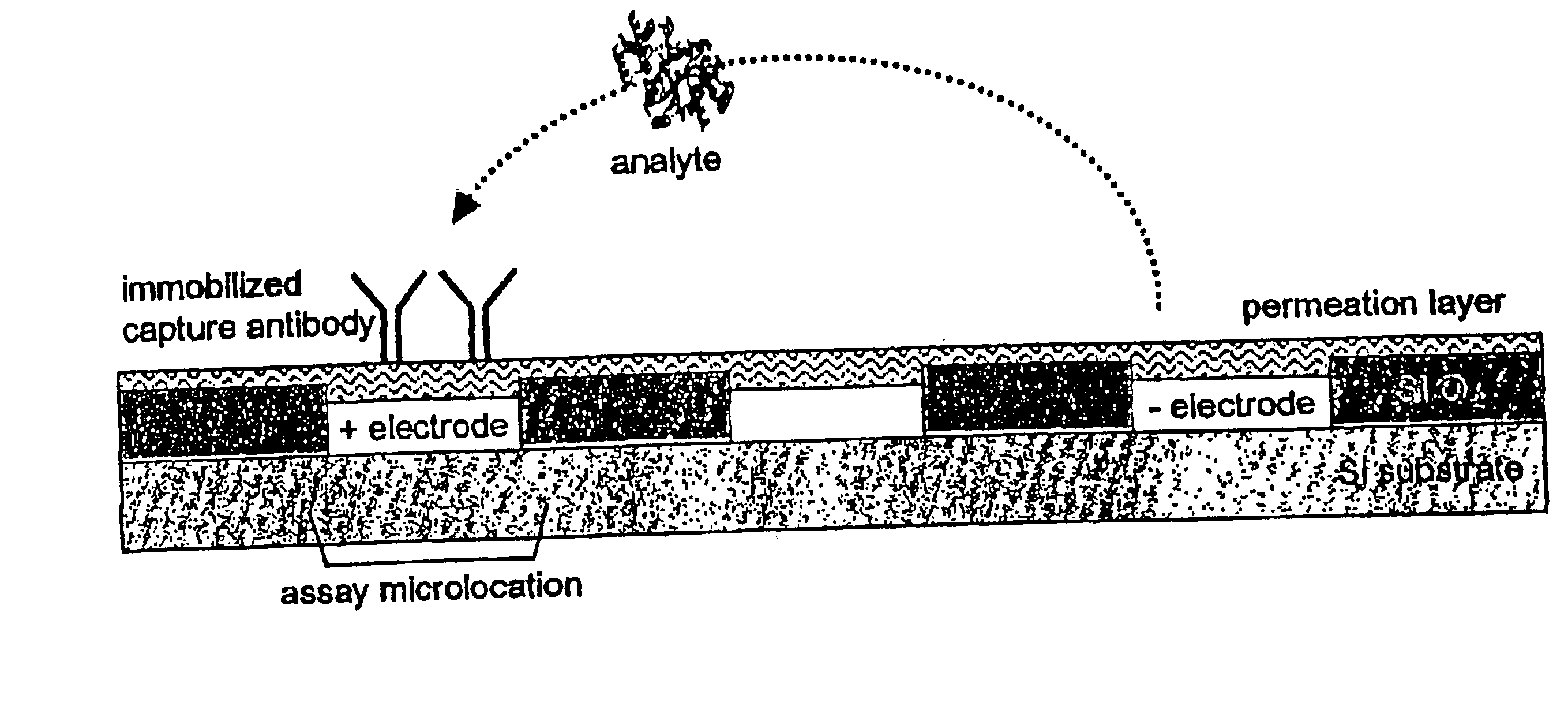
This invention relates to devices and methods for performing active, multi-step molecular and biological sample preparation and diagnostic analyses employing immunochemical techniques. It relates generally to bioparticle separation, bioparticle enrichment, and electric field-mediated immunochemical detection on active electronic matrix devices utilizing AC and DC electric fields. More specifically, the invention relates to devices and methods for sample preparation / manipulation, immunoimmobilization, and immunoassays, all of which can be conducted on one or more active electronic chip devices within a single system. These manipulations are useful in a variety of applications, including, for example, detection of pathogenic bacteria and biological warfare agents, point-of-care diagnostics, food or medical product quality control assays, and other biological assays.
Owner:GAMIDA FOR LIFE +1
Method and apparatus for performing high-voltage contactless conductivity (HV-CCD) electrophoresis
InactiveUS20050109621A1Reduce adhesionSludge treatmentVolume/mass flow measurementAnalyteCapillary electrophoresis
A chip-based capillary electrophoresis assembly including: a holder including a frame for removably receiving a chip; a capillary electrophoresis microchip dimensioned to fit onto the holder, and comprising a body and a separation channel defined in the body; and a pair of adhesive detection electrodes integrated with an electronic conductometric detection circuit, wherein the assembly is assemblable by disposing the capillary electrophoresis microchip on the holder and removably placing the adhesive electrodes on the microchip body near the separation channel. A label-free analyte conductometric detection method and a label-free capillary electrophoresis immunoassay method are also described.
Owner:HAUSER PETER C
Apparatus and methods for multi-analyte homogeneous fluoro-immunoassays
InactiveUS6979567B2Reduce nonspecific bindingEasy to prepareBioreactor/fermenter combinationsBiological substance pretreatmentsMulti analyteLaser light
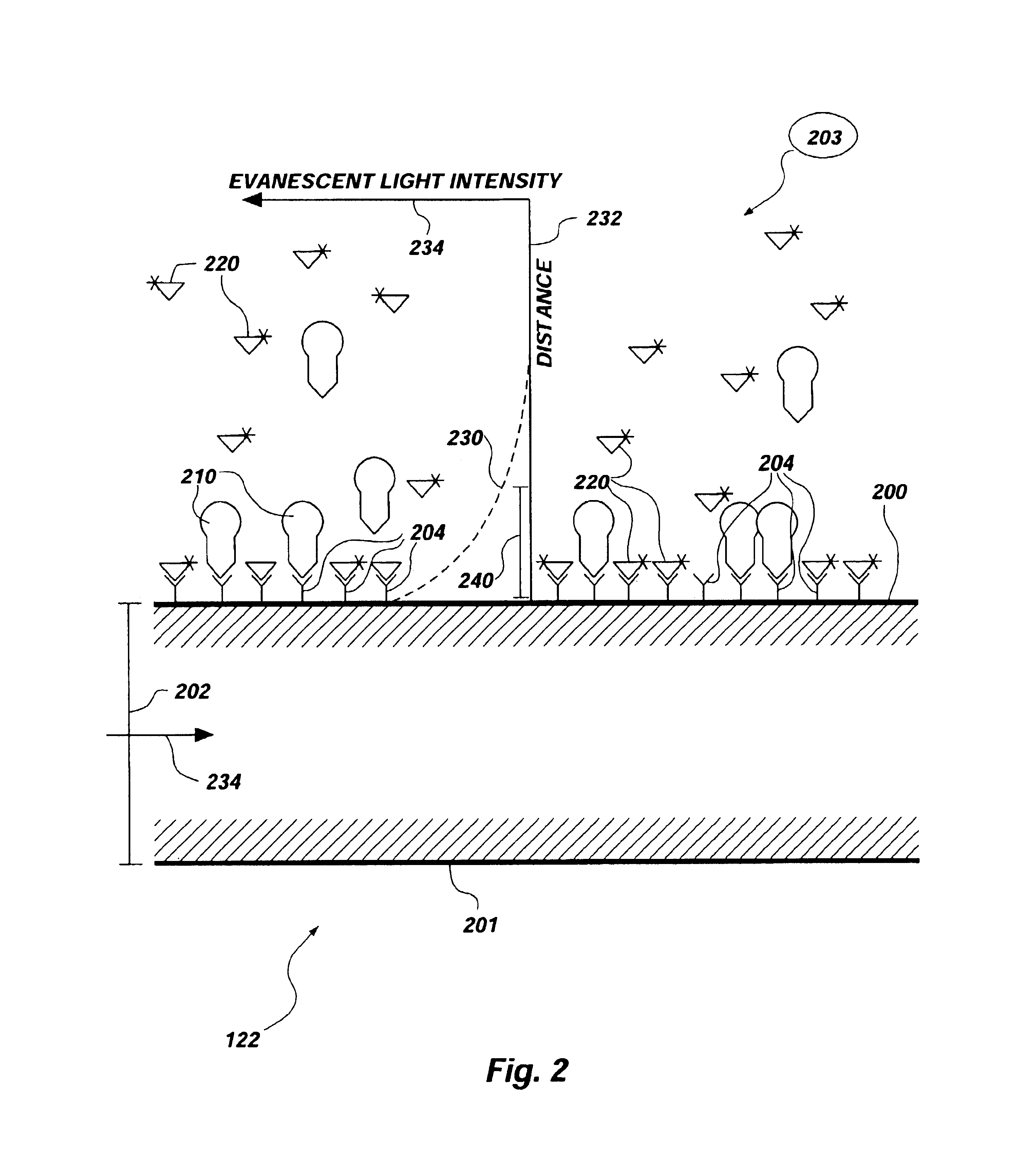
Methods and apparatus for evanescent light fluoroimmunoassays are disclosed. The apparatus employs a planar waveguide with an integral semicylindrical lens, and has multi-analyte features and calibration features, along with improved evanescent field intensity. A preferred embodiment of the biosensor and assay method has patches of capture molecules, each specific for a different analyte disposed adjacently within a single reservoir. The capture molecules are immobilized to the patches on the waveguide surface by site-specific coupling of thiol groups on the capture molecules to photo-affinity crosslinkers, which in turn are coupled to the waveguide surface or to a nonspecific binding-resistant coating on the surface. The patches of different antibodies are produced by selectively irradiating a portion of the waveguide surface during the process of coupling the photo-affinity crosslinkers, the selective irradiation involving a mask, a laser light source, or the like.
Owner:BIOCENTX
