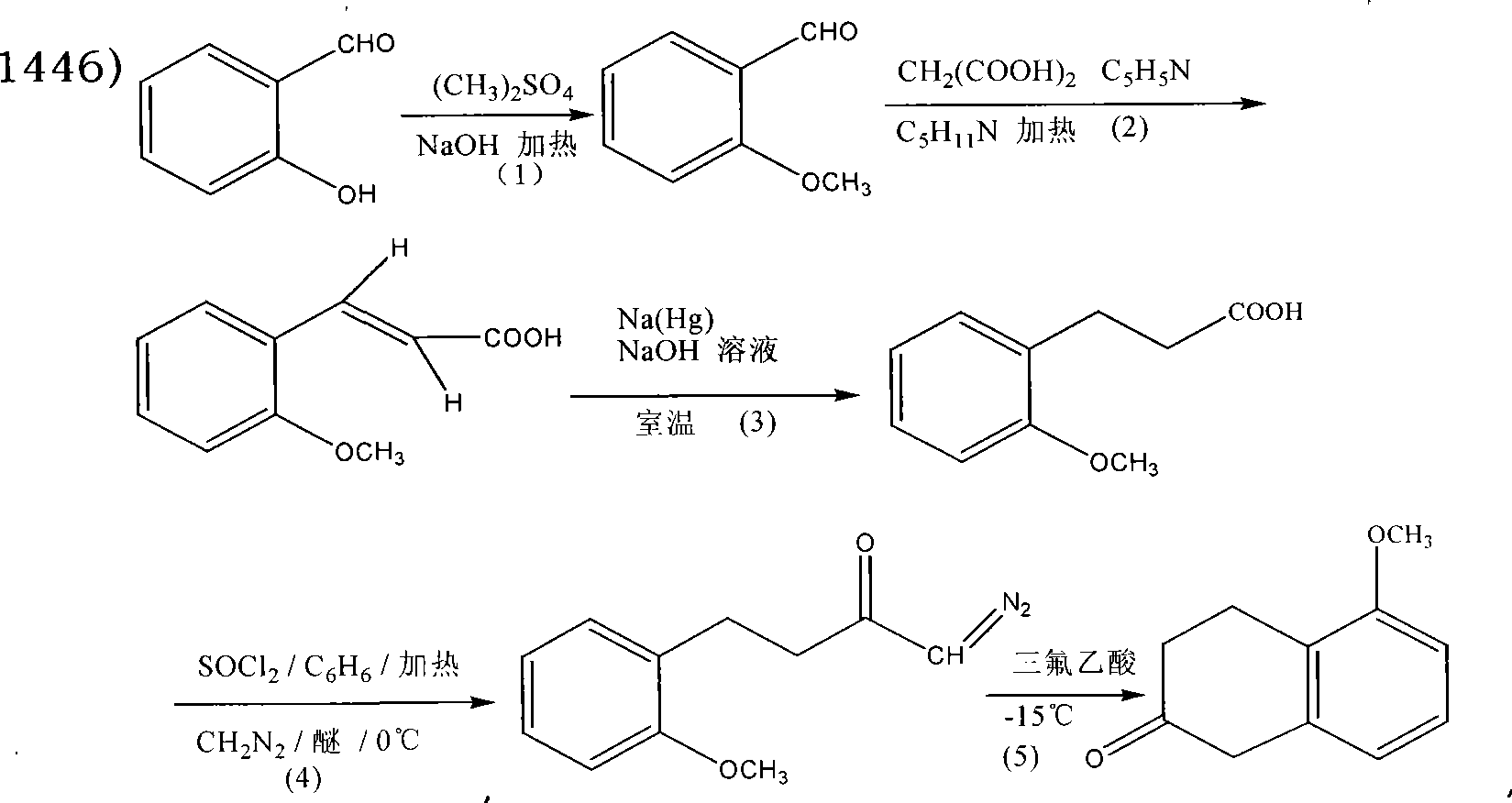
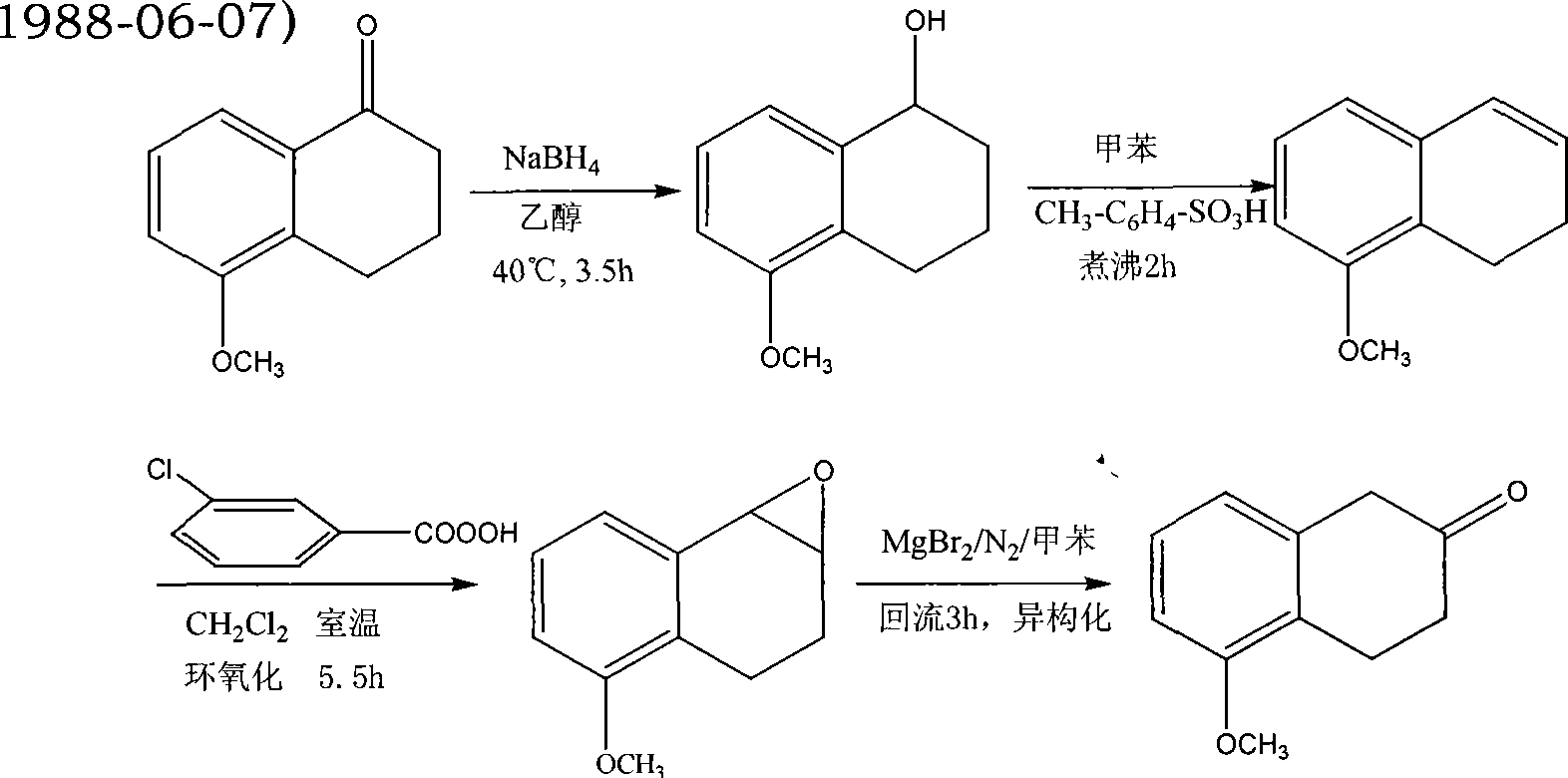
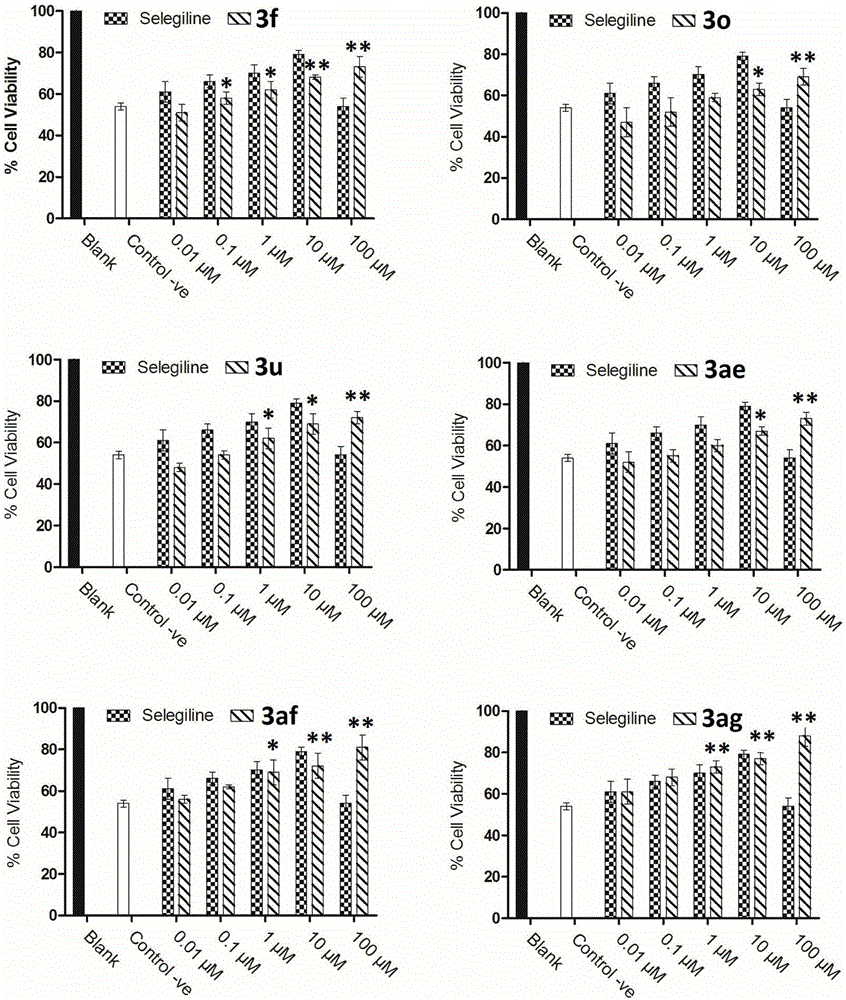
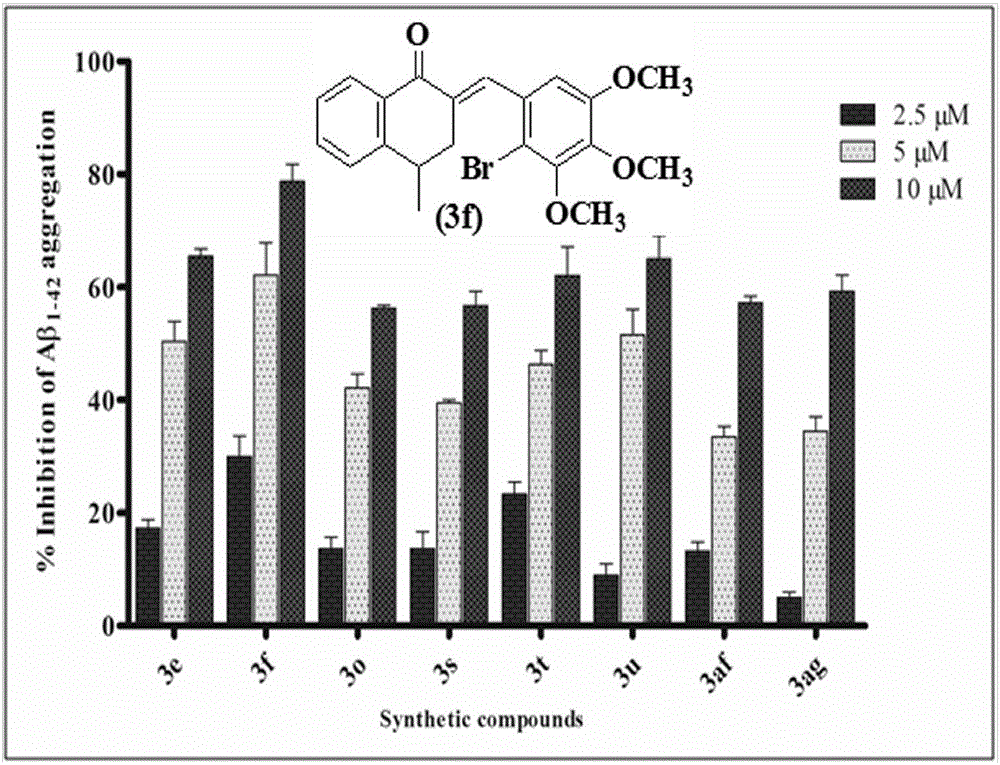
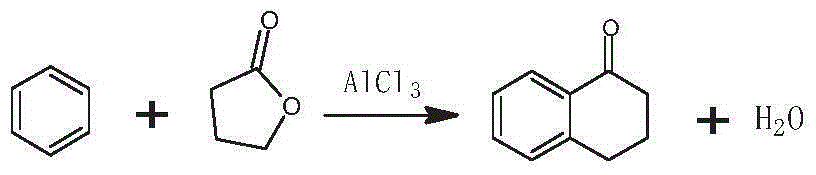Patents
Literature
94 results about "Tetralone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetralone may refer to either of two chemical isomers: 1-Tetralone 2-Tetralone
