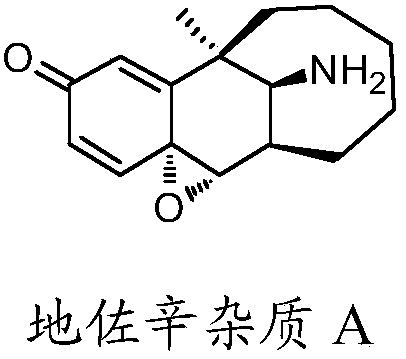
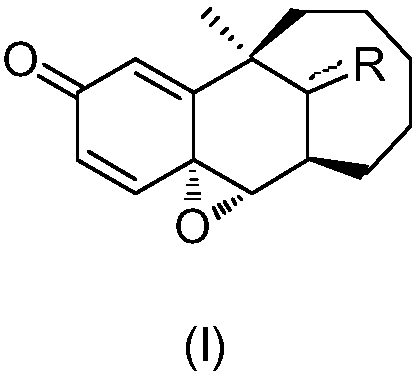
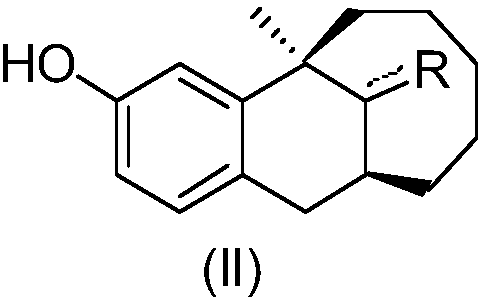
Preparation method of dezocine impurity A and homologues thereof
A technology of homologue, dezocine, applied in the direction of organic chemistry, etc., to achieve the effect of simple and easy-to-obtain reagents and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1 g (1.0 equivalent) of dezocine, 0.1 g (0.05 equivalent) of scandium trifluoromethanesulfonate, 1,8-diazabicyclo[5.4.0]-undeca-7 into a 25 mL single-necked round bottom bottle -ene 0.93g (1.5 equivalents), dichloromethane 10mL, 70% tert-butanol peroxide 2.62g (5.0 equivalents), and stirred at room temperature for 5h to stop the reaction. The reaction solution was diluted with 40 mL of dichloromethane, followed by 20 mL of 5% NaOH aqueous solution, 5% Na 2 S 2 o 3 Wash with 20 mL of aqueous solution, separate the liquids, and wash the organic phase with anhydrous Na 2 SO4 2 g was dried, concentrated to dryness under reduced pressure, and the residue was separated by column chromatography to obtain 93 mg of dezocine impurity A as an off-white solid. The NaOH aqueous extract was adjusted to pH 7 with acetic acid, stirred in an ice-water bath for 30 min, filtered, and the filter cake was dried to recover 0.52 g of dezocine. The dezocine charging amount is 1.0g, th...
Embodiment 2
[0025] Add 20.0 g (1.0 equivalent) of dezocine, 2.3 g (0.1 equivalent) of titanium tetraisopropoxide, 6.0 g (0.5 equivalent) and 200 mL of dichloromethane into a 500 mL three-necked flask, and lower the temperature of the reaction solution to -10°C , slowly add 62.0 g (4.0 equivalents) of 80% cumene hydroperoxide dropwise for 2.0 h. After the dropwise addition, the reaction temperature is slowly raised to room temperature for 3 h, and then the reaction temperature is raised to 75° C. for 1 h. Stop the reaction, extract the reaction solution with 70 mL of 5% NaOH aqueous solution, filter the insoluble matter, separate the liquids, extract the organic phase twice with 5% NaOH aqueous solution, 5% NaOH 2 S 2 o 3 Extract once with 200mL of aqueous solution, once with 50mL of saturated saline, use starch-KI test paper to detect that there is no peroxide residue in the organic phase, separate the liquids, and wash the organic phase with anhydrous MgSO 4 5.0 g was dried, concentrat...
Embodiment 3
[0027] Add 2 g (1.0 equivalent) of dezocine (1.0 equivalent), 0.23 g (0.1 equivalent) of titanium tetraisopropoxide, 0.6 g (0.5 equivalent) of DBU, and 10 mL of isopropyl acetate into a 50 mL single port, and lower the temperature of the reaction solution to -10 ° C. Slowly add 6.2 g (4.0 equivalents) of 80% cumene hydroperoxide dropwise, and react at 60° C. for 3 h after the dropwise addition. Stop stirring, extract the reaction solution with 20mL of 5% NaOH aqueous solution, filter the insoluble matter, separate the liquids, and use 10mL of 5% NaOH aqueous solution, 5% NaOH solution for the organic phase successively 2 S 2 o 3 Wash with 30 mL of aqueous solution, use starch-KI test paper to detect that there is no peroxide residue in the organic phase, separate the liquids, concentrate the organic phase to dryness, and separate the residue by column chromatography to obtain 192 mg of dezocine impurity A. Combine the aqueous NaOH extracts, adjust the pH to 7 with acetic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com