Whole-blood labeled immunoassay method and instant detection system
ActiveCN103575882AReduce lossesReduce sensitivityChemiluminescene/bioluminescenceMagnetic beadSystem structure
The invention in particular relates to a whole-blood labeled immunoassay method for quickly collecting a target object from whole-blood through specificity binding reaction and labeling and monitoring the target object by a labeling technology, and an instant detection system. The method comprises the following steps: 1) capturing magnetic beads; 2) eluting blood; 3) labeling a detection antibody; 4) eluting the detection antibody; 5) detecting. The system comprises a microfluidic chip, a magnetic field generation device arranged above or below the microfluidic chip and a detection device, wherein the microfluidic chip comprises a microchannel, the magnetic beads arranged in the microchannel, a waste liquid outlet and an eluent storage pond; the microchannel is separated into a first reaction region, a second reaction region and a detection region in stages by a separation mechanism. The technical scheme provided by the invention has the characteristics of high sensitivity, high repeatability, simple system structure, low cost and the like, and can detect the target object from trace blood samples quickly and quantitatively.
Owner:SUZHOU HUAMAI XINGWEI MEDICAL TECH +1
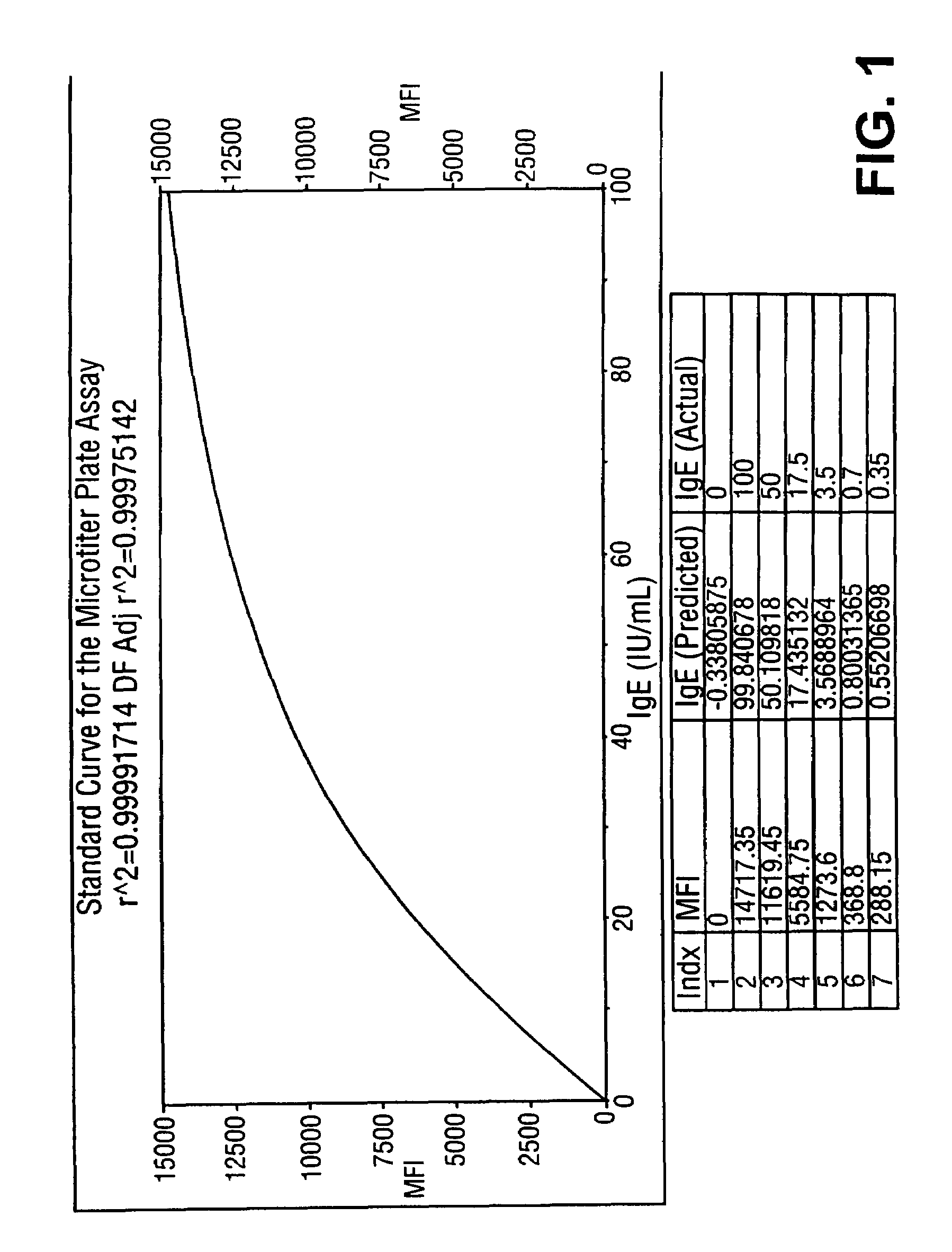
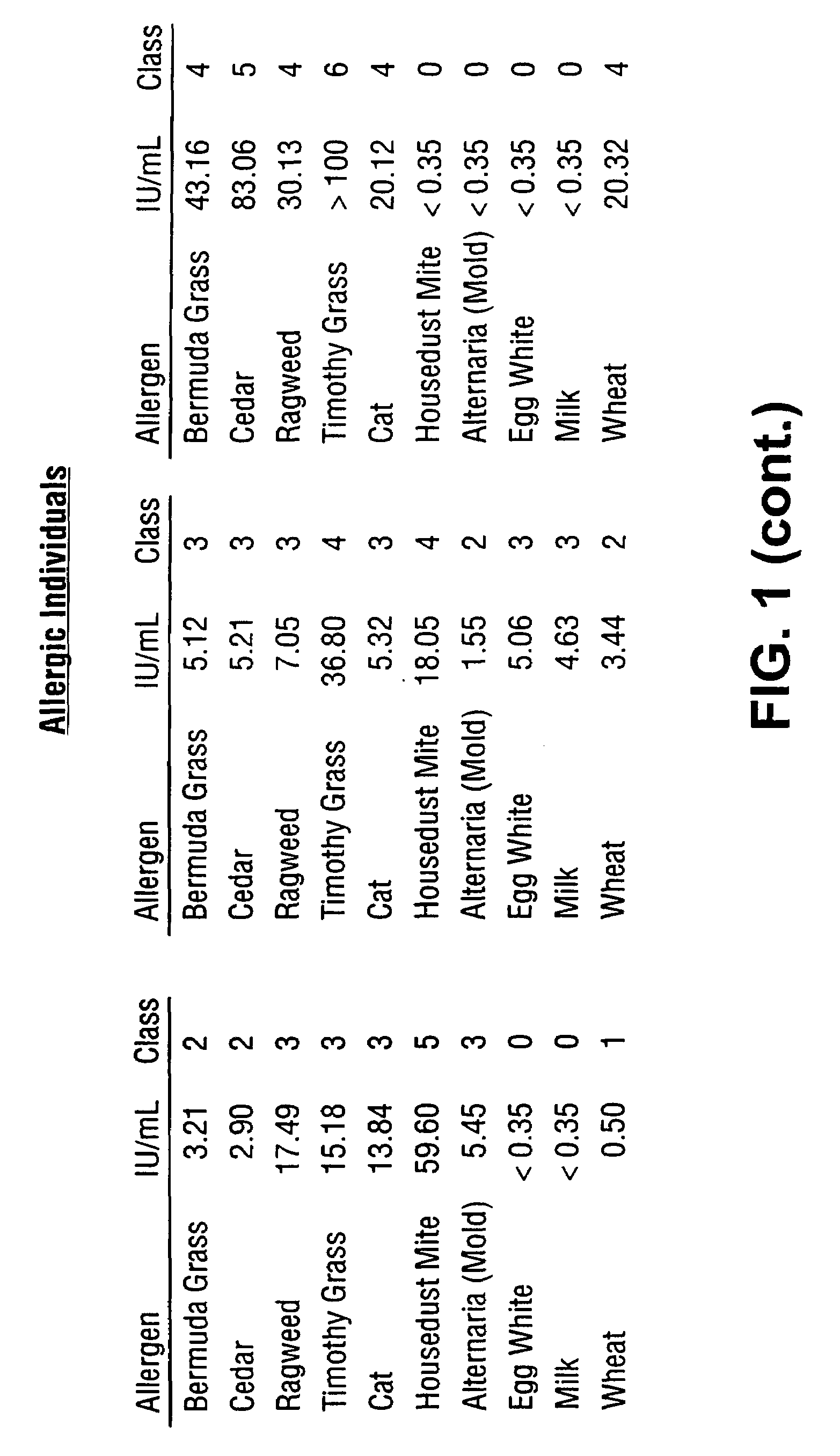
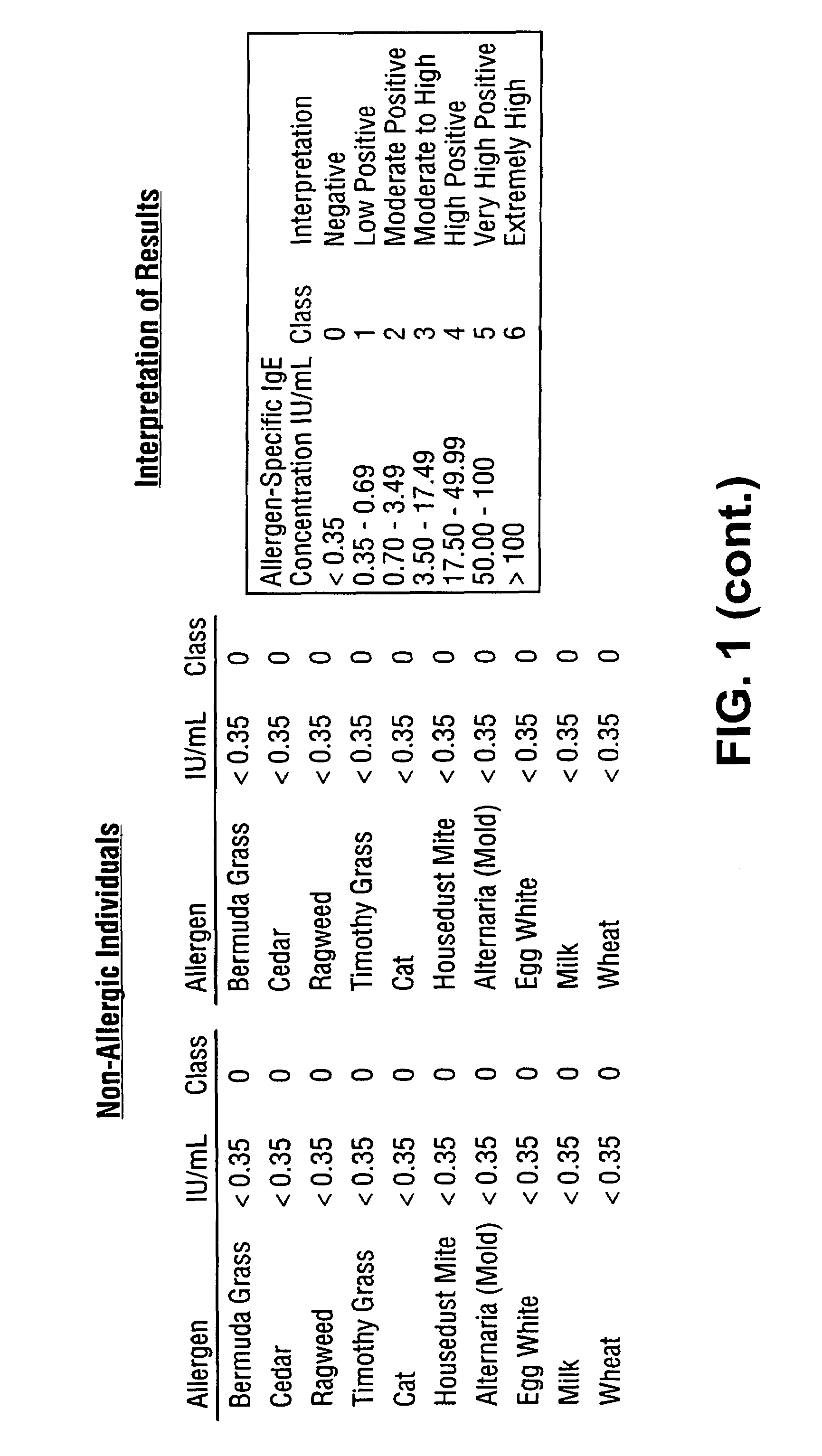
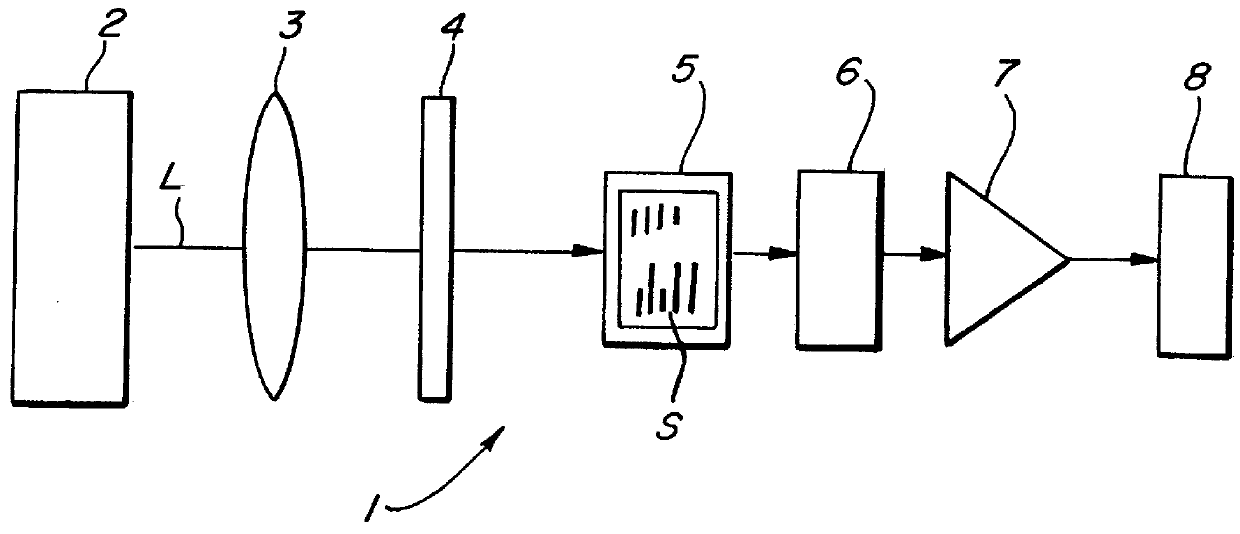
Homogeneous immunoassays for multiple allergens
InactiveUS7491553B2Chemiluminescene/bioluminescenceBiological testingQuantitative determinationMicroparticle
A homogeneous immunoassay method and system for quantitative determination of total immunoglobulin E and specific antibody levels to a plurality of allergens, in which a relatively small sampling of blood is required. The method utilizes relatively small microparticles in aqueous suspension. The immunoassay procedure is an immunometric sandwich procedure preferably utilizing biotin-streptavidin signal amplification techniques and R-phycoerytherin fluorescent labels.
Owner:IMMUNETECH
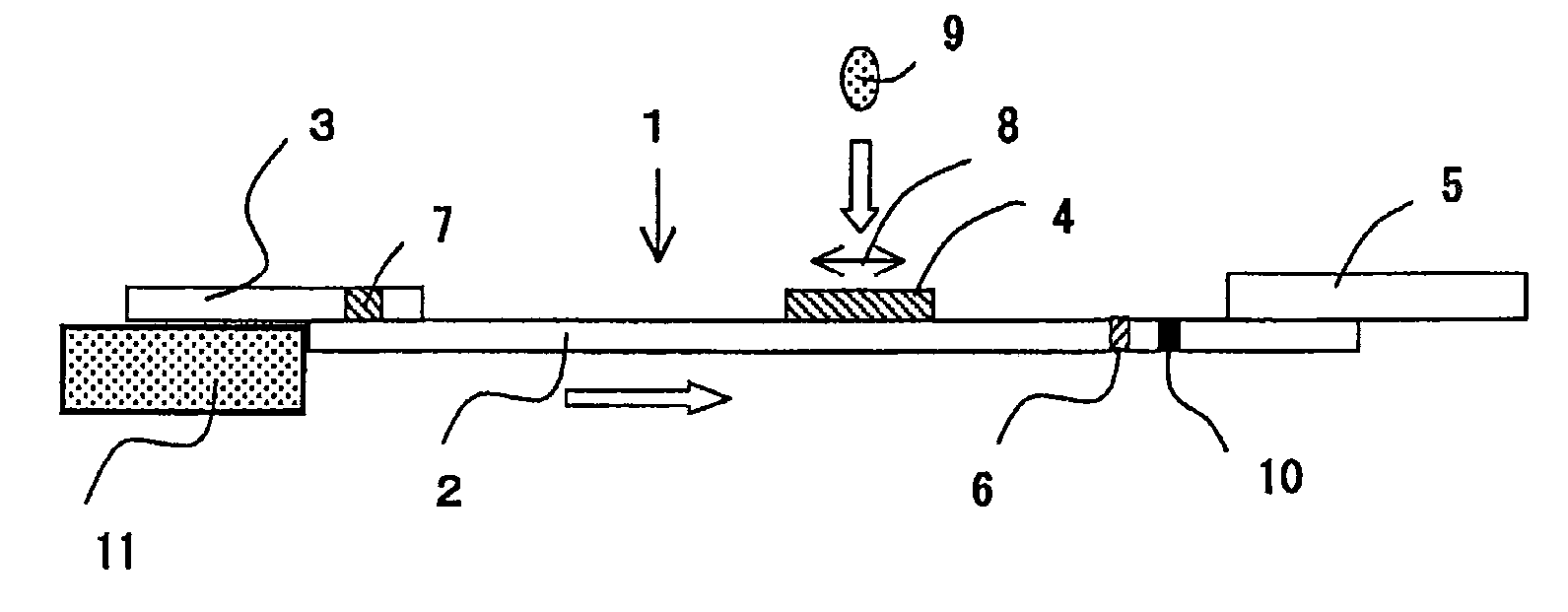
Immunoassay method for lysed whole blood
InactiveUS6030845AIntuitive effectEasy to separateMaterial analysis by optical meansBiological testingAntigenAgglutination
An immunoassay method in which blood can be measured even without pretreatment by a centrifuge etc. In the present invention, antibodies or antigens in a sample are subjected to agglutination reaction with insoluble carriers onto which antigens or antibodies specifically reacting with the antibodies or antigens in the sample have been imrobilized and the resulting agglutination mixture is determined for the change in its absorbance or in its scattered light by irradiation with light, wherein said sample is whole blood and the whole blood is forcibly lysed.
Owner:HORIBA LTD
Immunoassay method for BNP
InactiveUS6828107B2Accurate diagnosisAccurate methodBiological material analysisEnzymologyImmunoassay methodChemistry
Owner:SHIONOGI & CO LTD
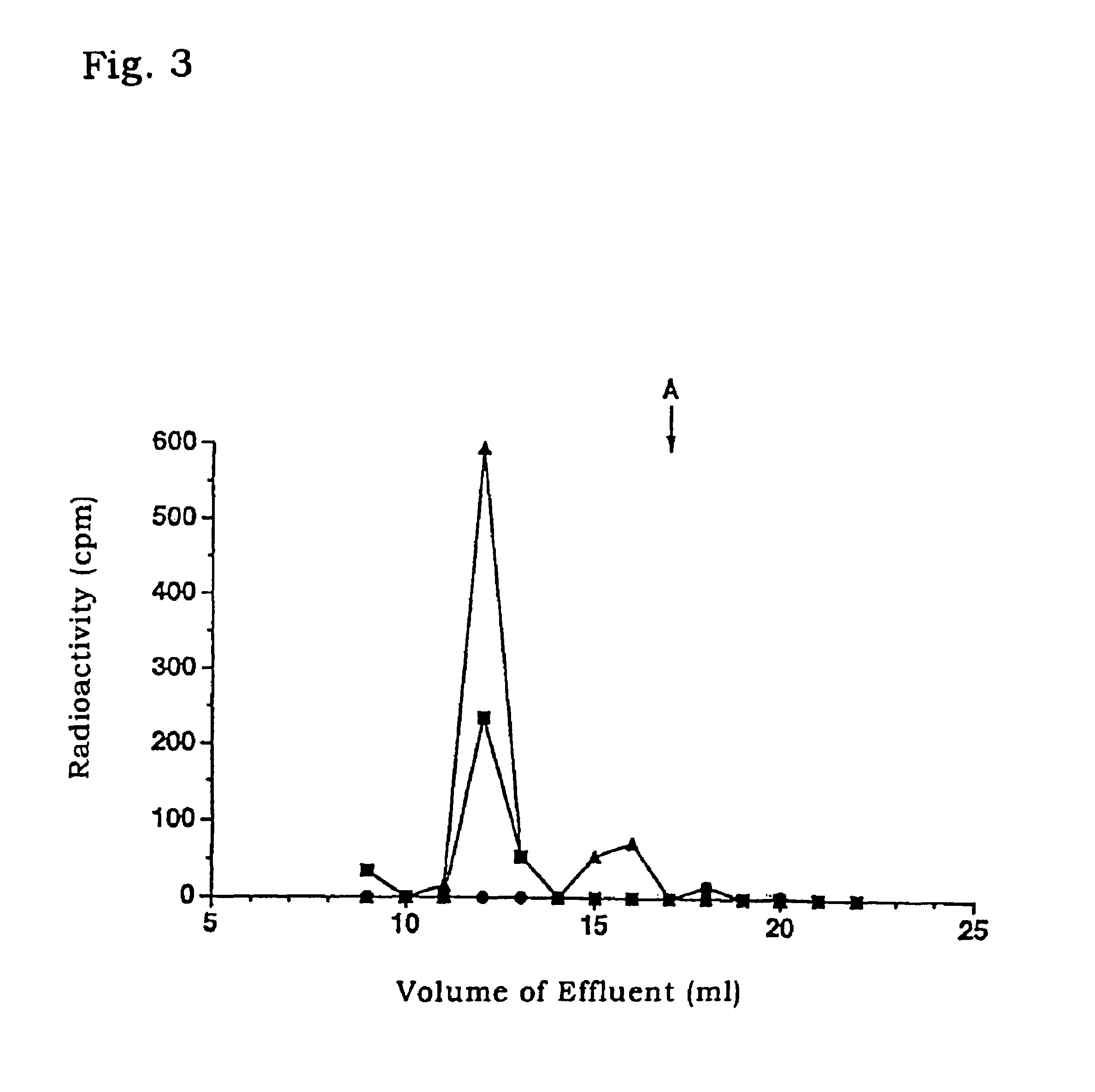
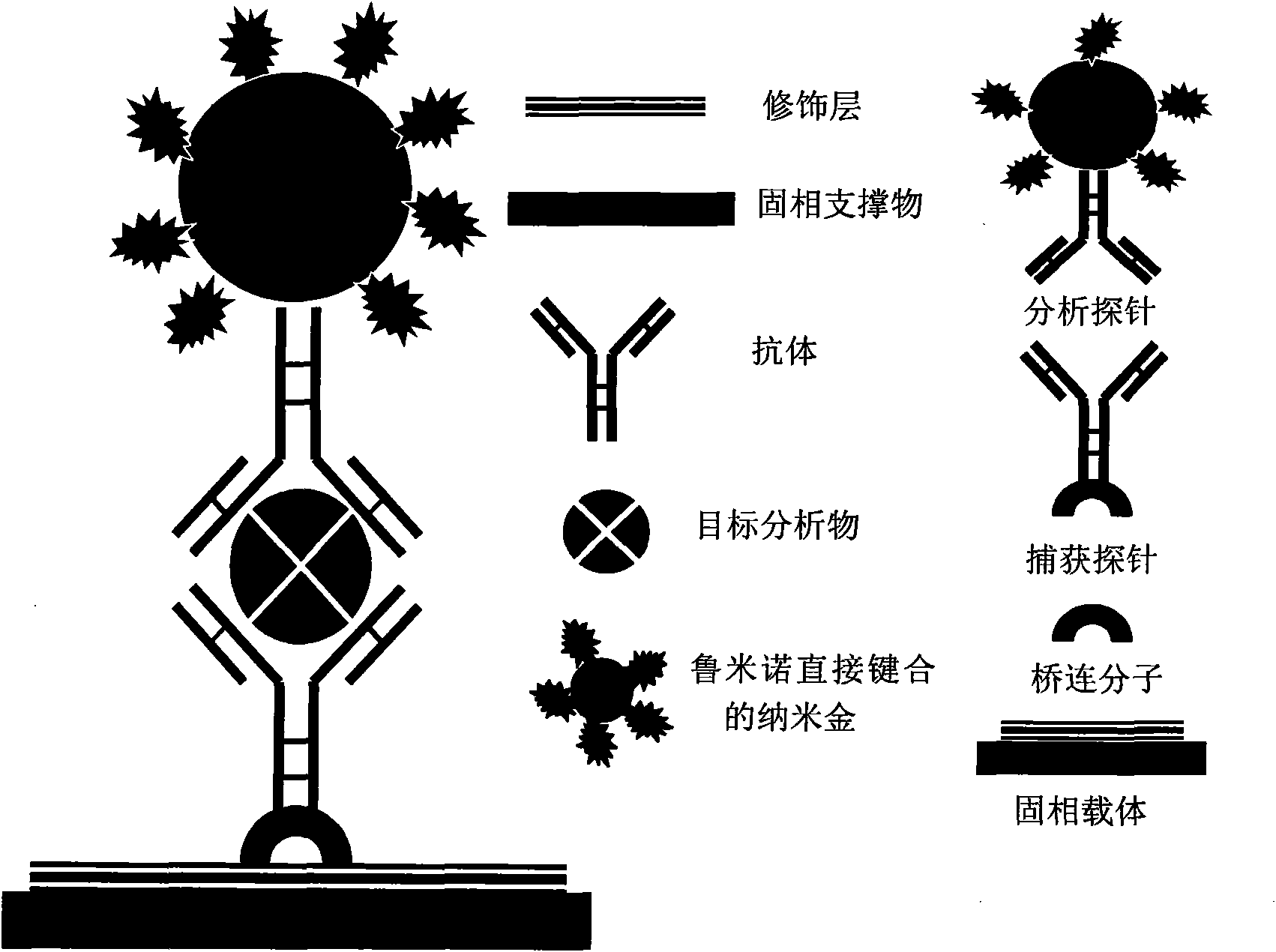