Preparation method of meso-porous alumina and catalytic synthesis of alpha-tetralone
InactiveCN101337186AEasy to recycleImprove conversion rateCarbonyl compound preparation by oxidationMetal/metal-oxides/metal-hydroxide catalystsTetraloneHydrogen
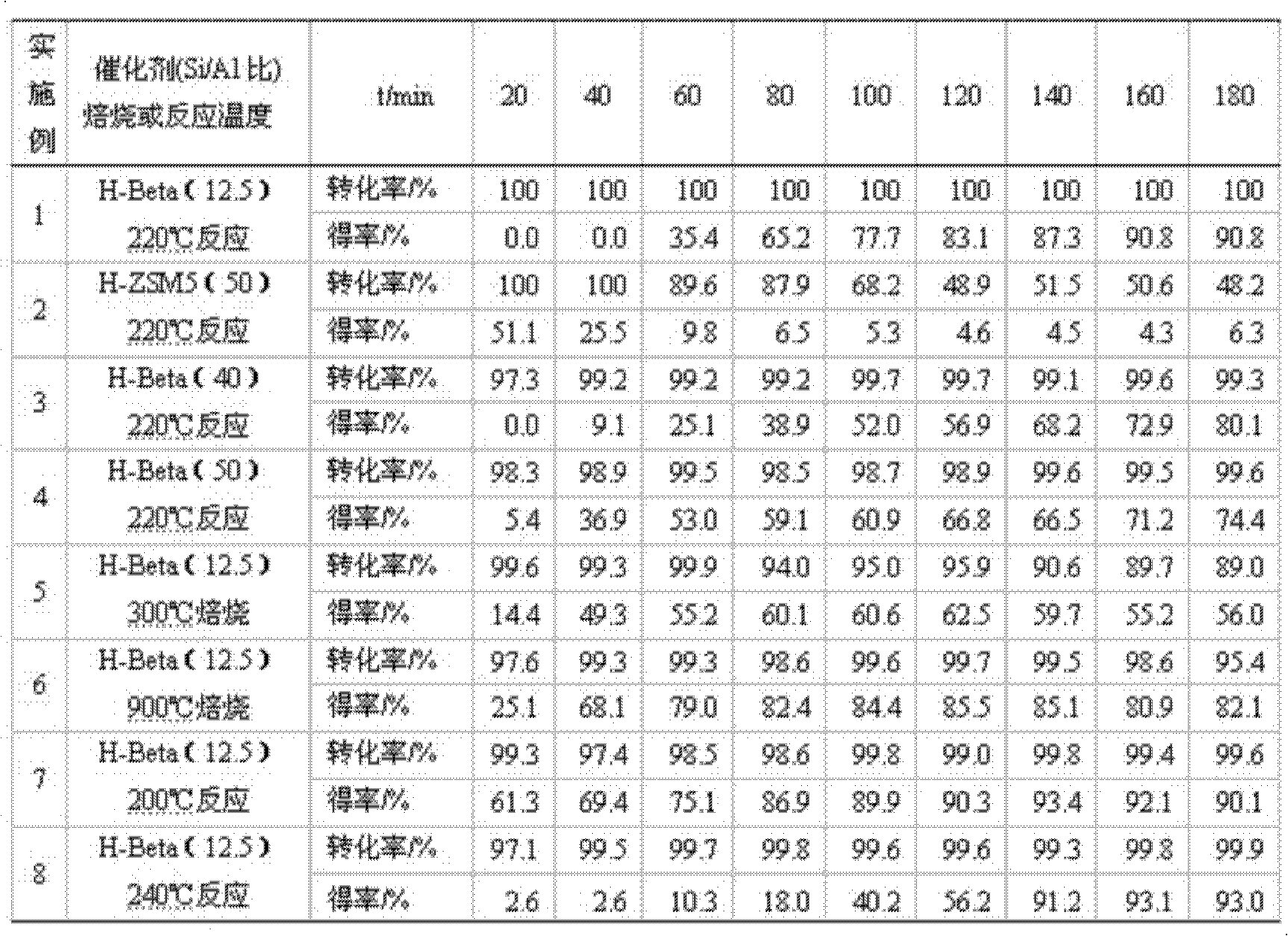
The invention provides a method for preparing cobalt-doped mesoporous alumina by taking a rubber latex as a biological template, and a method for catalytically oxidizing tetralin into tetralone under liquid phase. The invention relates to the method for preparing the cobalt-doped mesoporous alumina by taking the rubber latex as the template, and the method for synthesizing tetralone. The invention aims to develop a method which is used for preparing the cobalt-doped mesoporous alumina by taking the rubber latex as the biological template and a high-conversion and high-selectivity method which is used for catalytically oxidizing tetralin into tetralone under the liquid phase, and comprises the following steps: a mesoporous material is taken as a heterogeneous catalyst, and tetralin is catalytically oxidized into tetralone under the low temperature liquid phase. The experimental results show that the conversion rate of the tetralin reaches 80.3 percent and the selectivity of tetralone reaches 74.5 percent.
