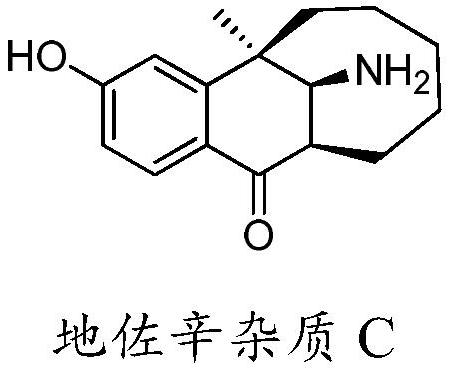
A kind of preparation method of dezocine impurity c
A technology of dezocine and impurities, which is applied in the field of medicinal chemistry, can solve problems such as difficult preparations, and achieve the effect of simple and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
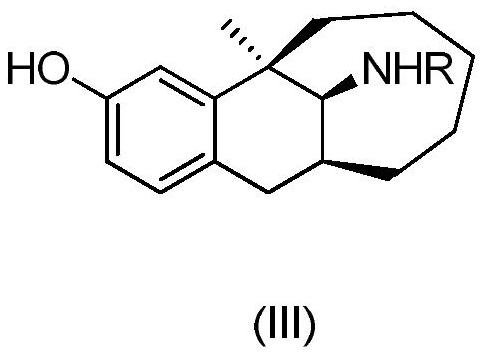
[0035] Synthesis of dezocine impurity C: dissolve dezocine impurity A (10.1 mg, namely R=H in formula (II)) in CH 2 Cl 2 (1mL), reduce the reaction temperature to -78°C with dry ice, add BF 3 (0.05mL, 33% ether solution, about 7.0 equivalents), after the addition, the reaction temperature was slowly raised to -20°C for 60 minutes, the reaction was stopped, and 5% NaHCO was added to the reaction solution 3 The aqueous solution is neutralized to neutral, the liquid is separated, and the aqueous phase is re-used with CH 2 Cl 2 (2 mL) was extracted twice, the organic phases were combined and then column chromatographed to obtain 5.3 mg of dezocine impurity C (formula I) as a white solid with a yield of 52%. The ratio of the amount (unit: mL) of the reaction solvent dichloromethane to the amount (unit: g) of dezocine impurity A is about 100:1. H NMR spectrum (DMSO-d 6 ,500MHz)δ8.29(s,1H),7.83(d,J=8.5Hz,1H),6.83(d,J=2.0Hz,1H),6.77(dd,J=8.5,2.0Hz,1H), 3.40(d,J=7.5Hz,1H),2.85(dd...
Embodiment 2
[0037] Synthesis of dezocine impurity C: dissolve dezocine impurity A (10.2 mg, ie R=H in formula (II)) in 10% sulfuric acid aqueous solution (0.2 mL, H 2 SO 4 The equivalent of about 6.0), after 5 minutes, dilute the reaction solution with 2mL of ice water, wash with 5% NaHCO 3 The aqueous solution is neutralized to neutral, adding CH 2 Cl 2 (5mL) was extracted twice, and the organic phases were combined and subjected to column chromatography to obtain 4.6mg of dezocine impurity C, a white solid, with a yield of 45%. The ratio of the amount (unit: mL) of the reaction solvent sulfuric acid aqueous solution to the amount (unit: g) of dezocine impurity A is about 20:1. The characterization data of dezocine impurity C are the same as in Example 1.
Embodiment 3
[0039] Synthesis of Dezocine Impurity C: Dissolve Dezocine Impurity A (30.4 mg, ie R=H in formula (II)) in dioxane (1 mL), add ZnO (0.1 g, 10.5 equivalents), and heat After reacting at 90°C for 6 hours, it was concentrated to remove the solvent and separated by column chromatography to obtain 24.6 mg of dezocine impurity C (Formula I) as a white solid with a yield of 81%. The ratio of the amount (unit: mL) of the reaction solvent dioxane to the amount (unit: g) of dezocine impurity A is 33:1. The characterization data of dezocine impurity C are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com