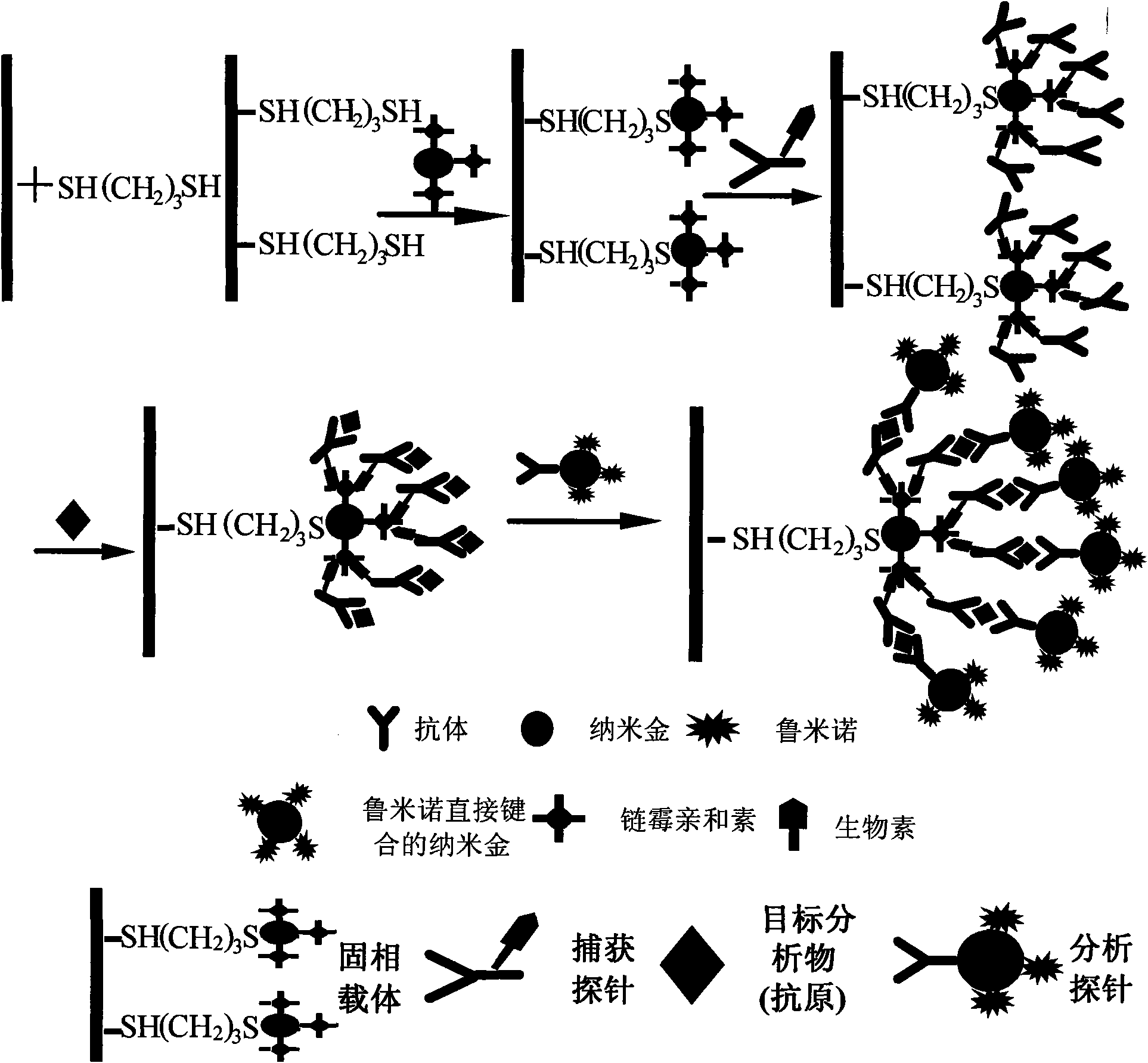
Application of nano-gold directly bonded with luminol in immunoassay
ActiveCN101900723ASimple methodFast wayAnalysis by electrical excitationBiological testingMulti analyteLinearity
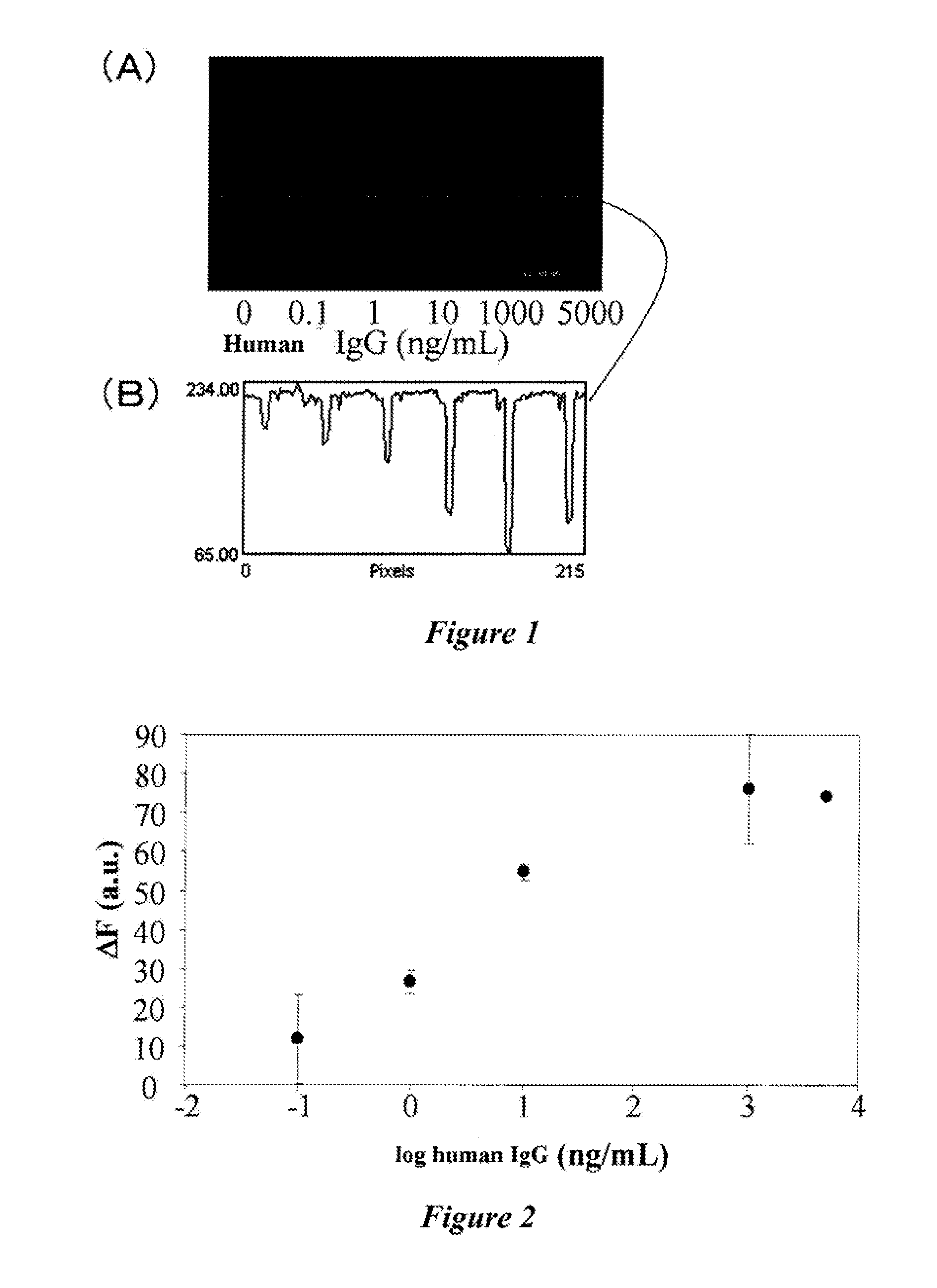
The invention discloses an application of nano-gold directly bonded with luminol in an immunoassay. The invention is characterized in that the immunoassay probe of a nano-gold directly bonded with luminol comprises antibodies which are labeled by the nano-golds directly bonded with luminol, wherein the nano-gold directly bonded with luminol are prepared through reducing chloroauric acid by the luminol at a single step; a chemiluminescence immunoassay method is based on the immunoassay probe of the nano-gold directly bonded with luminol; and a kit is used for carrying out the immunoassay method. The chemiluminescence immunoassay method of the invention has the advantages of high sensitivity (for instance, the detection limit can reach 1.0pg / mL for detecting human IgG), wide range of linearity, good repeatability, simple operation, low cost and the like, can be applied to detect multi-analyte in various samples and has key application prospect in fields, such as clinical diagnosis and cure, drug analysis, food security detection, environmental monitoring and the like.
Owner:UNIV OF SCI & TECH OF CHINA
Particle having magnetic material incorporated therein, process for producing the same, particle for immunoaasay and method of immunoassay
InactiveUS20060188932A1Magnetic homogeneityImprove dispersion stabilityNanomagnetismPharmaceutical non-active ingredientsDispersion stabilityOragene
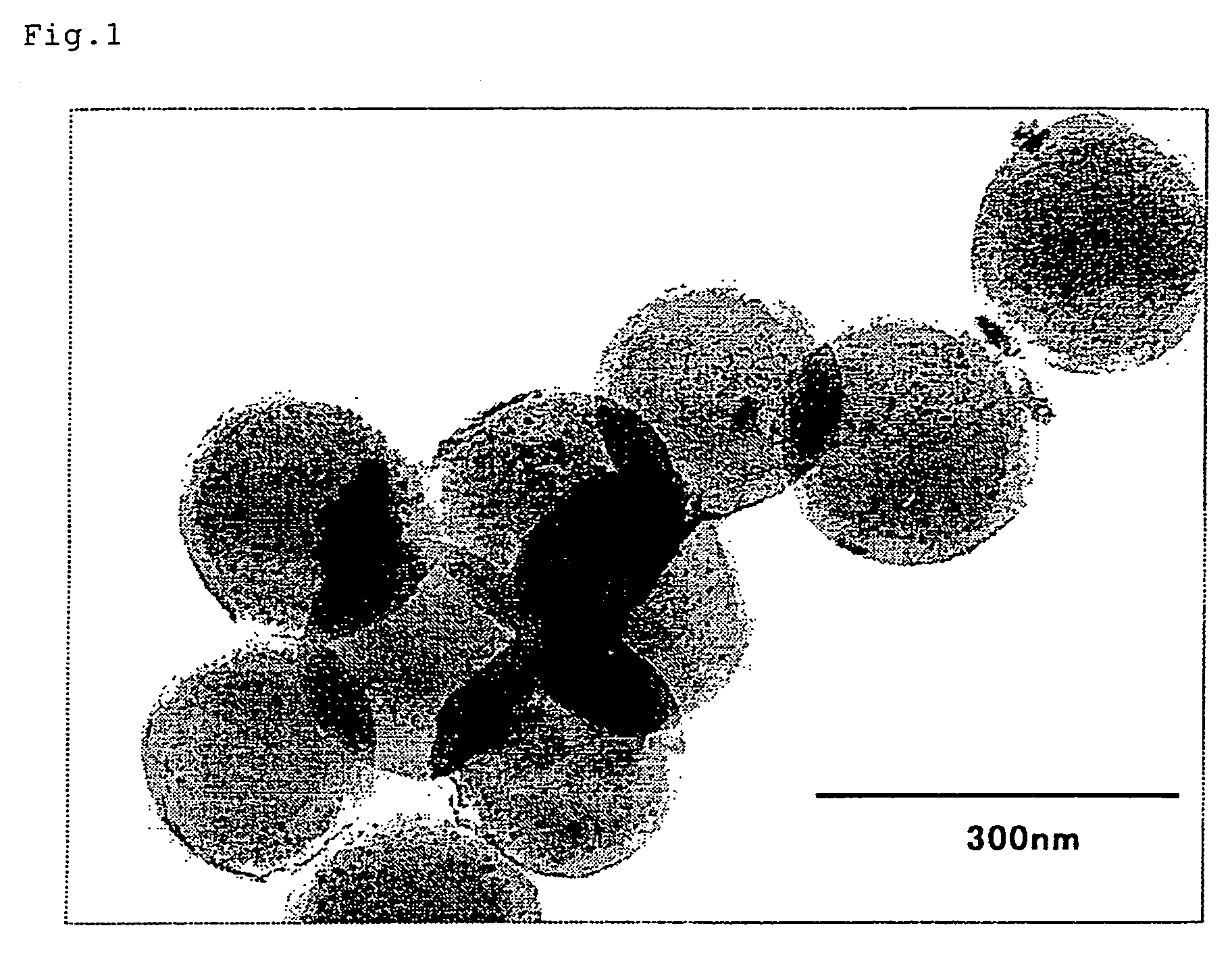
It is an object of the present invention to provide magnetic substance-encapsulated particles which have uniform magnetism, high dispersion stability and a narrow particle size distribution, a method of producing the same, particles for immunoassay formed by using the magnetic substance-encapsulated particles and a method of immunoassay in which the magnetic substance-encapsulated particles or the particles for immunoassay are used. The present invention relates to a magnetic substance-encapsulated particle, which comprises an organic polymer material and a magnetic substance having an average particle size of 1 to 30 nm, the magnetic substance being contained within a particle in a state of being dispersed.
Owner:SEKISUI CHEM CO LTD
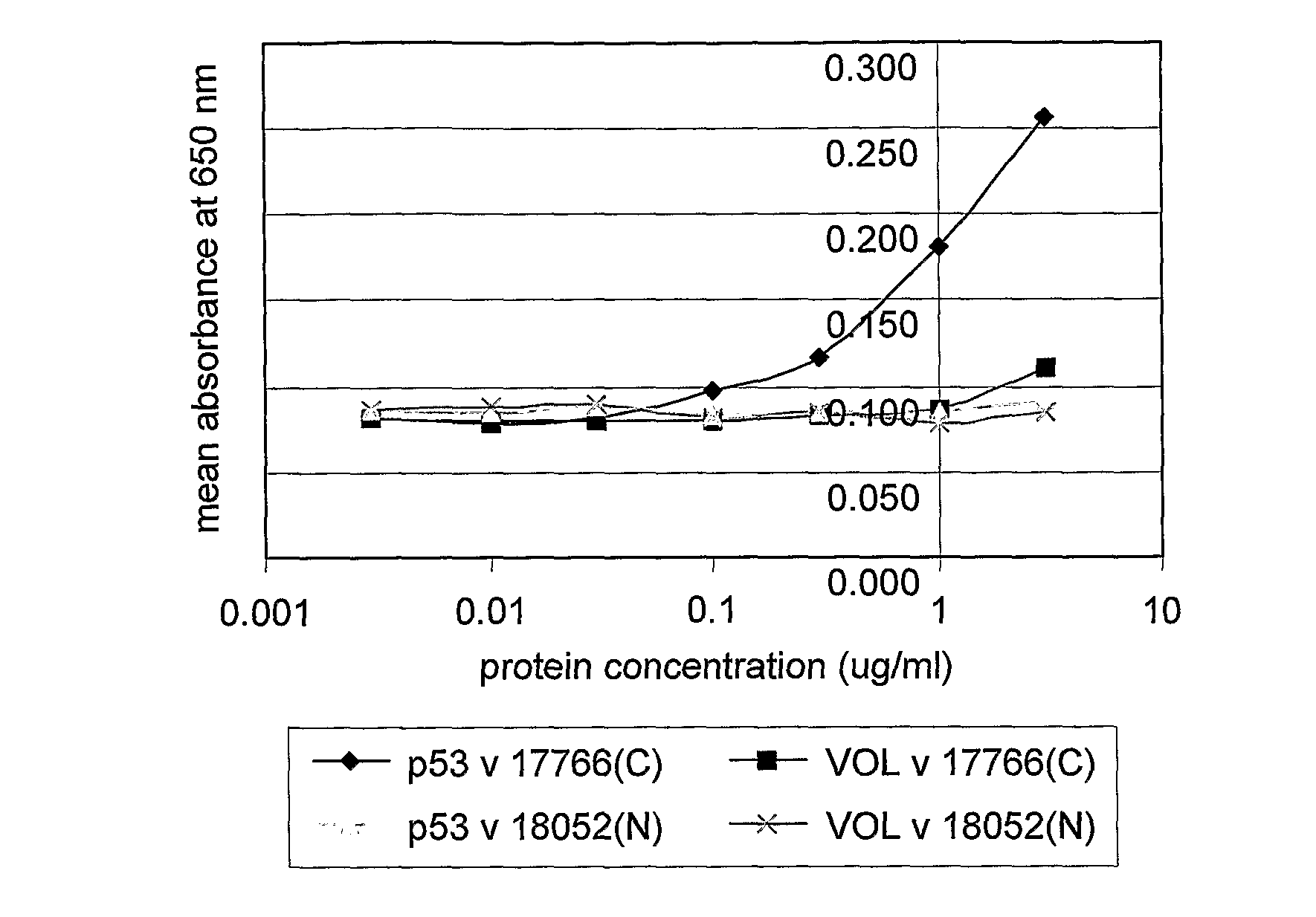
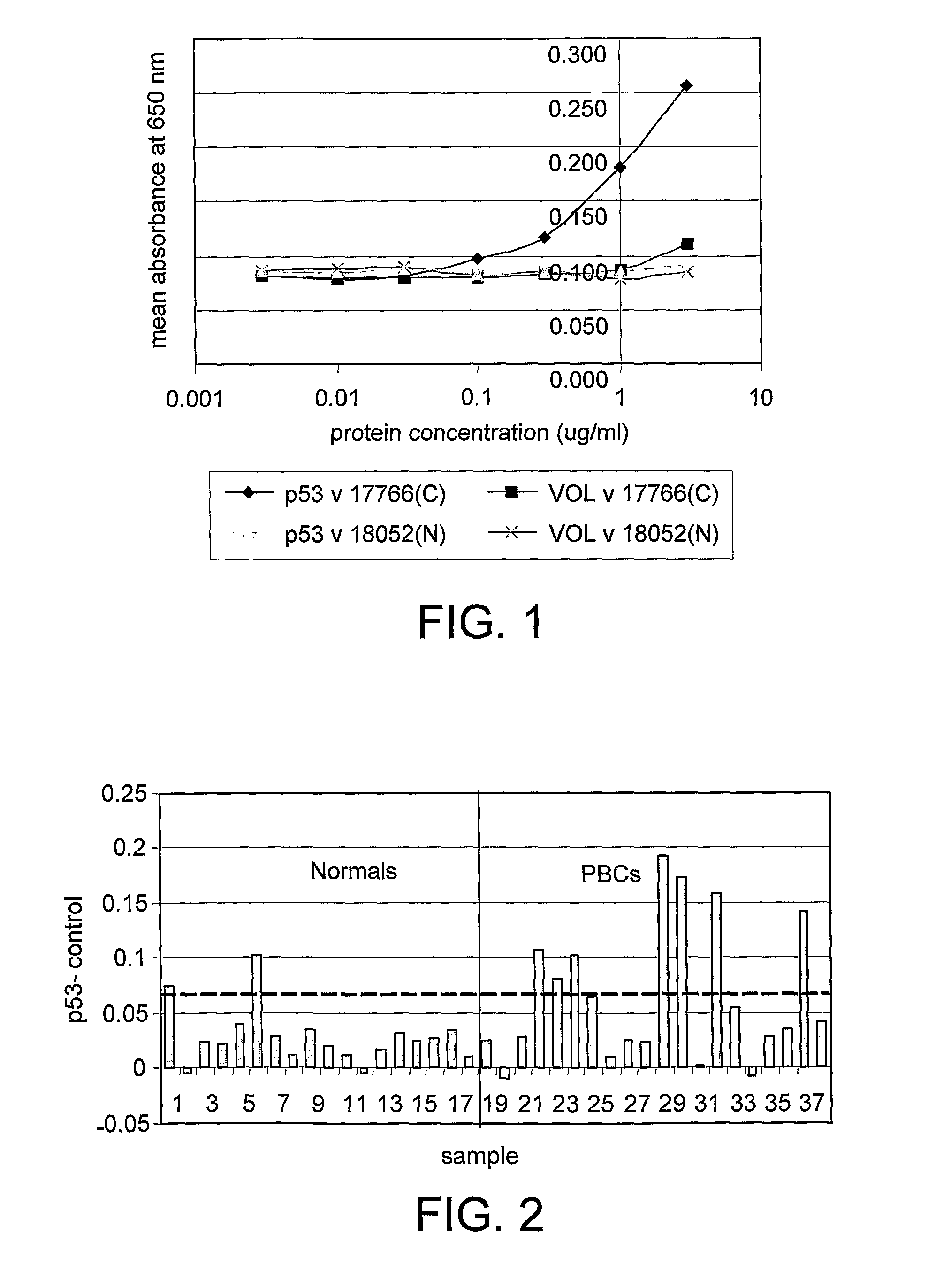
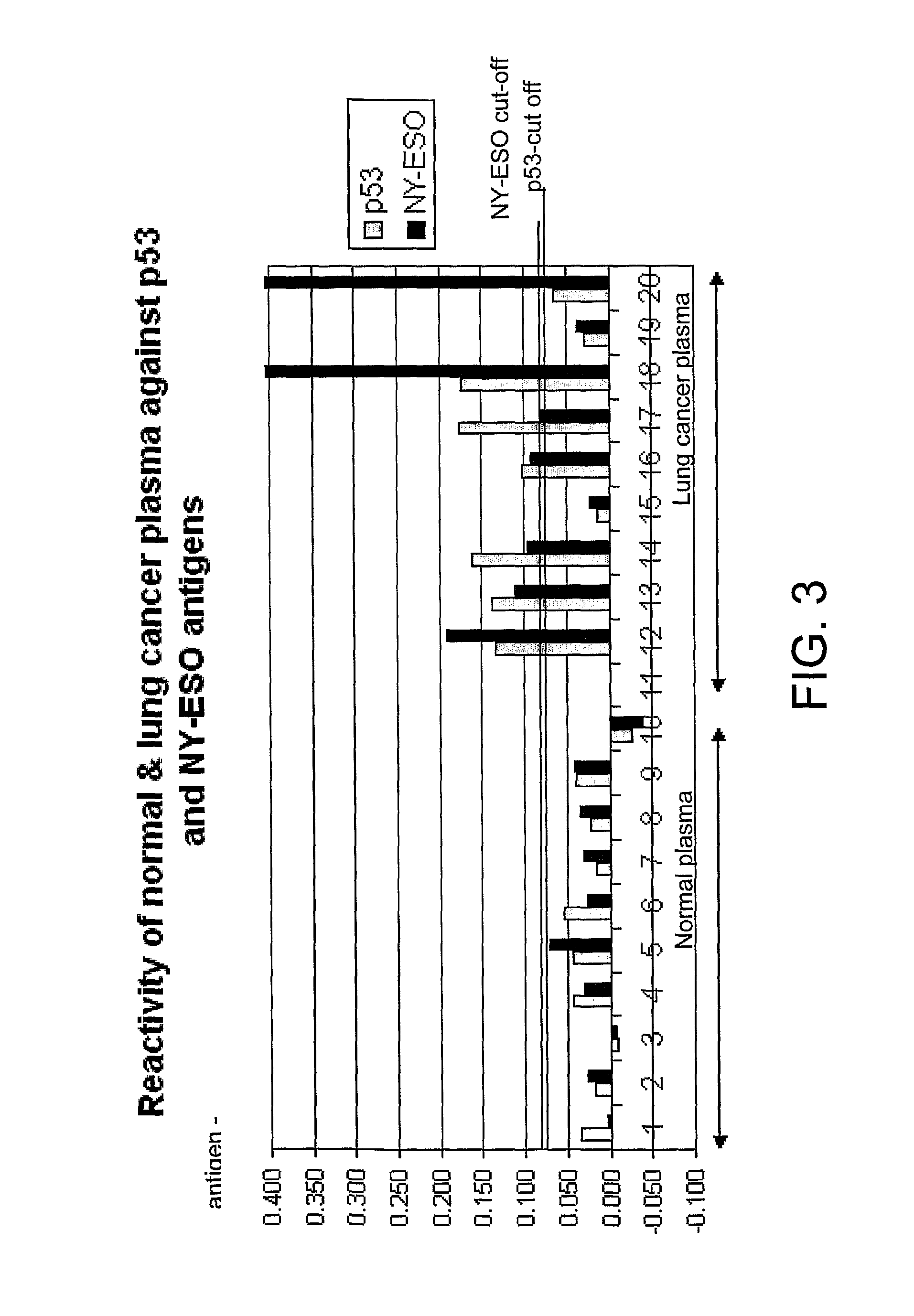
Immunoassay Methods
InactiveUS20080213921A1High sensitivityReduce morbidityDisease diagnosisBiological testingAntigenImmunoassay method
The invention generally relates to the field of diagnostic or prognostic assays and in particular relates to assays for the detection of antibodies in a sample comprising patient bodily fluid, wherein such antibodies are used as biological markers of a disease state or disease susceptibility. The assay is based on cross-titration of both the patient bodily fluid to be tested for the antibody and an antigen used to detect the antibody by specific binding.