Owner:YUNNAN UNIV
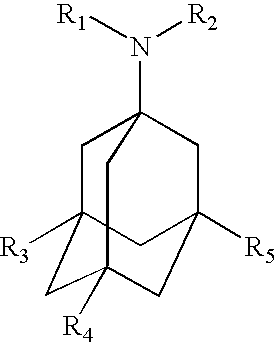
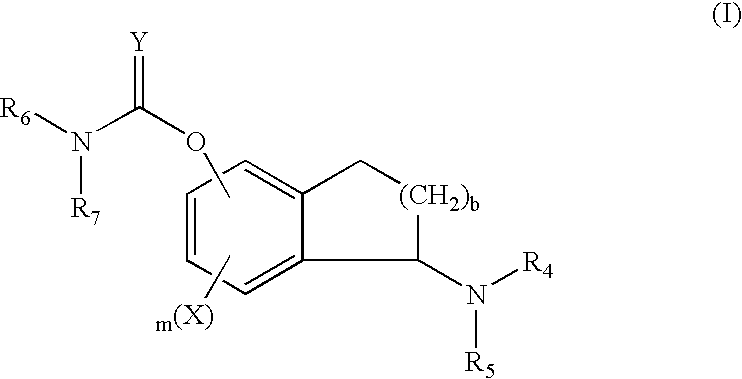
Cyclic amine derivative or salt thereof
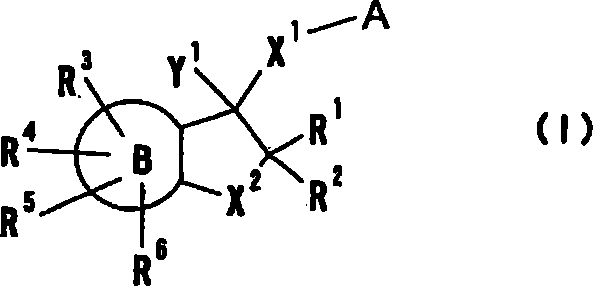
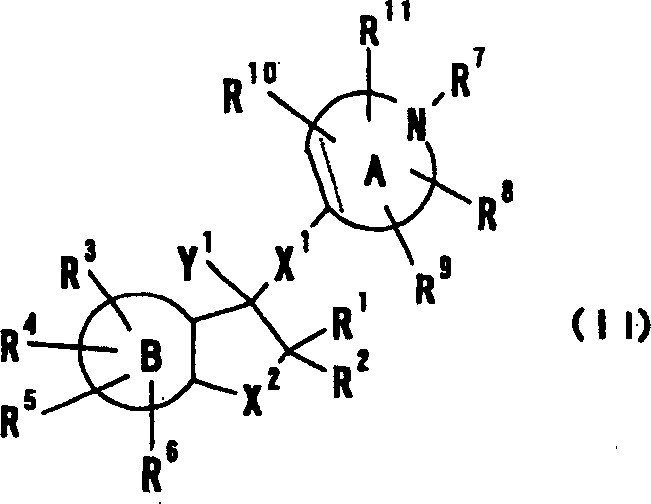
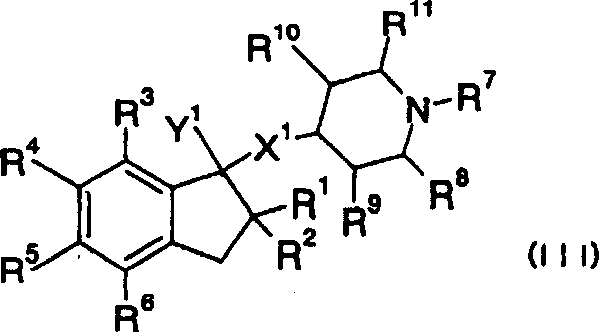
Provided are compounds which are an NMDA antagonist having a broad safety margin and are useful as a treating agent or a preventing agent for Alzheimer's disease, cerebrovascular dementia, Parkinson's disease, ischemic apoplexy, pain, etc. Concretely provided are an amine derivative or its salt characterized in that the amine-containing structure A therein bonds to a 2- or 3-cyclic condensed ring (e.g., indane, tetralone, 4,5,6,7-tetrahydrobenzothiophene, 4,5,6,7-tetrahydrobenzofuran, 7,8-dihydro-6H-indeno[4,5-b]furan, 2,3-dihydro-1H-cyclopenta[1]naphthalene) via X1 (bond or lower alkylene); and an NMDA antagonist containing it as an active ingredient thereof.
