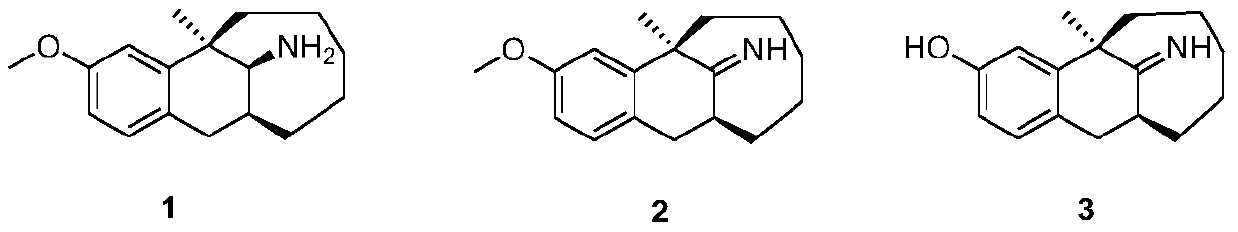
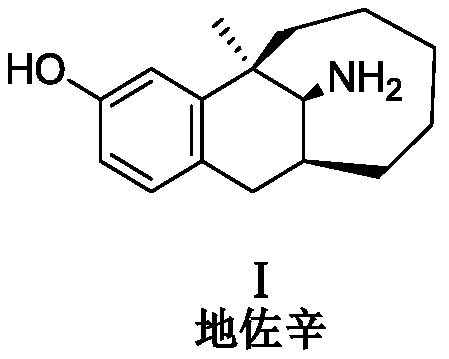
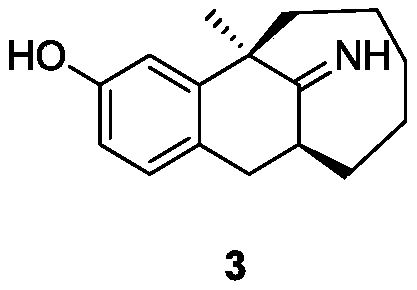
Preparation method of dezocine impurities
A technology of dezocine and impurities, applied in the field of medicine, can solve the problem of no standard product sales, etc., and achieve the effects of improving clinical drug safety, high yield and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Formula 1 compound (5.0g, 19.3mmol) and 60ml of 1,2-dichloroethane were added to a 250ml reaction flask, and N-chlorosuccinimide (3.8g, 28.95mmol) was slowly added dropwise at room temperature, stirred Reaction 3h. DBU (2 mL) was added, the temperature was raised to 40°C-50°C, and the reaction was completed after 12 hours of reaction. Add water (75mL) to the reaction flask, filter with a Buchner filter, remove the solids, wash with saturated brine, dry and spin dry to obtain the crude compound of formula 2 (4.8g, light yellow solid), which can be used directly without further purification react in the next step.
[0048] Dissolve the compound of formula 2 (4.8g) in dichloromethane (55mL), slowly add aluminum chloride (5.13g, 38.6mmol), then dropwise add thiophenol (4.24g, 38.6mmol), and heat up to 30-350°C, keep warm for 6h. Cool the reaction system in an ice bath to about 0°C, slowly add ammonia water dropwise to quench the reaction until the pH>7, keep the system t...
Embodiment 2
[0051] The compound of formula 1 (5.0g, 19.3mmol) and 60ml of 1,2-dichloroethane were added to a 250ml reaction flask, and N-bromosuccinimide (4.05g, 23.16mmol) was slowly added dropwise at room temperature, stirred Reaction 2h. Triethylamine (2 mL) was added, the temperature was raised to 50°C-60°C, and the reaction ended after 18 hours of reaction. Add water (75mL) to the reaction flask, filter with a Buchner filter, remove the solid matter, wash with saturated brine, dry and spin dry to obtain the crude compound of formula 2 (4.9g, light yellow solid), which is used directly without further purification react in the next step.
[0052] The compound of formula 2 (4.9 g) was dissolved in dichloromethane (55 mL), and aluminum chloride (7.7 g, 57.9 mmol) was added slowly, followed by ethanethiol (3.58 g, 57.9 mmol). After the addition, the temperature was raised to 30-35°C, and the reaction was kept for 4 hours. Cool the reaction system in an ice bath to about 0°C, slowly ad...
Embodiment 3
[0054] Formula 1 compound (5.0g, 19.3mmol) and 80ml of 1,2-dichloroethane were added to a 250ml reaction flask, and N-iodosuccinimide (7.3g, 32.81mmol) was slowly added dropwise at room temperature, stirred Reaction 1.5h. Pyridine (2 mL) was added, the temperature was raised to 50°C-60°C, and the reaction was completed after 18 hours of reaction. Add water (100mL) to the reaction flask, filter with a Buchner filter, remove the solid matter, wash with saturated brine, dry and spin dry to obtain the crude compound of formula 2 (4.7g, light yellow solid), which is used directly without further purification react in the next step.
[0055] The compound of formula 2 (4.7g) was dissolved in 1,2-dichloroethane (55mL), ethanethiol (3.58g, 57.9mmol) was added, and aluminum chloride (7.7g, 57.9mmol) was added slowly. After the addition, the temperature was raised to 35-40°C, and the reaction was kept for 2 hours. Cool the reaction system in an ice bath to about 0°C, slowly add ammoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com