Owner:ONCIMMUNE
Homogeneous luminescence immunoassay method for quantitatively analyzing multiple components simultaneously and kit used for method
InactiveCN103837675ALess specimenShorten detection timeBiological testingFluorescence/phosphorescenceBiotin-streptavidin complexMicrosphere
The invention provides a homogeneous luminescence immunoassay method for quantitatively analyzing multiple components simultaneously and a kit used for the method. Receptor microspheres containing various different fluoresceins are adopted, and antibody molecules for capturing different biological markers to be measured are enveloped in the method; in a measuring process, the multiple biological markers in a sample to be measured are combined with the corresponding antibody molecules on the surfaces of the receptor microspheres and biotinylated antibodies in a detection system respectively to form double-antibody sandwich compositions which are respectively connected with donor microspheres labeled with streptavidin; when the donor microspheres are irradiated by exciting light, all types of the receptor microspheres send out optical signals with different wavelengths; the intensities of the light with different wavelengths are respectively detected, so that the biological markers to be measured can be accurately quantified. The kit comprises the receptor microspheres containing chemiluminescence reagents and the fluoresceins, the biotinylated antibodies and the donor microspheres containing photosensitive substances. The homogeneous luminescence immunoassay method and the kit have the beneficial effects that simultaneous and quantitative measurement on multiple components is realized, and the detection cost is reduced.
Owner:TIANJIN NANKAI HOSPITAL
Magnetic microparticle-packing unit, microfluidic device including the same, and immunoassay method using the microfluidic device
InactiveUS20080171400A1Bacterial antigen ingredientsLaboratory glasswaresCentre of rotationEngineering
A magnetic microparticle-packing unit using centrifugal force, a microfluidic device including the same, and an immunoassay method using the microfluidic device are provided. The magnetic microparticle-packing unit includes a rotary body controllably rotating; a microfluidic channel which includes a curved portion in which the microfluidic channel first extends away from the rotation center of the rotary body and then turns toward the rotation center of the rotary body.
Owner:SAMSUNG ELECTRONICS CO LTD
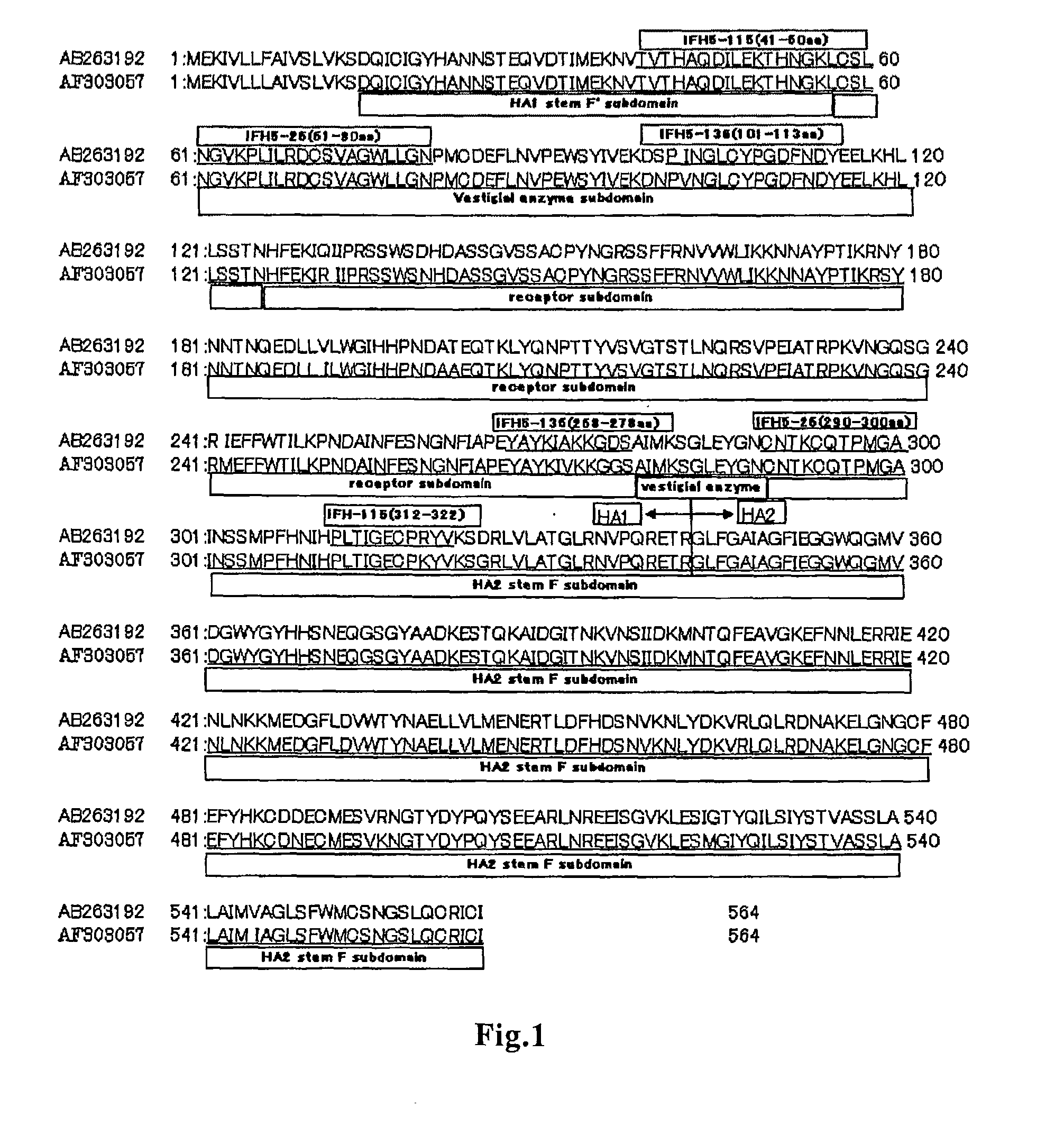
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
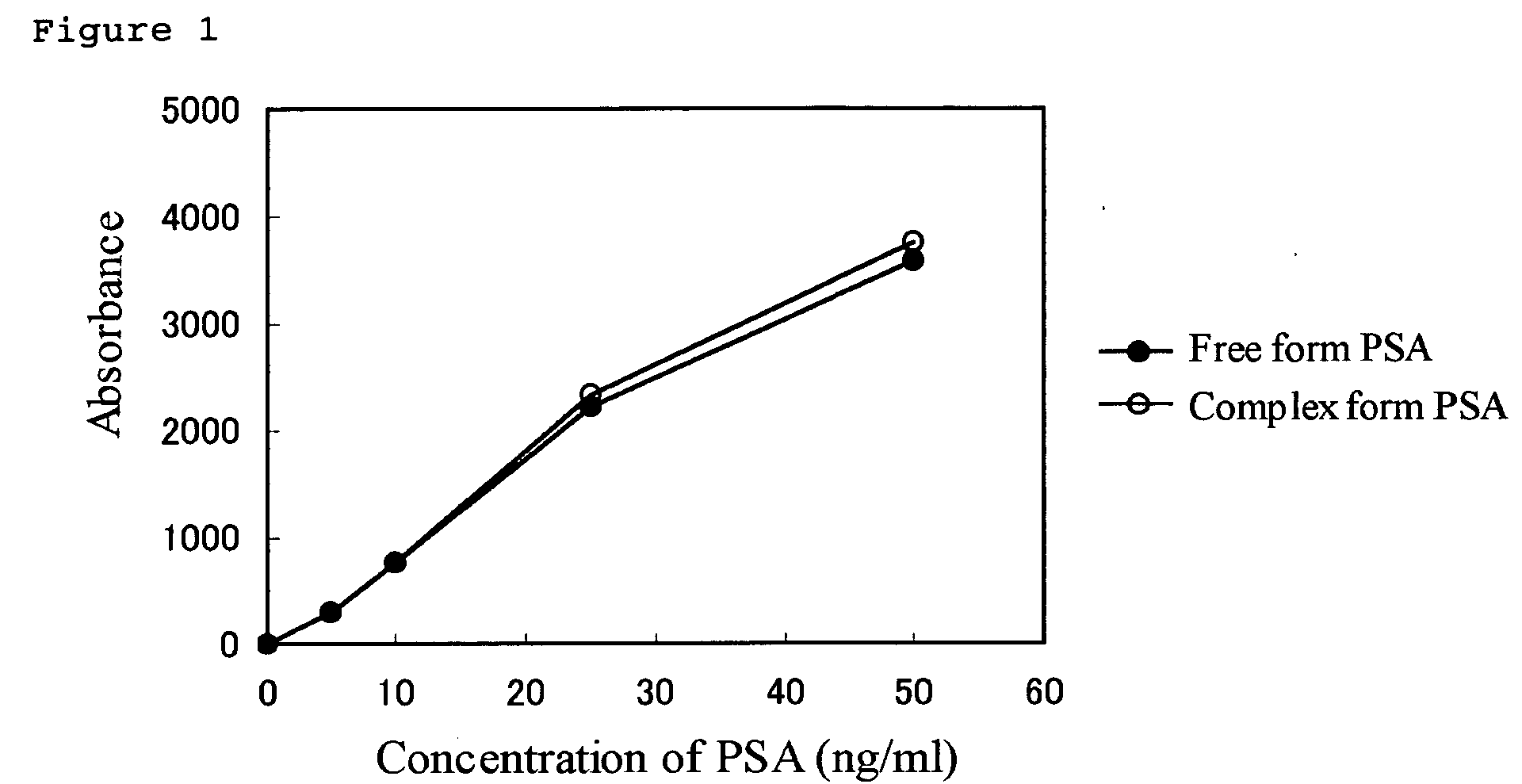
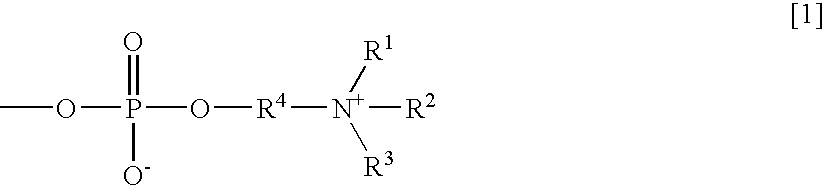
Reagent for an immunoassay
InactiveUS20050069967A1Highly sensitive and highly preciseAccurate measurementBiological testingParticle suspension analysisFree formMonoclonal antibody
The present invention relates to (1) A reagent for an immunoassay of a target substance existing in a free form and a bound form in a specimen, comprising a latex 1 which is immobilized with a monoclonal antibody 1 for the target substance, and a latex 2 which has a different mean particle size from the latex 1 and is immobilized with a monoclonal antibody 2 having a different recognition site for the target substance from the antibody 1, (2) An immunoassay method comprising reacting the target substance with the reagent of (1) and determining an amount of the substance based on the result of an agglutination reaction among the target substance, the latex1 and the latex2, and (3) A reagent kit comprising a reagent of (1) and a reagent containing an agglutination accelerator for an antigen-antibody reaction.