Owner:ASTELLAS PHARMA INC
Tetralone-based monoamine reuptake inhibitors
ActiveUS20070197588A1Increase synaptic availabilityImprove usabilityBiocideNervous disorderSynaptic cleftTetralone
The invention relates to novel tetralone based amines and their use in the treatment of central nervous system (CNS) disorders, such as depression, attention deficit hyperactivity disorder (ADHD) and Parkinson's disease. The invention further relates to pharmaceutical compositions containing the compounds and compositions of the invention as well as methods of inhibiting reuptake of one or more monoamine, such as such as dopamine and norepinephrine, from the synaptic cleft, and methods of modulating one or more monoamine transporter.
Owner:SUNOVION PHARMA INC
Polyamide-imide insulating varnish and preparation method thereof
The invention relates to a polyamide-imide insulating varnish. The polyamide-imide insulating varnish is characterized in that the varnish comprises the following components in parts by weight: 100-120 parts of polyamide-imide solution and 2-5 parts of sepiolite material, wherein the polyamide-imide solution mainly comprises the following components in parts by weight: 20-35 parts of polyamide-imide resin and 20-60 parts of organic solvent; the organic solvent is one or a combination of N-methylpyrrolidone, chlorobenzene, bromobenzene, dichlorobenzene, dibromobenzene, 1-tetralone, fenchone, phorone and isophorone; and the sepiolite material mainly comprises sepiolite. The polyamide-imide insulated enamel wire provided by the invention forms a homogeneous stable system, the surface friction coefficient is below 0.30, and each index meets the International Electrotechnical Commission (IEC) standard requirement; and the polyamide-imide insulating varnish is suitable to be used as the enamel wire insulating varnish for coating the surface of the copper wire.
Owner:GUANGDONG JINGDA REA SPECIAL ENAMELED WIRE CO LTD
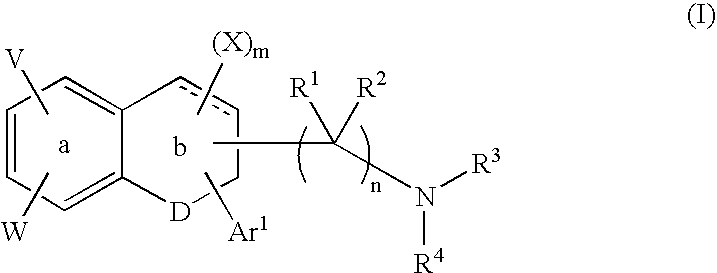
