A kind of preparation method of dezocine key intermediate
An intermediate, dezocine technology, applied in the field of medicinal chemistry, can solve the problems of high cost of raw materials and preparations, harsh reaction conditions, long synthesis cycle, etc., and achieve the effect of shortening the synthesis cycle, low cost of raw materials, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
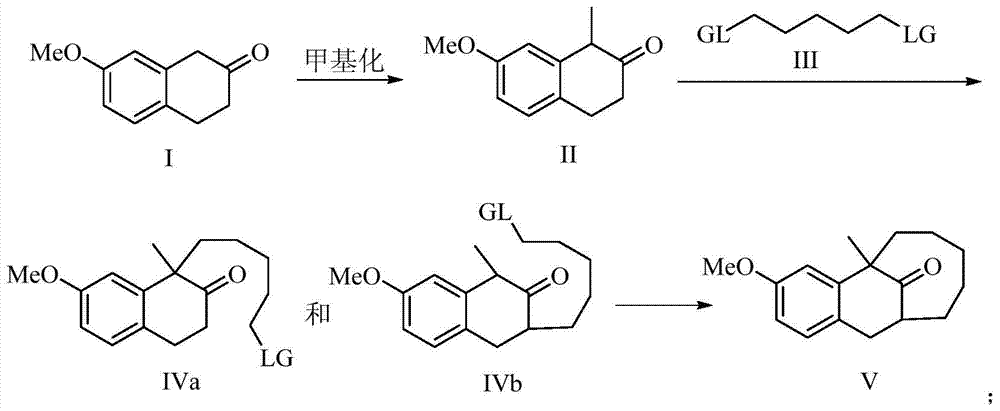
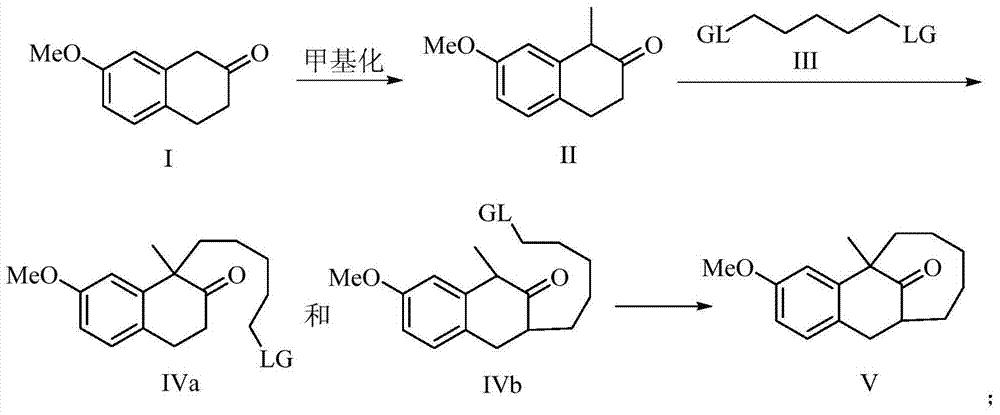
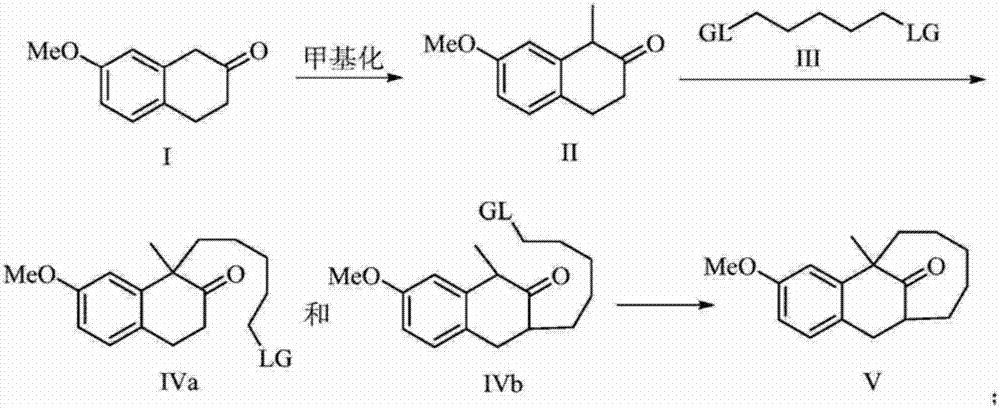
[0019] The preparation method of dezocine key intermediate V of the present invention, its synthetic route is as follows:
[0020]
[0021] Using 7-methoxy-2-tetralone as a raw material, first methylate at the benzylic position, and then carry out alkylation at the ortho position of the carbonyl to obtain the key intermediate V.
Embodiment 1
[0022] Embodiment 1, preparation methylation product II
[0023] Dissolve 7-methoxy-2-tetralone I (17.6g, 0.10mol) in 30ml of dichloromethane, cool the reaction system to 0°C, and add N,N-diisopropyl to the reaction system in batches Ethylamine (13.6ml, 0.1mol), at this temperature, slowly add cooled methyl iodide (6.22ml, 0.1mol) dropwise into it over 20 minutes. After the dropwise addition, the temperature was naturally raised to room temperature, and the reaction was carried out at room temperature for 4 hours. After TLC spotting, the raw material spots basically disappeared. The pH was then adjusted to neutral with saturated ammonium chloride solution, followed by three extractions with 25 ml each of EtOAc. The combined organic phases were washed with saturated sodium chloride, dried over magnesium sulfate, concentrated, and purified by column chromatography to obtain 16.2 g of methylated product II with a yield of 85%.
Embodiment 2
[0024] Example 2, preparation of carbonyl unilateral alkylation products IVa and IVb
[0025] The methylated product II (15.2g, 80mmol) was dissolved in 35ml tetrahydrofuran (THF), the reaction system was cooled to 0°C, and Et was slowly added to the reaction system 3 N (22.2ml, 0.16mol), stirred at this temperature for 30 minutes. Then warm up to room temperature, add dibromopentane III (12.2ml, 88mmol) to the reaction system at this temperature, then heat up to 40-45°C, keep this temperature for 12 hours, monitor by TLC, the methylation raw material basically disappears Finally, the pH value was adjusted to be neutral with 1mol / L hydrochloric acid solution, and then extracted three times with 90ml of ethyl acetate. The combined organic phases were washed with saturated sodium chloride, dried over sodium sulfate, concentrated under reduced pressure, and passed through a column to obtain carbonyl unilaterally alkylated products IVa and IVb. The combined mass is 22.4g, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com