Owner:WAKO PURE CHEMICAL INDUSTRIES
Immunoassay method and device with magnetically susceptible bead capture
ActiveUS20120031773A1Improving low-end sensitivityElimination of user induced errorImmobilised enzymesBioreactor/fermenter combinationsPoint of careAnalyte
Owner:ABBOTT POINT CARE
Immunoassay Methods
ActiveUS20080305476A1Strong specificityHigh sensitivityMicrobiological testing/measurementDisease diagnosisTest sampleBody fluid
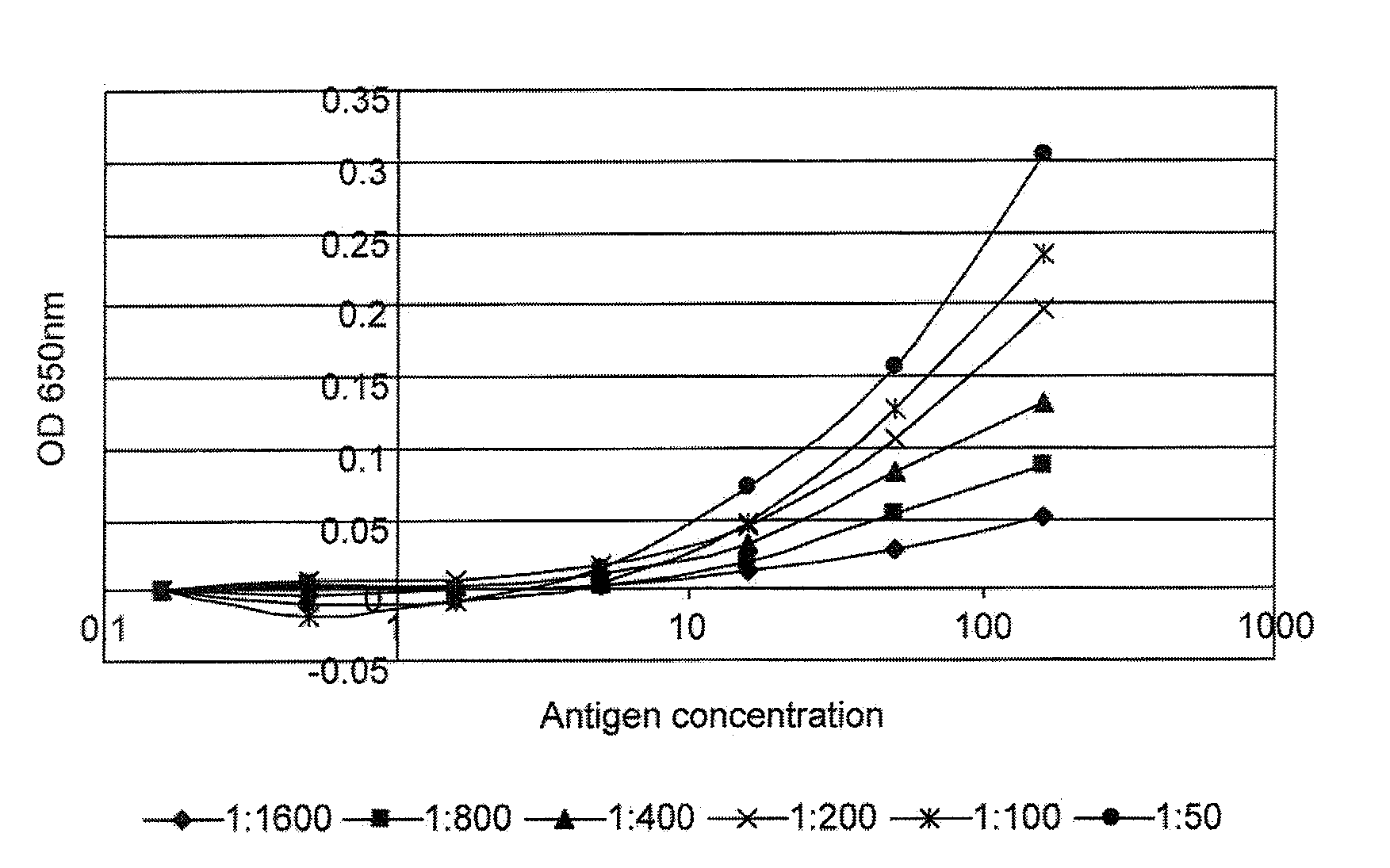
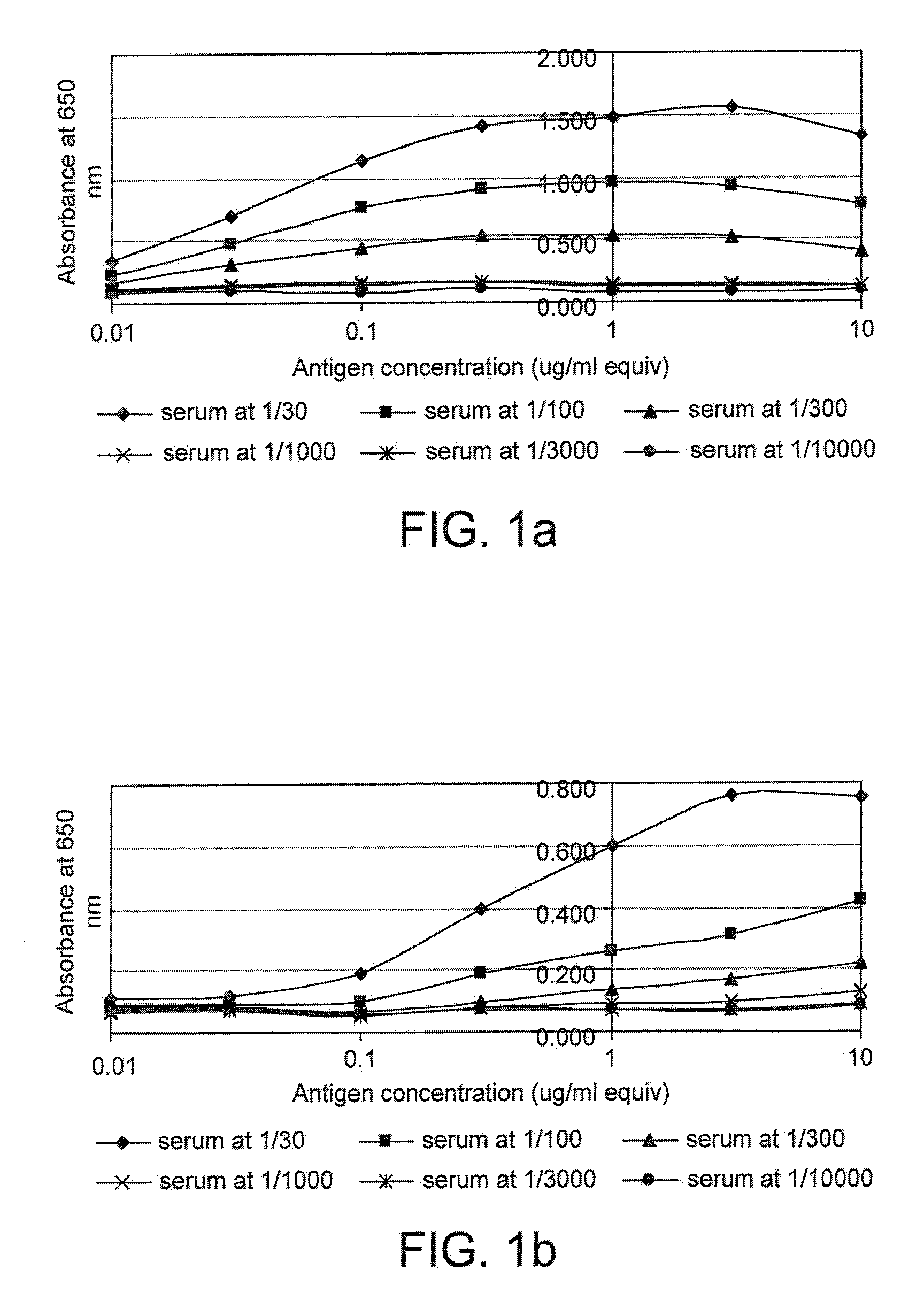
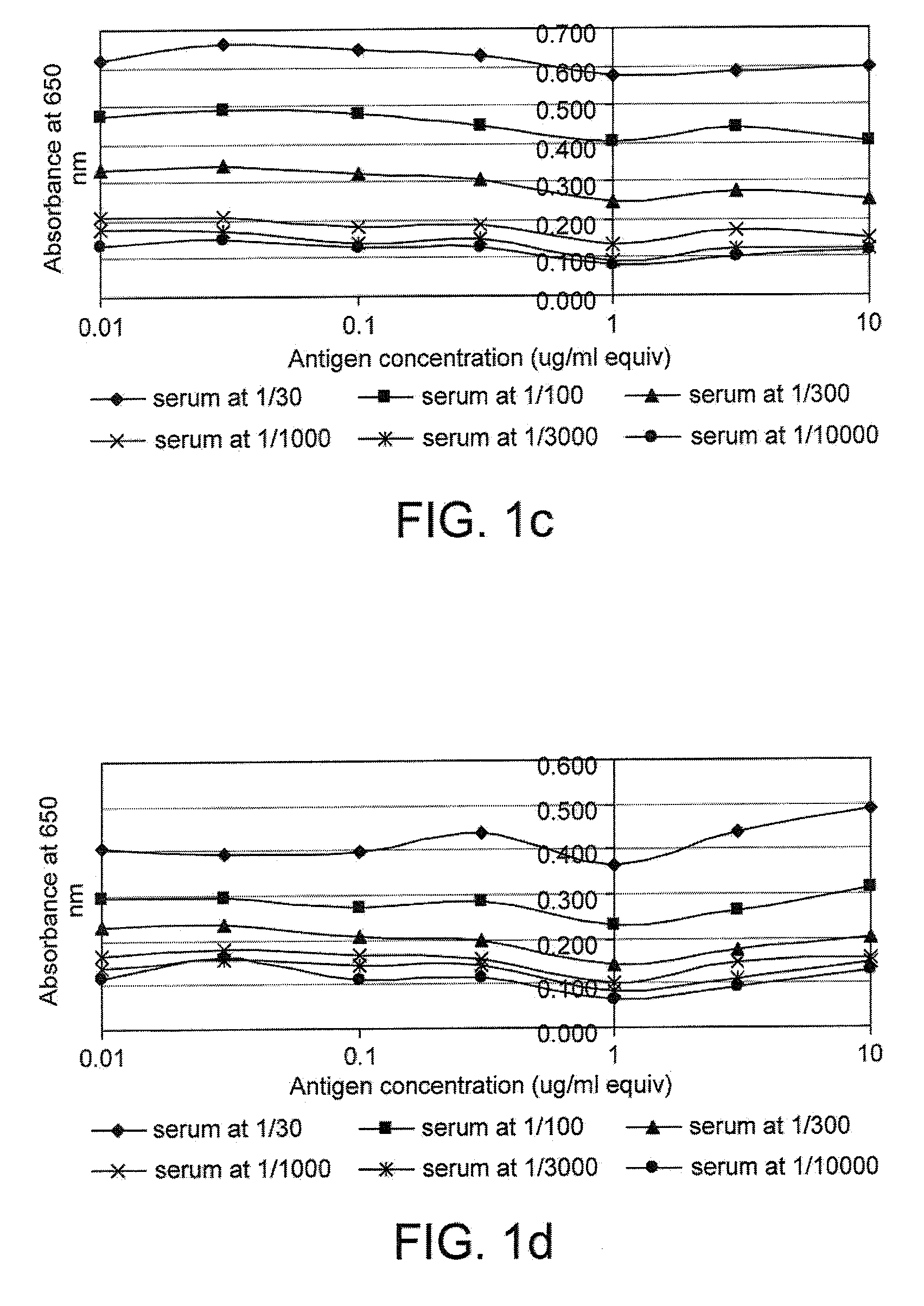
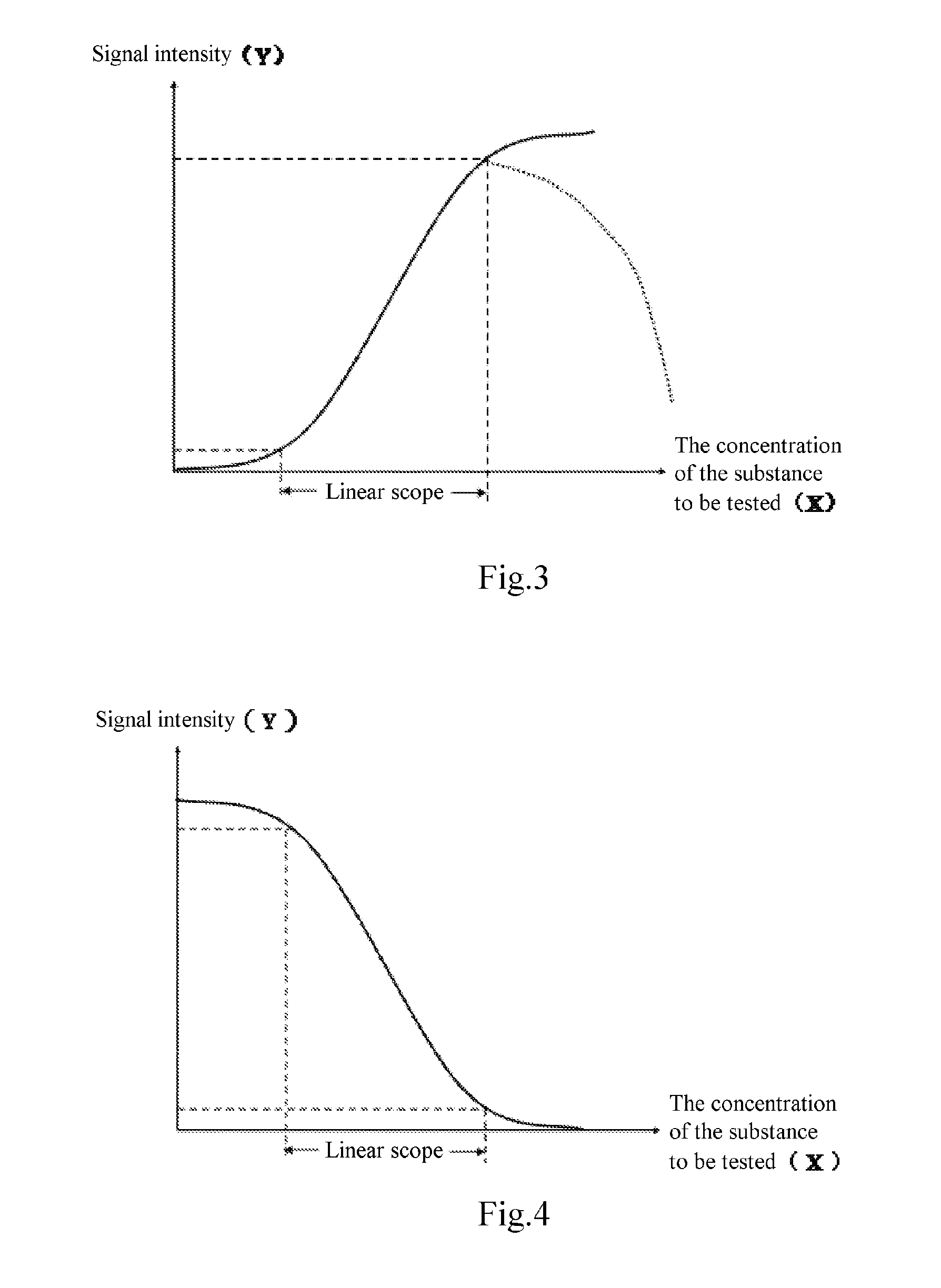
The invention relates to a method of detecting a disease state or disease susceptibility in a mammalian subject which comprises detecting an antibody in a test sample comprising a bodily fluid from said mammalian subject wherein said antibody is a biological marker of a disease state or disease susceptibility, the method comprising: (a) contacting said test sample with a plurality of different amounts of an antigen specific for said antibody, (b) detecting the amount of specific binding between said antibody and said antigen, (c) plotting or calculating a curve of the amount of said specific binding versus the amount of antigen for each amount of antigen used in step (a) and (d) determining the presence or absence of said disease state or disease susceptibility based upon the amount of specific binding between said antibody and said antigen at each different antigen concentration used.