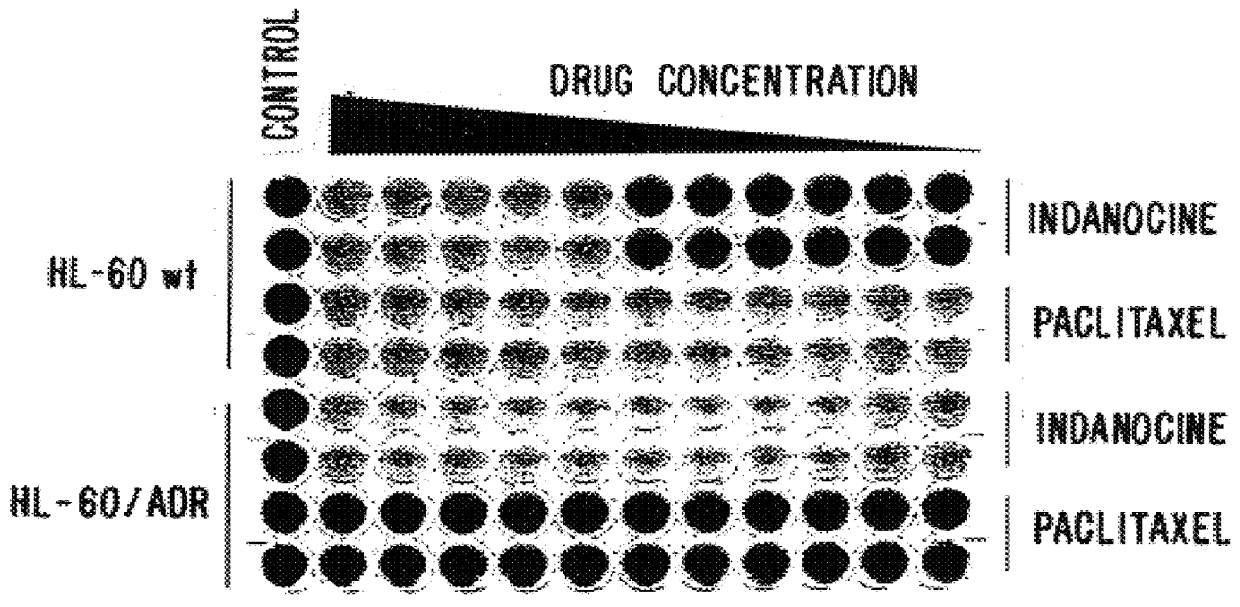
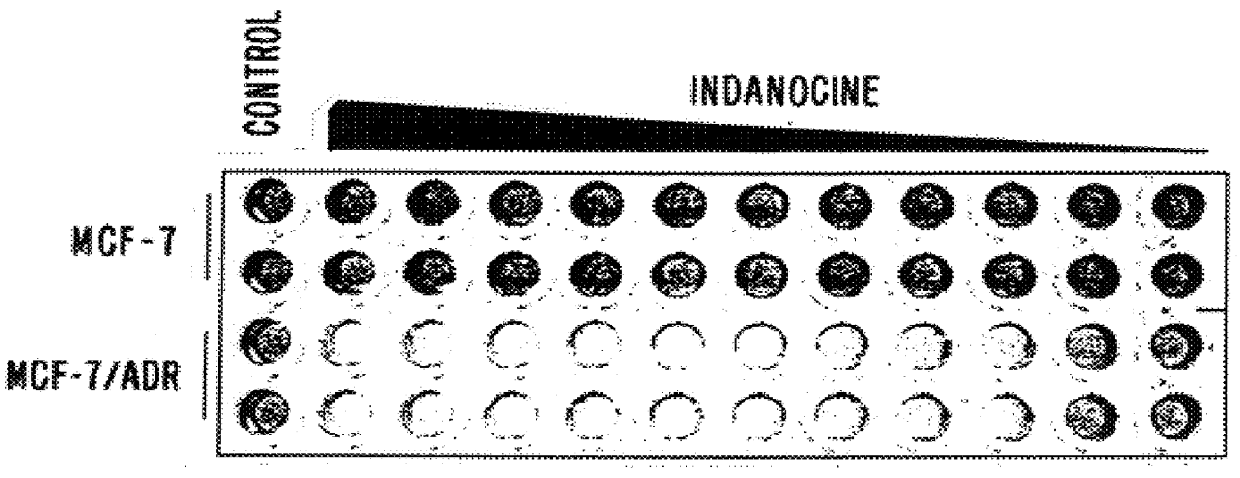
Indanone and tetralone compounds for inhibiting cell proliferation
A new family of indanone and tetralone tubulin-binding compounds (TBs) is disclosed. Unlike classical TBs, which inhibit mitosis among affected dividing cells, the TBs of the invention possess two unique properties: (1) they induce apoptosis among stationary phase (non-dividing) malignant cells, yet do not impair the viability of normal nonproliferating cells; and, (2) they affect cells which have acquired MDR more powerfully than they affect cells without MDR. Thus, the TBs of the invention provide means to target malignant cells for chemotherapy, even after previous therapies have failed, without affecting normal cells and tissues in the host.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing alpha-tetralin ketone by catalyzed oxidation tetrahydronaphthalene
InactiveCN101177386AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetraloneTetralin
The invention relates to a method of preparing Alpha-tetralone by catalysis and oxidation of tetralin, comprising following steps: quinonyl compounds and N-hydroxyphthalimade are combined to be double-component non-metallic catalysis system; air or oxygen is used as oxygen source to prepare Alpha-tetralone through catalysis and oxidation of tetralin with high efficiency under a certain condition; the applied quinonyl compounds comprise organic compounds, such as benzoquinones, naphthoquinones and anthraquinones and substituted derivatives of the organic compounds. The invention has the advantages of mild preparation conditions for the method of preparing the Alpha-tetralone, high efficiency and environmental friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
New composite method for triptolide
ActiveCN101638426AMethod route shortEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsSteroidsChemical synthesisComposite field