Owner:ONCIMMUNE
Homogeneous immunoassay kit, detection method and application thereof
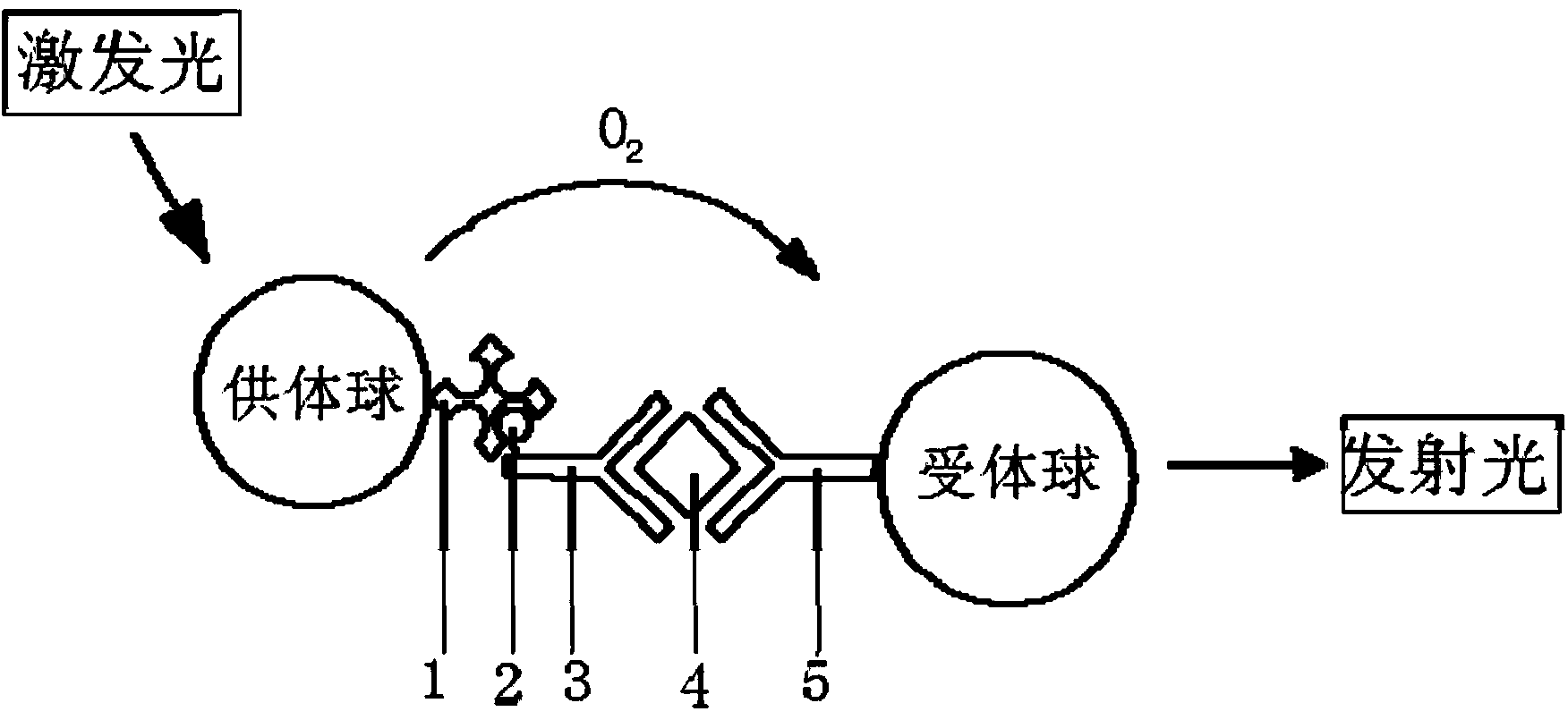
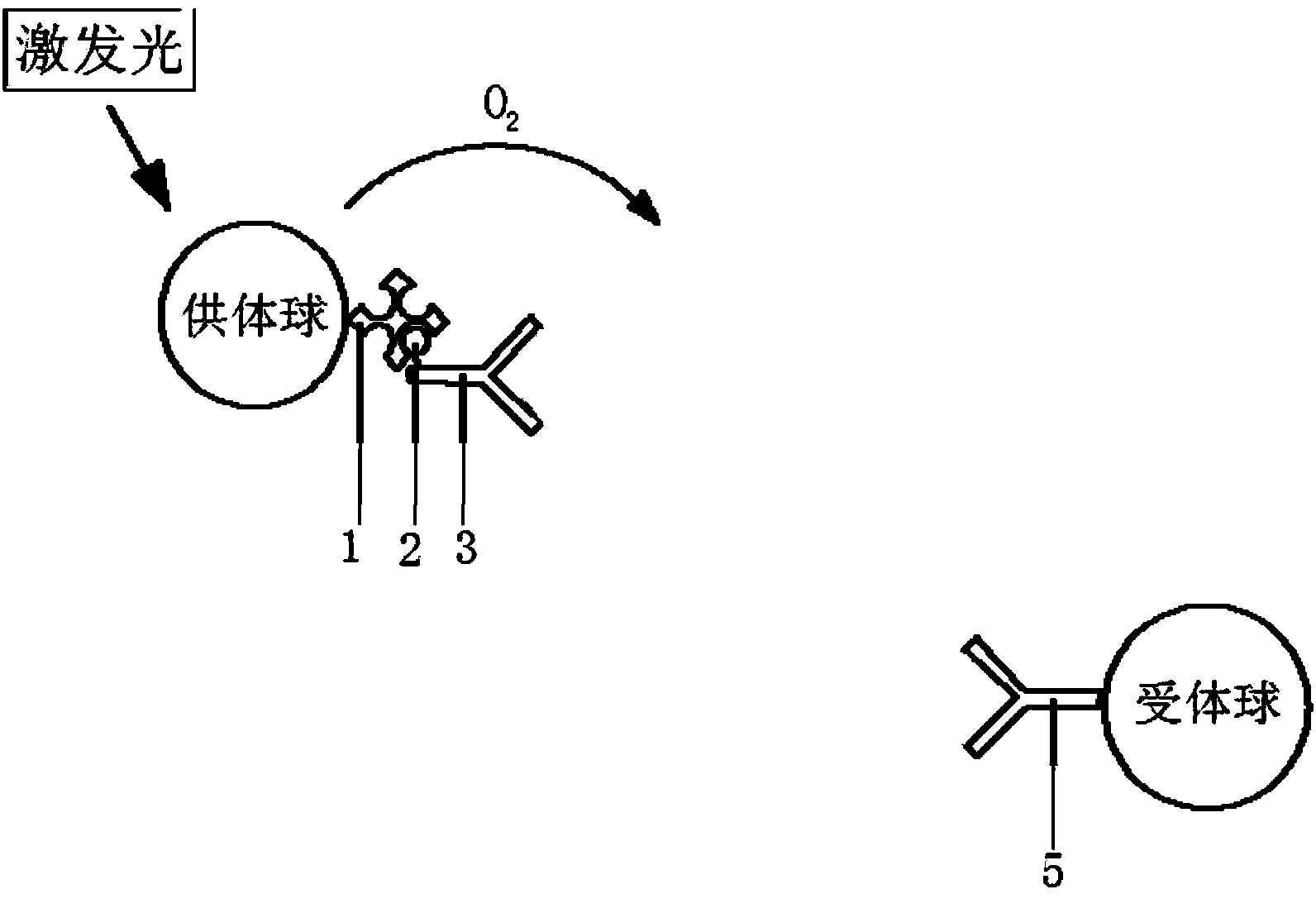
ActiveCN108051585AReduce difficultyAvoid cross reactionChemiluminescene/bioluminescenceBiological material analysisBiotin-streptavidin complexAntigen
The invention relates to a homogeneous immunoassay kit, detection method and application thereof in the technical field of biology. The kit comprises a reagent I, a reagent II, a reagent III and a reagent IV, wherein the reagent I contains a first counterpart, and the first counterpart is a known antigen or a known antibody specially combined with an antigen to be detected in a sample to be detected; the reagent II contains a receptor which can react with singlet oxygen to generate a detectable signal and a second counterpart combined with the receptor; the reagent III contains a third counterpart specially combined with the first counterpart; the reagent IV contains a donor which can generate the singlet oxygen in an excitation state; the surface of the third counterpart is coated with biotin, and the surface of the donor is coated with streptavidin. By utilizing combined detection of the kit and the homogeneous immunoassay method of the antigen and the antibody, the screening difficulty of raw materials is reduced, and the detection sensitivity is promoted at the same time.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
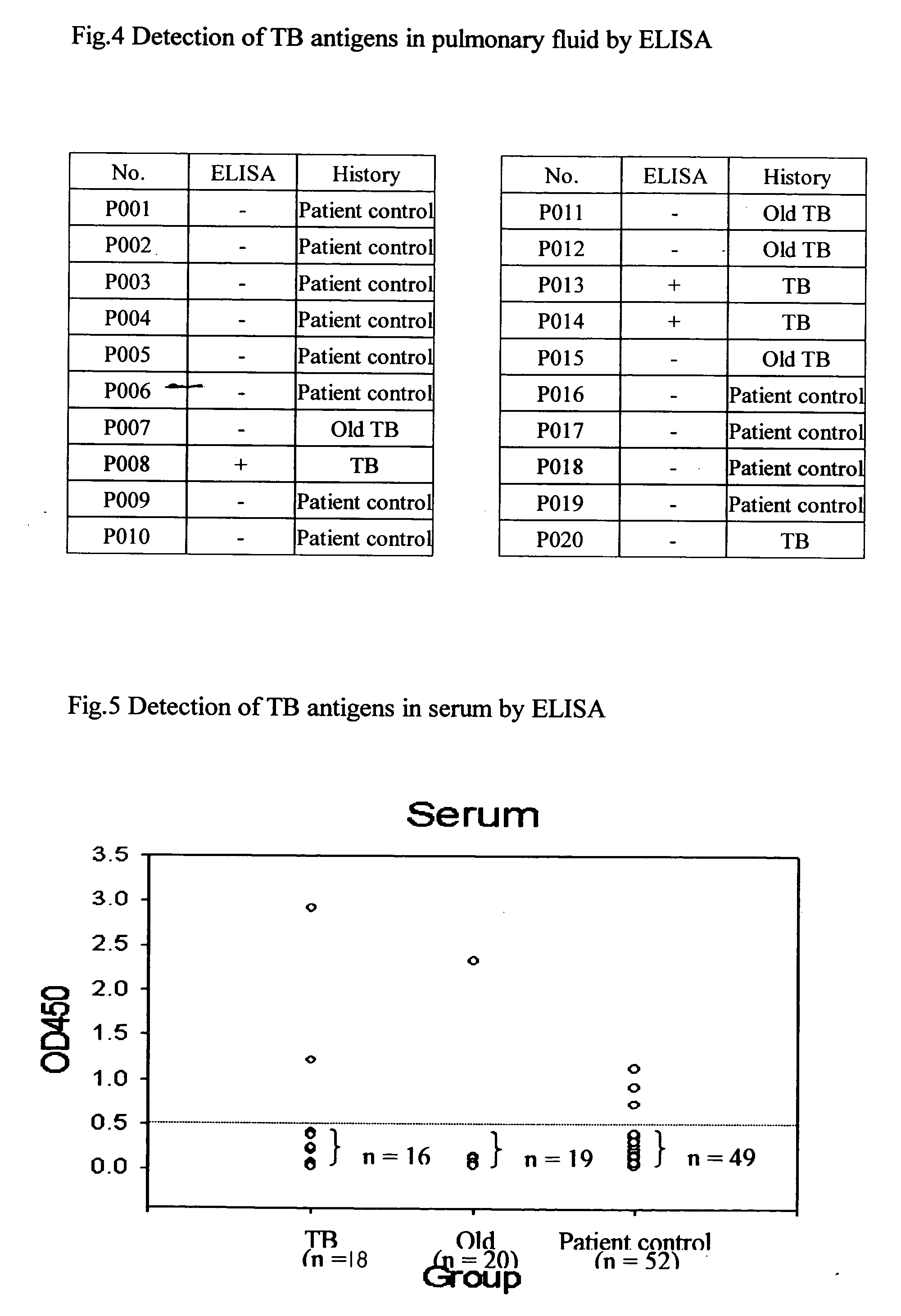
Method for detection of Mycobacterium tuberculosis antigens in biological fluids
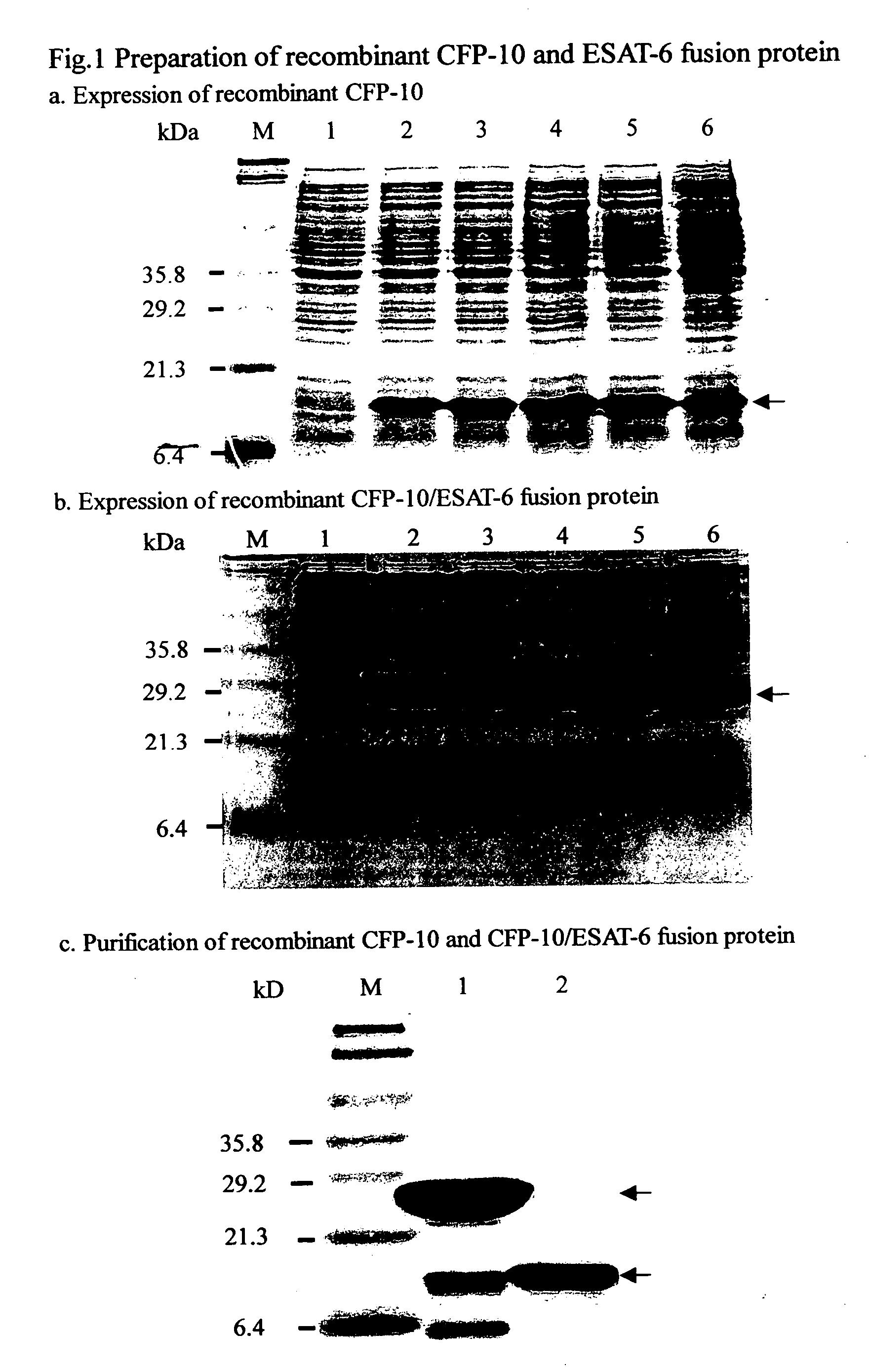
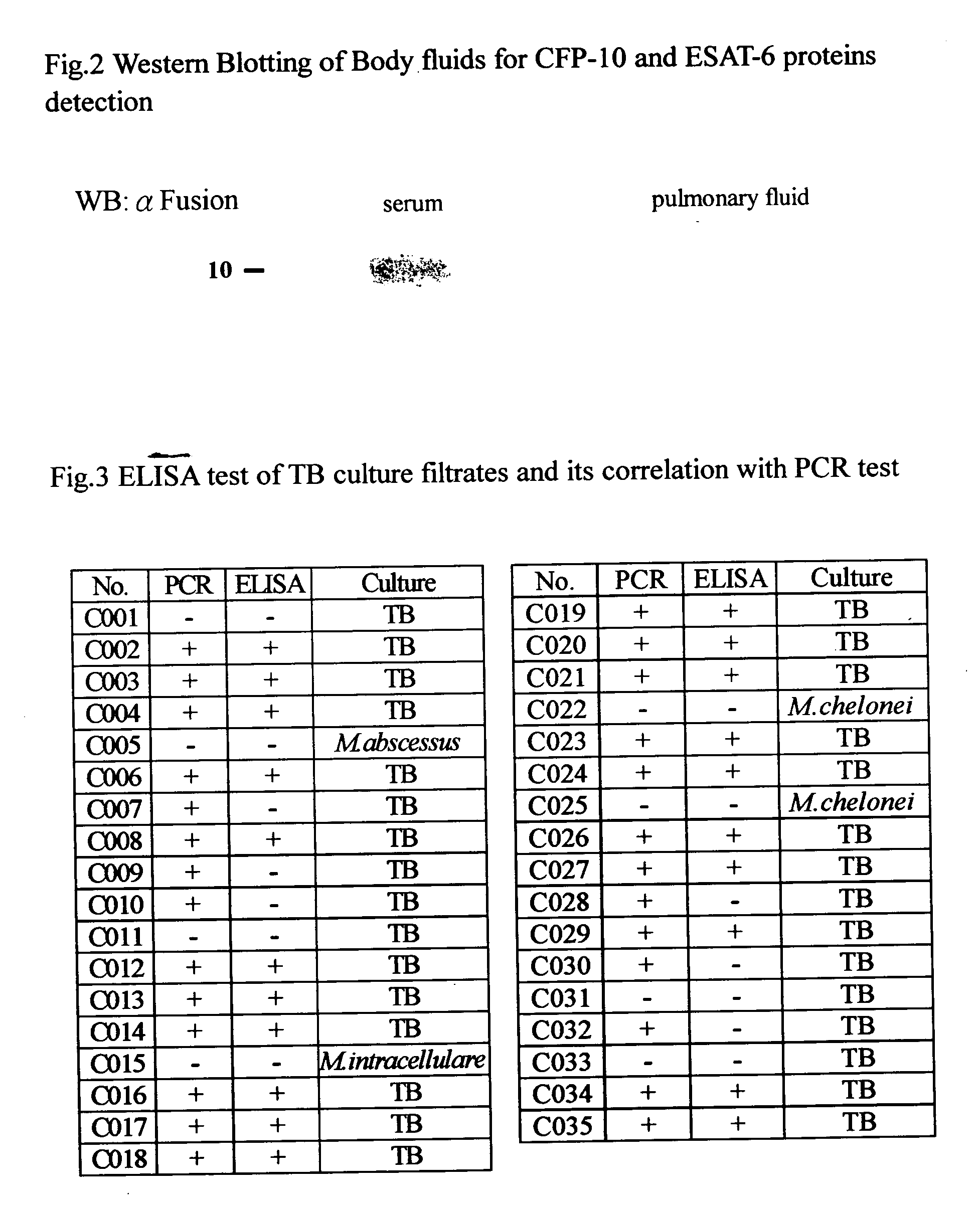
A method for detection of mycobacterium tuberculosis antigens in biological fluids provides immunoassay methods, diagnostic kits, and an immunochromatoraphic assay device for detection of Mycobacterium tuberculosis antigens in biological specimens, preferably body fluids and tissues. The preferred body fluids are blood, serum, plasma, urine, pulmonary fluid, sputum, cerebrospinal fluid, and the preferred tissue is the lung biopsy specimen. The immunoassays require two primary antibodies against RD1, RD2, or RD3 of Mycobacterium tuberculosis. At least one of the primary antibodies is attached to a solid carrier. Optional, a second antibody against an animal species producing one of the primary antibodies can be added. Either the other primary antibody or the secondary antibody is labeled with a detection agent, which can be an enzymatic marker, a fluorescent or luminescent agent, a radio active label or a color particle. The biological specimens may be used directly, concentrated or diluted for the immunoassays.
Owner:CHANG GUNG UNIVERSITY
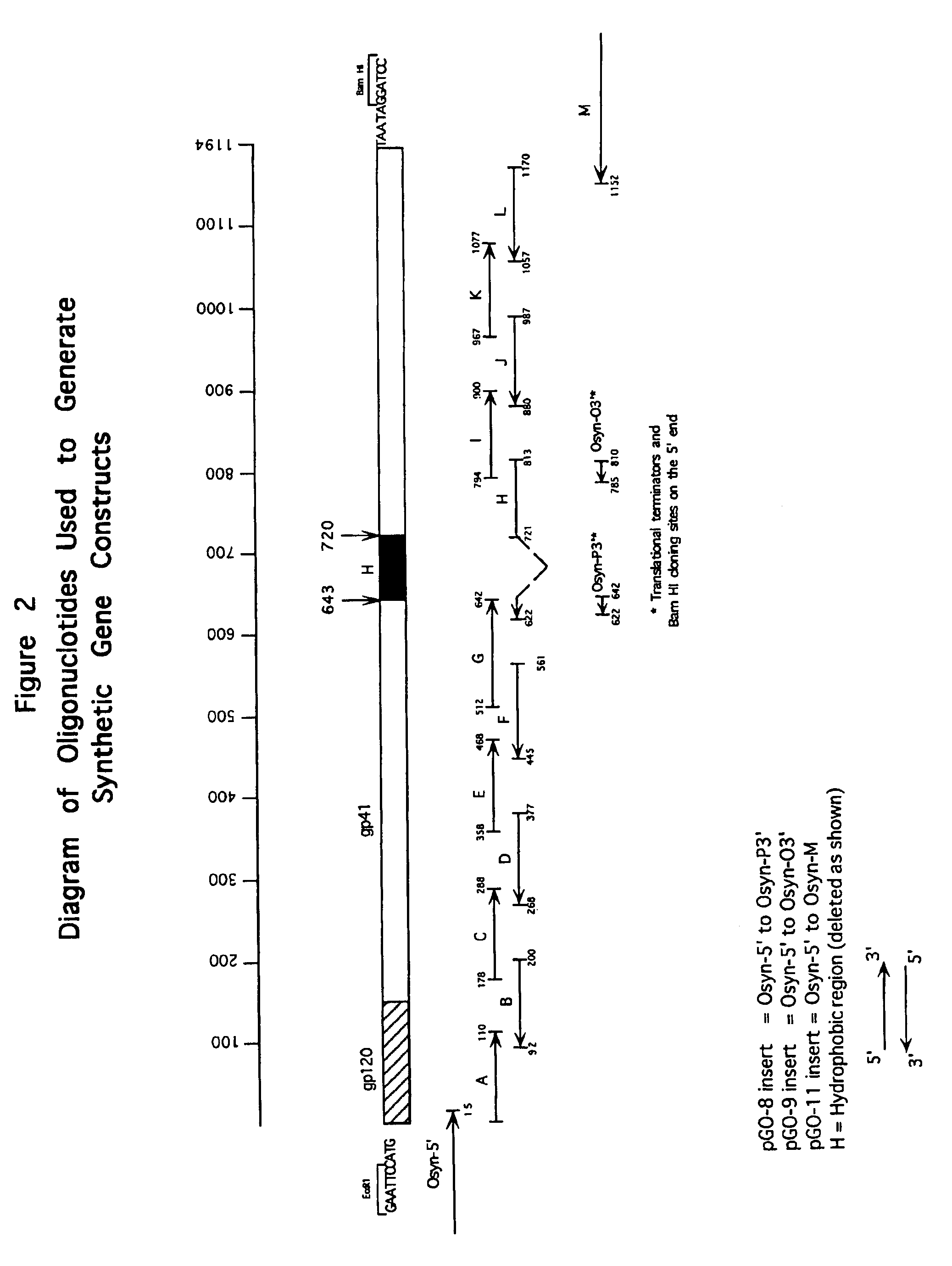
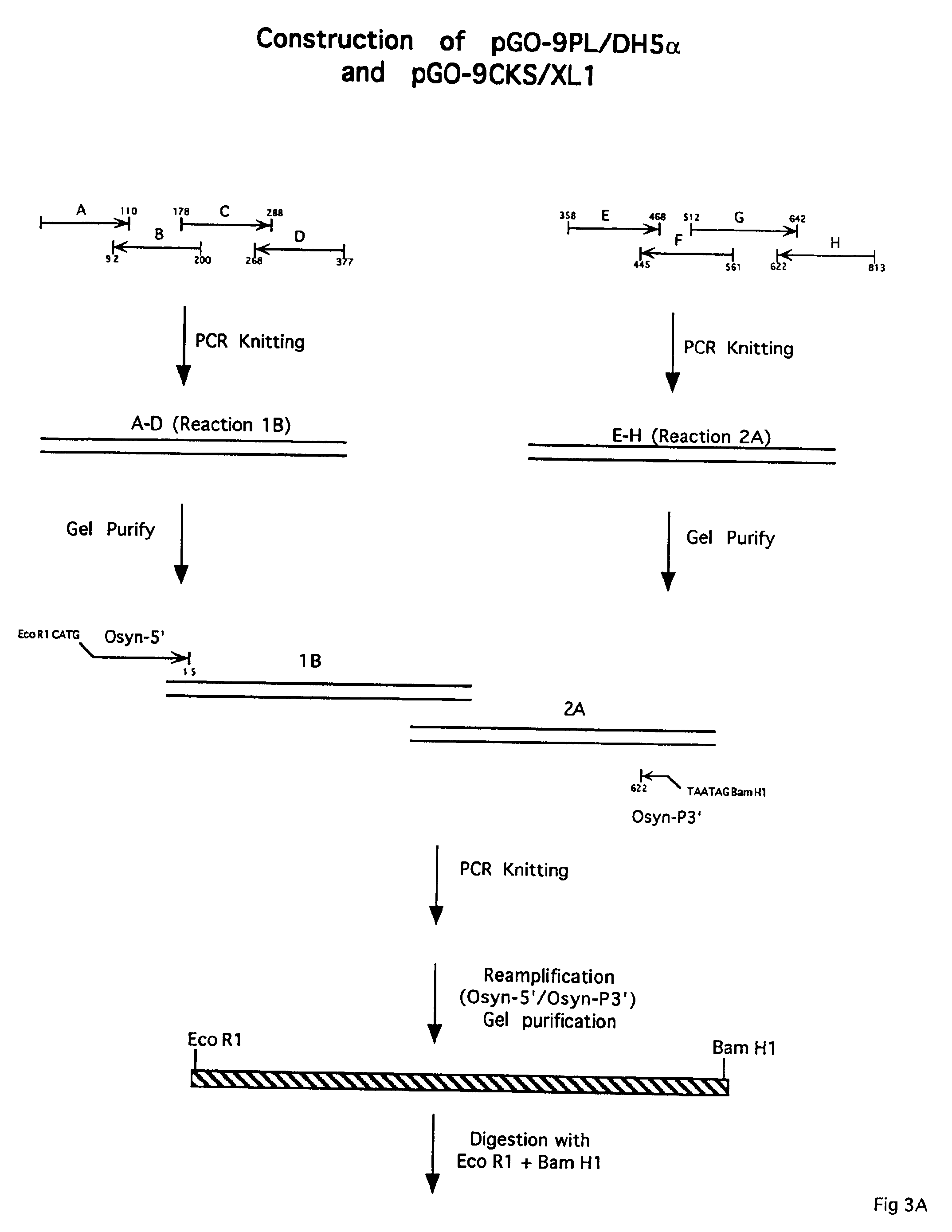
Antigen constructs useful in the detection and differentiation of antibodies to HIV
Isolated HIV-1 Group O env polypeptides obtained from the HIV-1 isolate HAM112 are claimed, as well as (a) antigen constructs comprising fusions of one or more of each of HIV-1 Group O env polypeptides and HIV-1 Group M env polypeptide and (b) further antigen constructs containing additional Group O sequences and especially the gp41 IDR of isolate HAM112. Also claimed are polynucleotide sequences encoding the above, expression vectors comprising the same, host cells transformed thereby, and immunoassay methods and kits utilizing the antigen constructs of the invention.
Owner:ABBOTT LAB INC
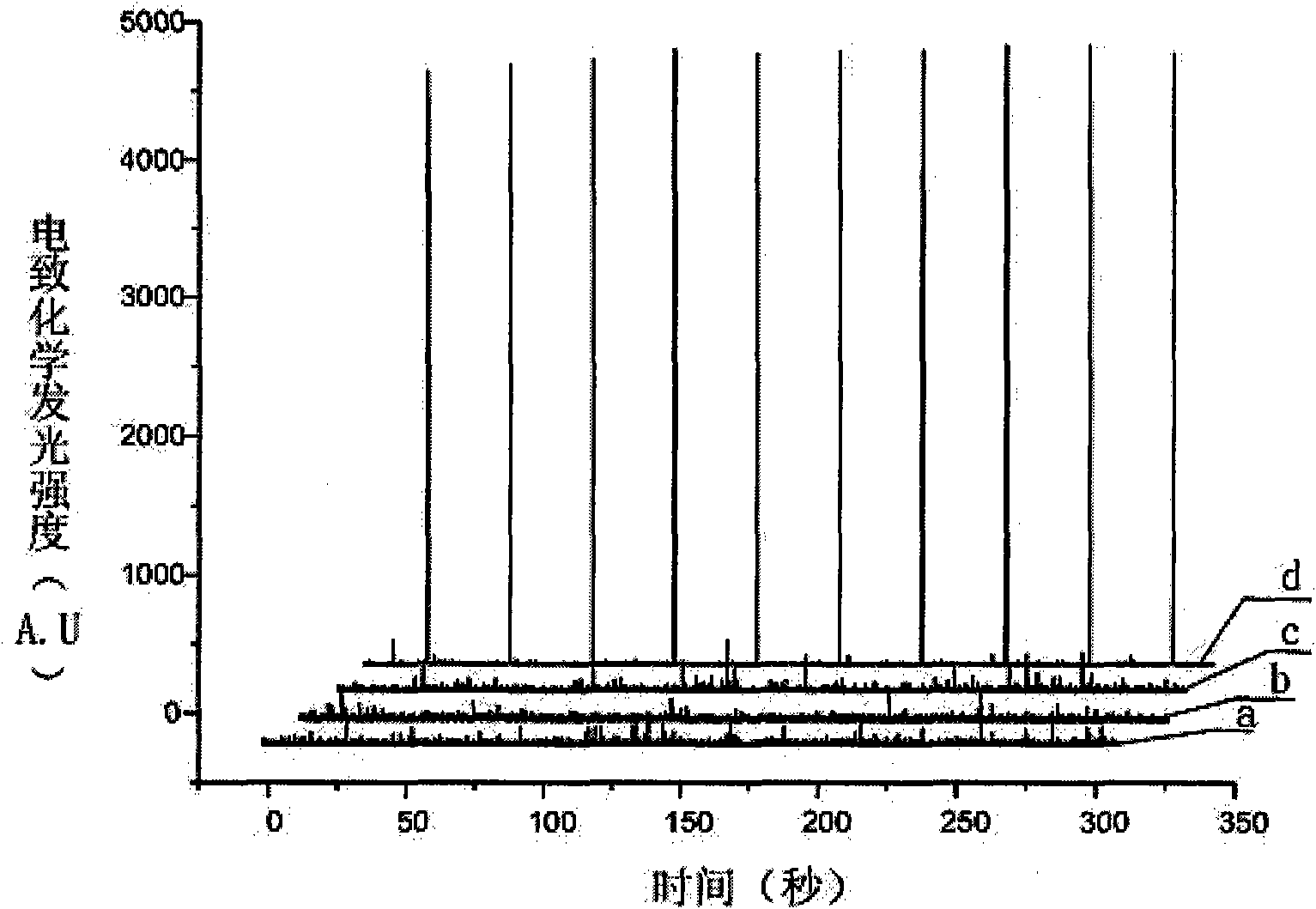
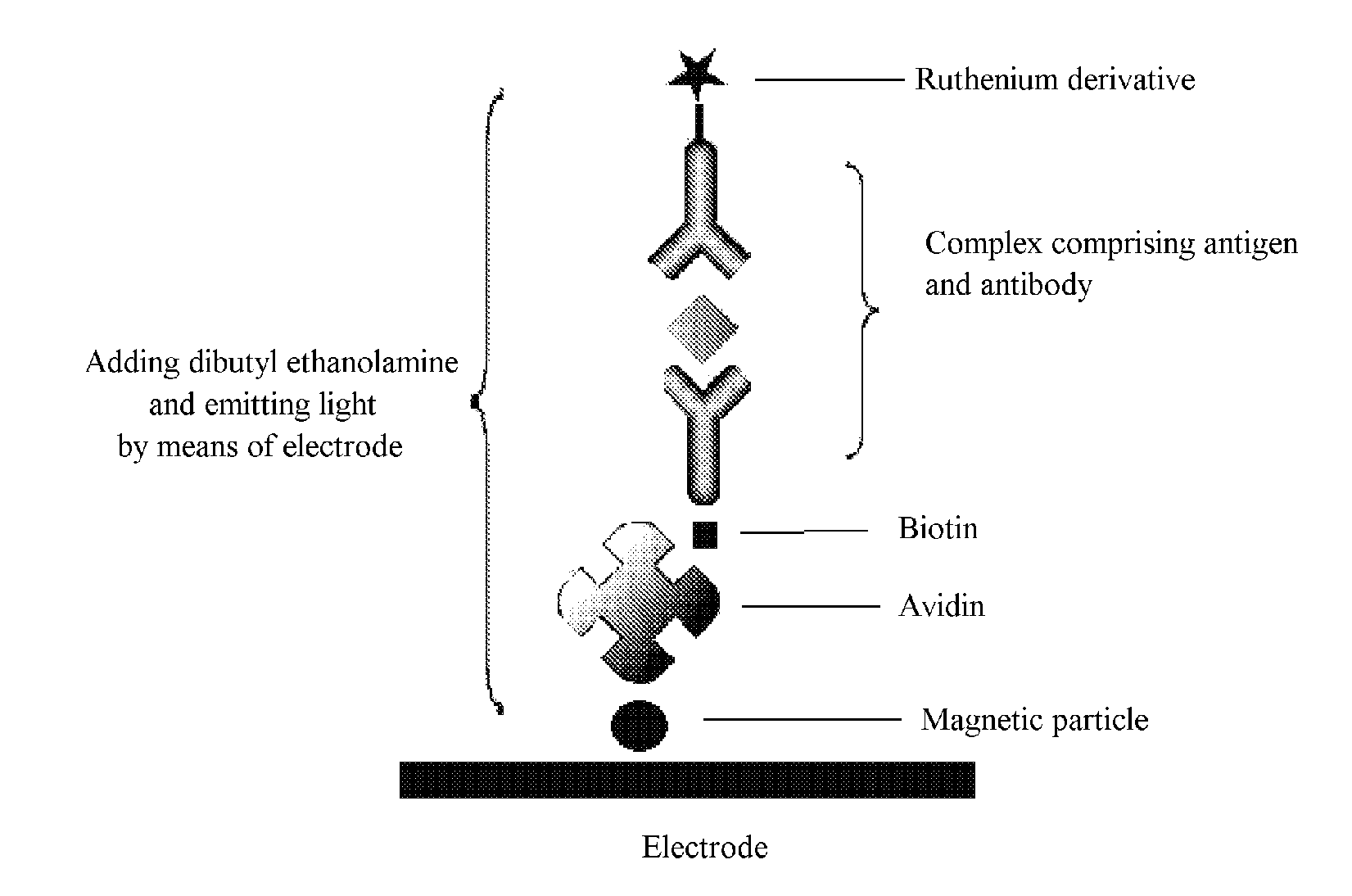
Electrochemiluminescence immunoassay method
ActiveUS20140072963A1Solve low luminous efficiencyRapid responseMicrobiological testing/measurementAzo dyesAntigenBiotin-streptavidin complex
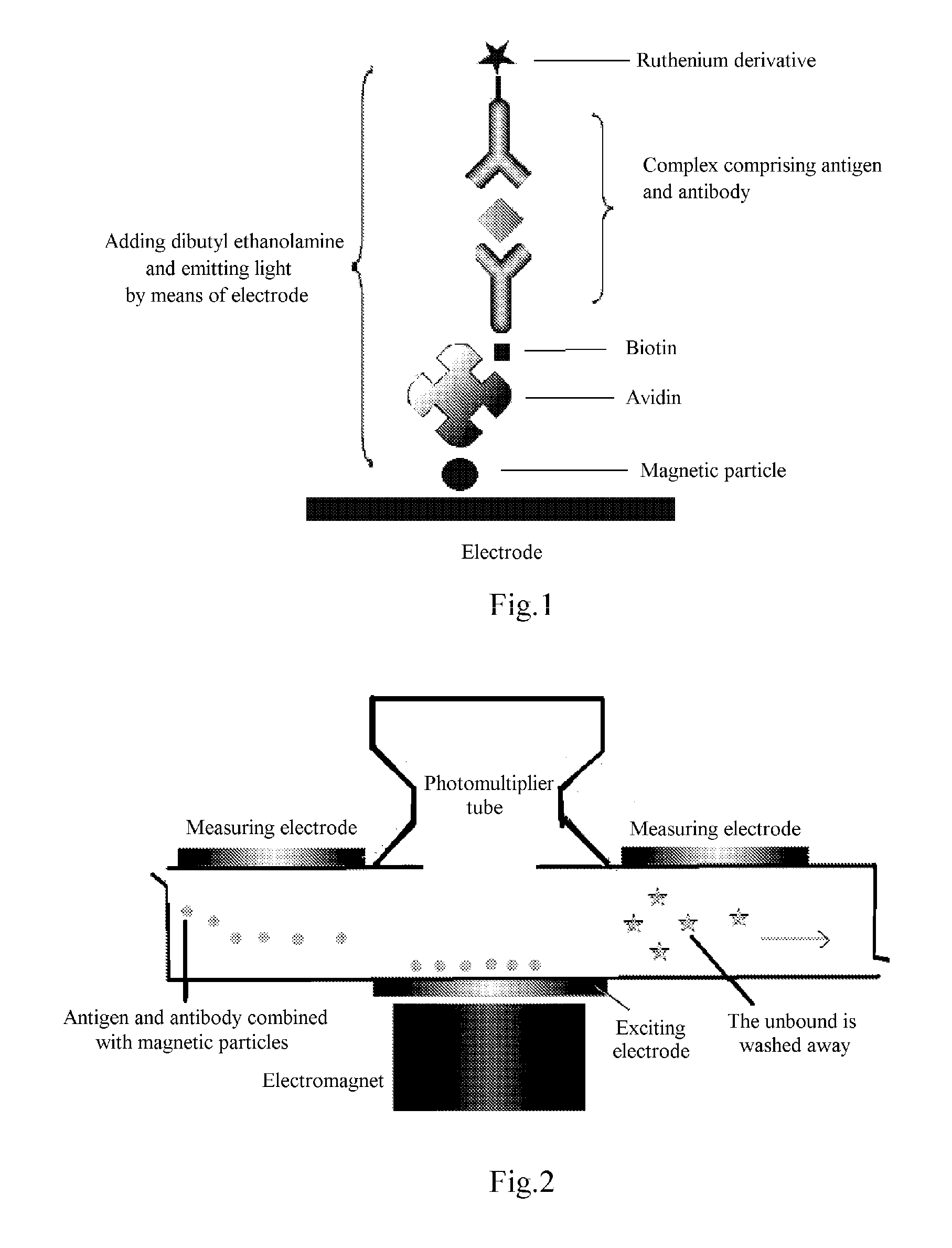
Disclosed is an electrochemiluminescence immunoassay method, using a full reaction of a Ru(bpy) marked protein-primary antibody, a biotinylated protein-secondary antibody to be tested, and a sample to be tested; addition of a Streptavidin-coated magnetic particle to form a complex comprising an antigen, an antibody, and a magnetic particle; adsorption to an electrode surface by the magnetic particle; addition of a dibutyl ethanolamine solution; testing by means of an electrochemical method. Also disclosed is a corresponding electrochemiluminescence immunoassay detection kit.
Owner:BEIJING UNIDIAG TECH
Capillary for immunoassay, and capillary immunoassay method using same
InactiveUS20110262940A1Delayed reaction timeHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsHydrophilic polymersAntibody conjugate
A capillary for an immunoassay is provided which comprises an insoluble layer of an oxidase formed on an inner wall surface of said capillary, said oxidase being conjugated to a first antibody, and a layer of a hydrophilic polymer formed on said insoluble layer, said hydrophilic polymer layer containing a second antibody conjugated to a peroxidase, wherein said first and second antibodies are capable of binding to the same antigen.
Owner:OSAKA PREFECTURE UNIV PUBLIC CORP
Oscillating immunoassay method and device
ActiveUS20120034624A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careAnalyte
Owner:ABBOTT POINT CARE
Method for pretreating sample and method for immunoassay of hcv
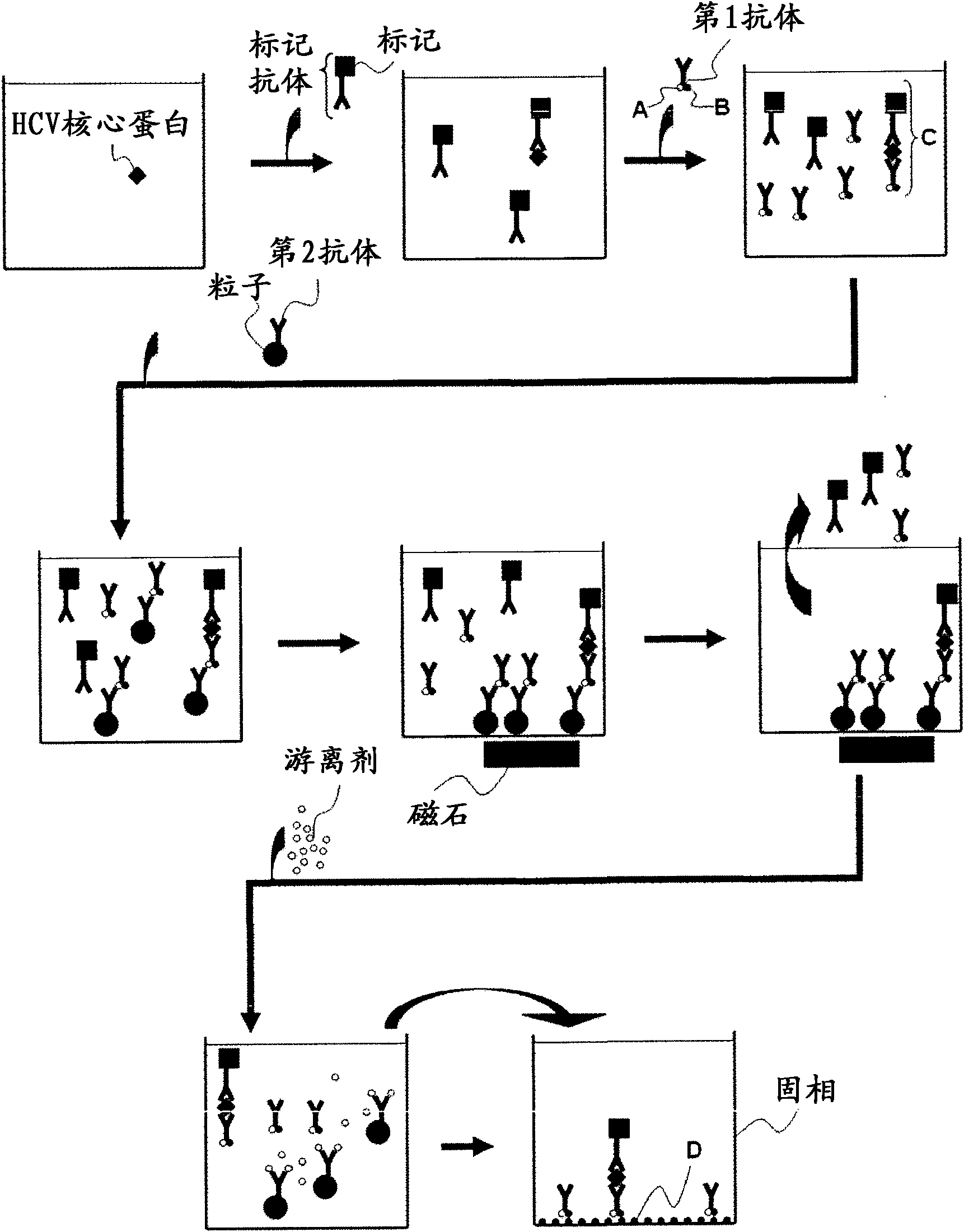
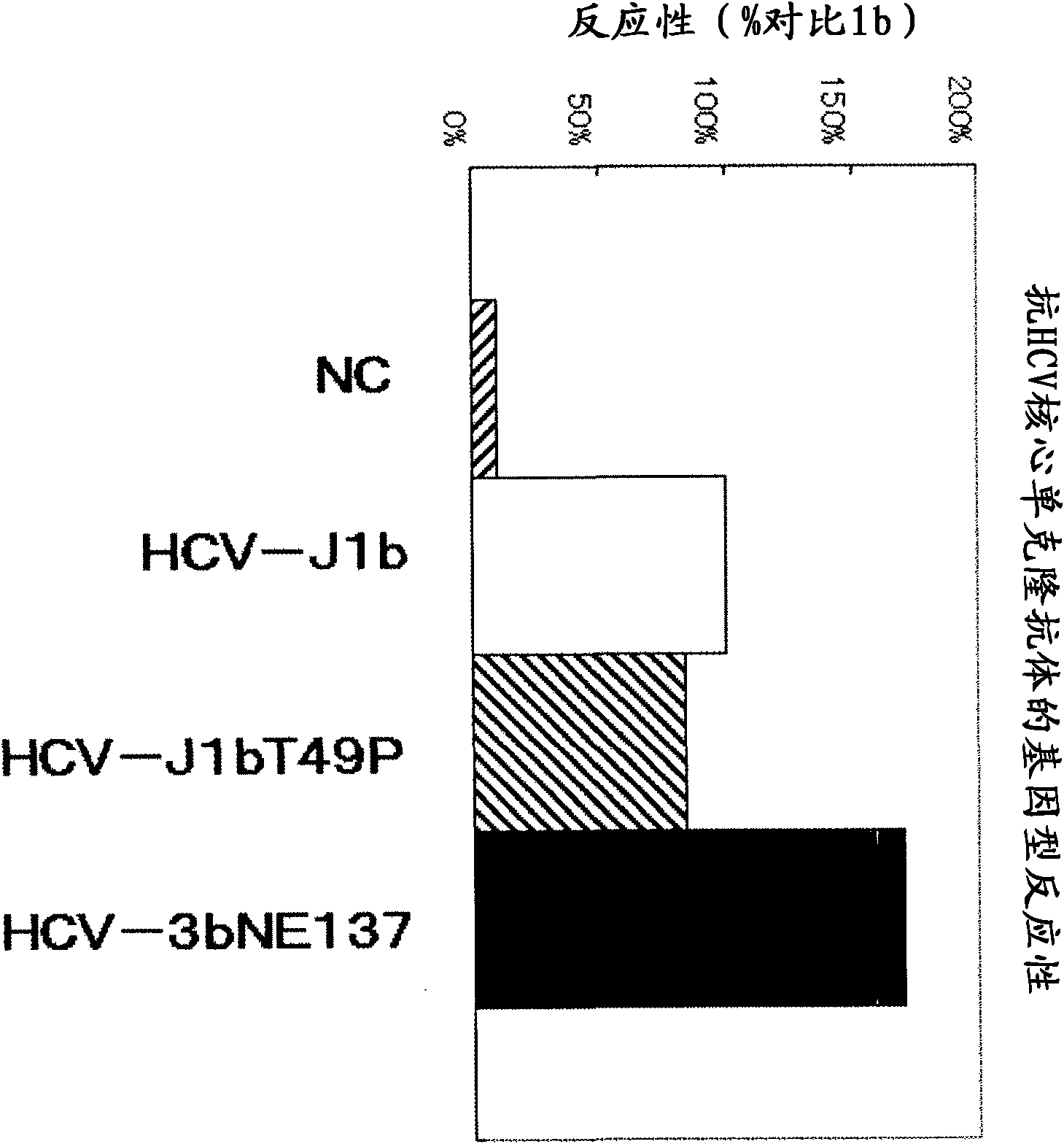
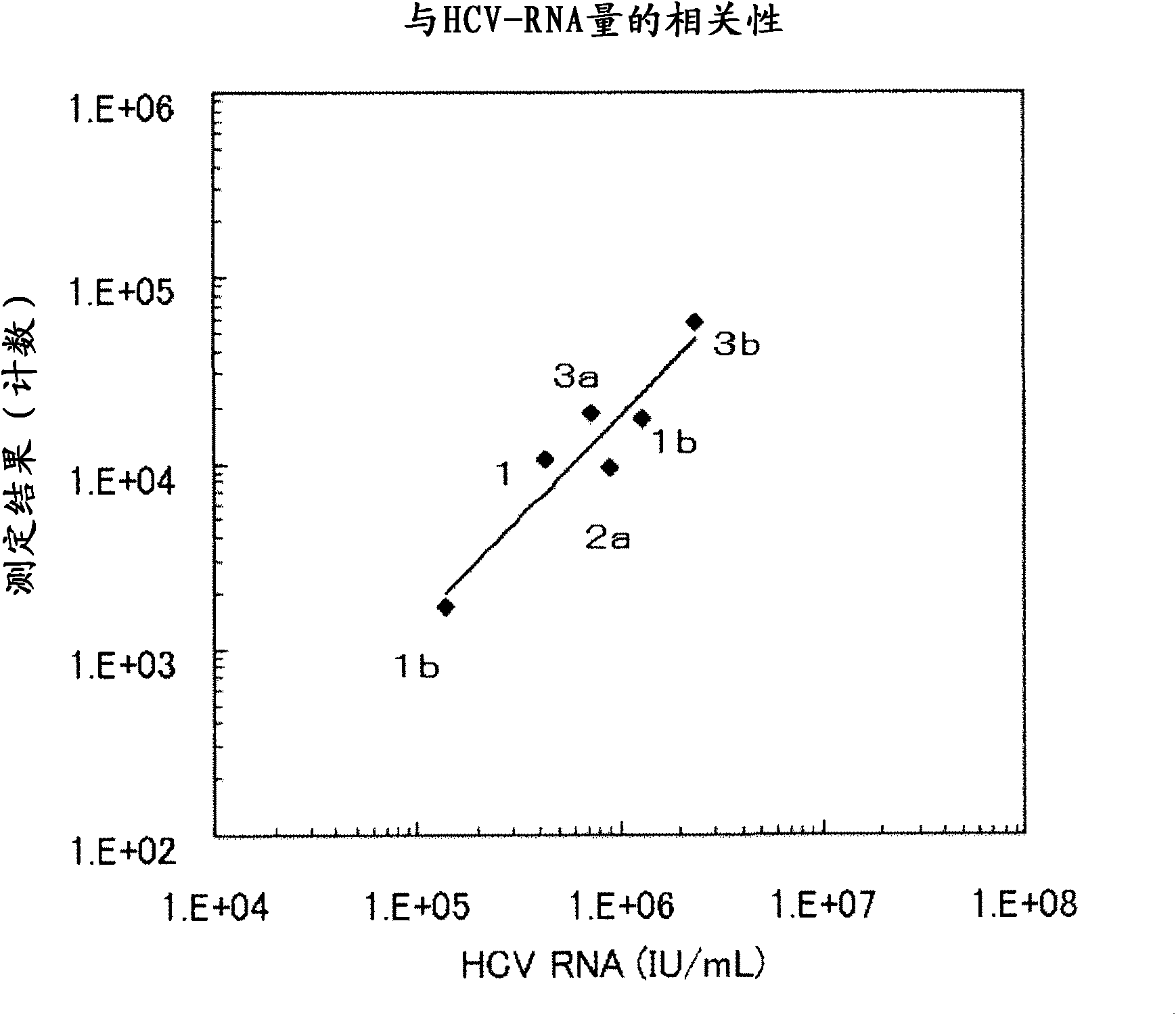
InactiveCN102081018APrevent agglutinationAgglutination does not occurPreparing sample for investigationTissue cultureProtein detectionVirus
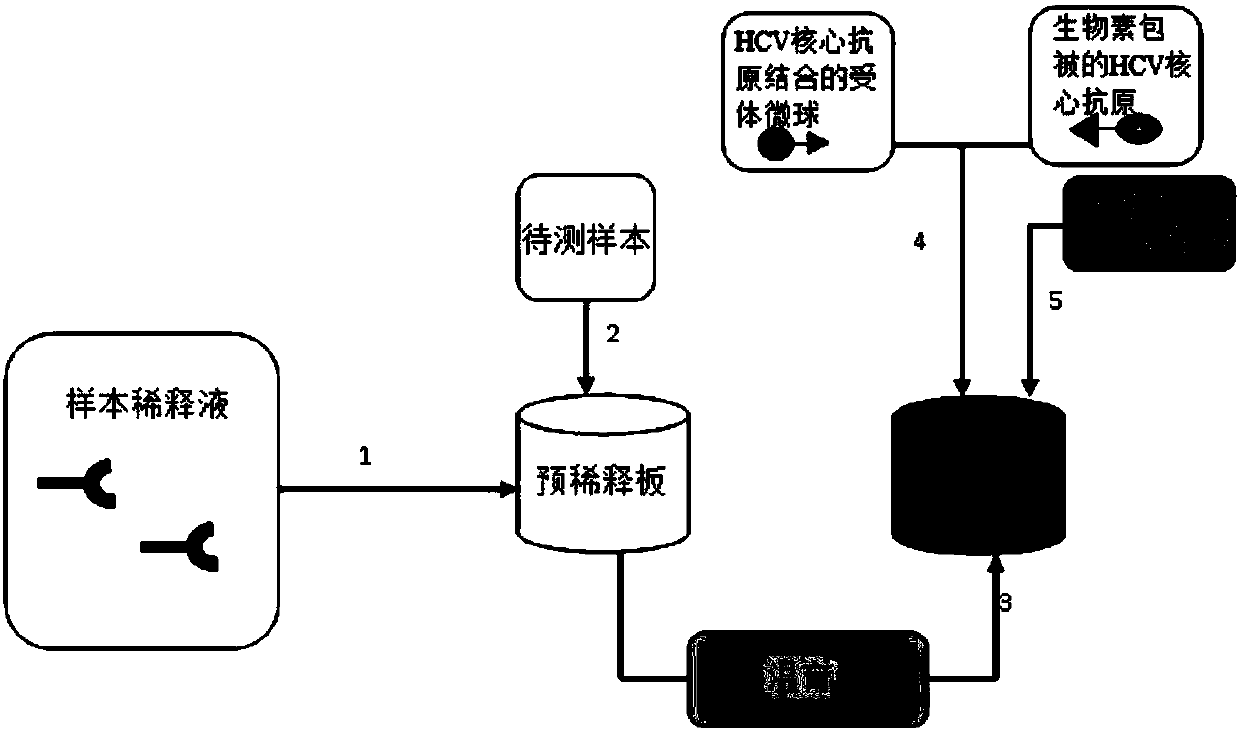
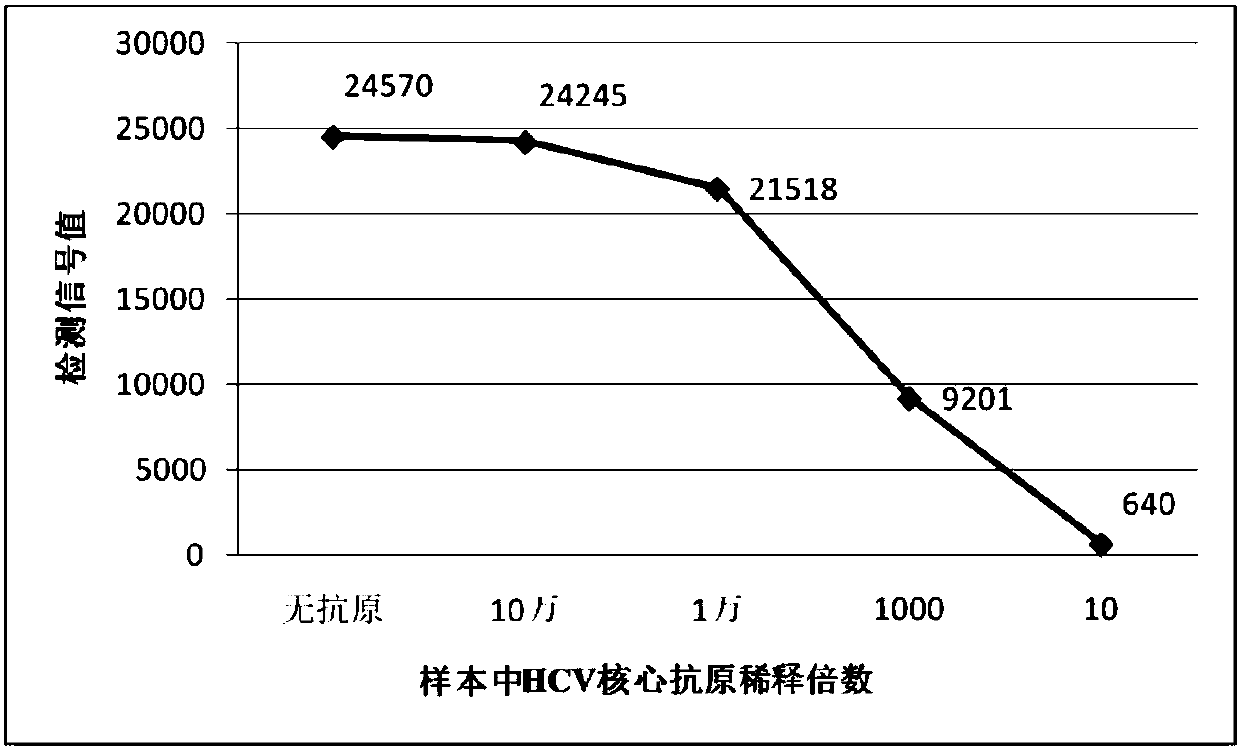
There are provided: a method for pretreating a sample for HCV core protein detection by an immunoassay using particles, which includes treating a sample suspected of containing hepatitis C virus (HCV) with an alkaline material-containing reagent and neutralizing the sample with an acid material-containing reagent, wherein at least one of the reagents contains a reducing agent; a reagent kit for HCV core protein detection; a method for determining the presence or absence of hepatitis C virus in a sample; and a method for immunoassay of HCV.
Owner:SYSMEX CORP
Ratiometric Immunoassay Method and Blood Testing Device
InactiveUS20120142025A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careAnalyte
The invention is to devices and methods for rapid determination of analytes in liquid samples. The devices and methods incorporate a sample dilution feature and multiple immunosensors for performing a ratiometric immunoassay on a first analyte and a second analyte, for example, hemoglobin and hemoglobin A1c or albumin and glycosylated albumin. The devices are preferably capable of being used in the point-of-care diagnostic field.
Owner:ABBOTT POINT CARE
Method for preparing polyclonal antibody against mouse nerve growth factor (NGF) and application thereof
ActiveCN101633694AHigh potencyImmunoglobulins against growth factorsPeptide preparation methodsMouse Submandibular GlandPolyclonal antibodies
The invention discloses a method for preparing polyclonal antibody against mouse nerve growth factor (NGF) and application thereof in an enzyme-linked immunoassay method. The polyclonal antibody which is prepared by using mouse submandibular gland NGF as an immunogen can be used for quantitatively detecting the mouse growth factor. Therefore, an assembled kit can determine fixed position and expression of different tissue cell nerve growth factors and detect NGF contents in different samples such as blood, cells, tissues, culture medium supernatant, and the like.
Owner:SINOBIOWAY BIOMEDICINE
Lateral flow assay and device using magnetic particles
A complex including magnetic particle bound to a metal colloid. The complex may be part of a reagent for use in a method for determining analytes. The reagent may include a binding partner specific for an analyte. The reagent may further include a first label that is distinguishable from a second label that is used to detect the analyte. The reagent is used in kits and methods for detecting analytes in samples. The methods include immunoassay methods, including method where the first label is used to calibrate the assay.
Owner:IDEXX LABORATORIES
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com