The invention provides a new and effective method for asymmetrically compositing triptolide, belonging to the chemical composite field. The method takes 5-methoxyl-2-tetralone and 2-(2-iodine ethyl)-1, 4-butyrolactone as initial materials and composites the triptolide by chiral induction. The method is brief and convenient in operation and has high yield. As the reaction condition is mild, the composite method is easily industrialized and is further used for large-scale synthesis of target compounds.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
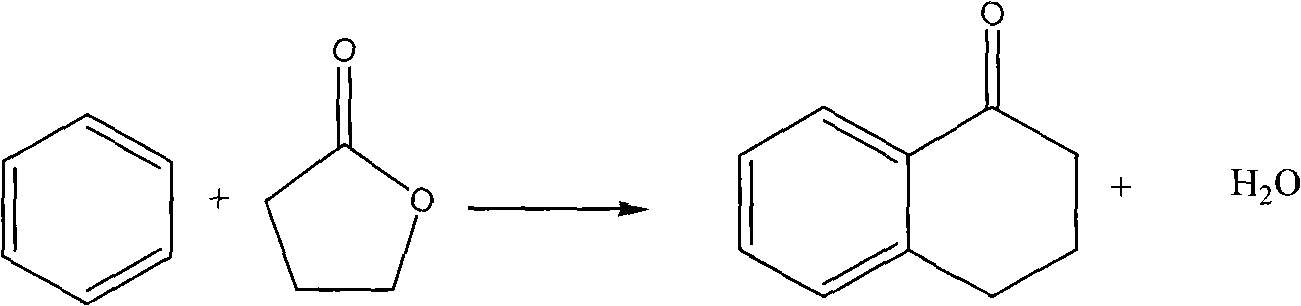
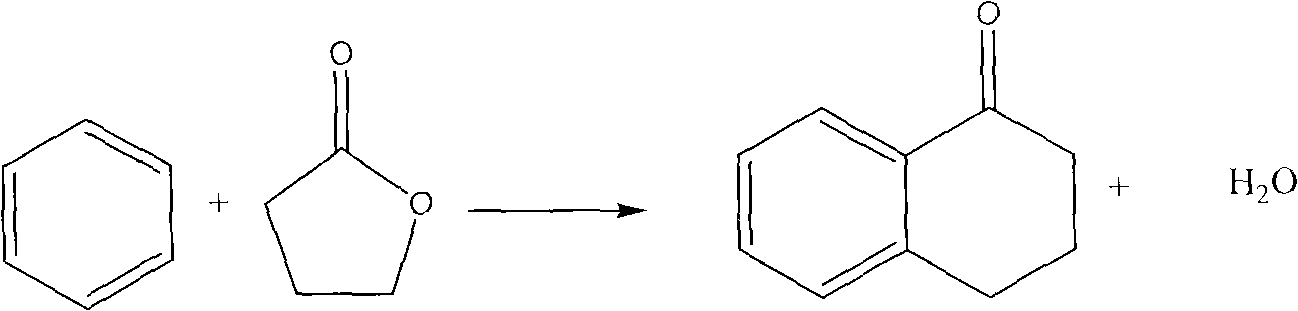
Method for synthesizing alpha-tetralone by gas solid phase reaction
InactiveCN101633611AAvoid the problem of being unable to reuse and discharge large amounts of acidic wastewaterImprove automationMolecular sieve catalystsCatalyst regeneration/reactivationTetraloneFixed bed
The invention relates to a method for synthesizing alpha-tetralone by gas solid phase reaction. Benzene and gamma-butyrolactone are taken as raw materials; a molecular sieve is taken as a catalyst; the alpha-tetralone is continuously synthesized in a fixed bed reactor by the gas solid phase reaction; the reaction temperature is 210-300 DEG C; the reaction is carried out under the condition that the liquid space velocity is 1-6.0 h<-1>; the average conversion rate of gamma-butyrolactone is 25-79%; and the average mole yield of alpha-tetralone is 5-40%. The catalyst which is devitalized can be reproduced through oxygenation ignition to recover the catalyst performance. The method for synthesizing alpha-tetralone has the characteristics of avoiding the problems of not reusing the catalyst and discharging a great amount of acidic waste water. A serialization synthetic process is beneficial to automation production and production efficiency and enhances the production efficiency.
Owner:ZHEJIANG UNIV
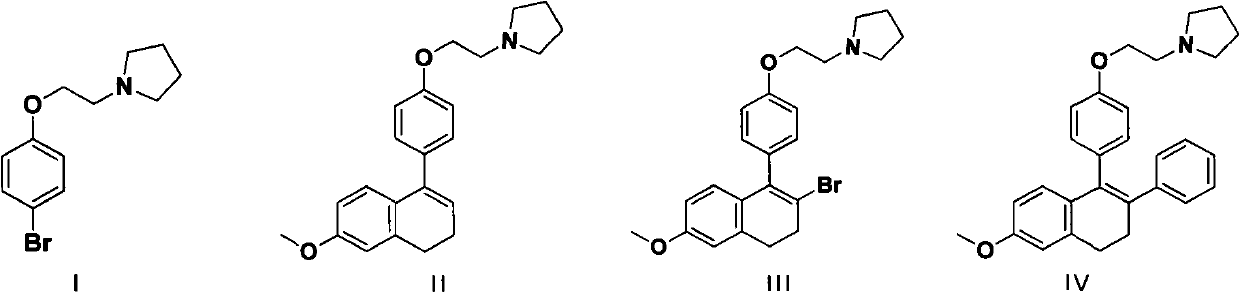
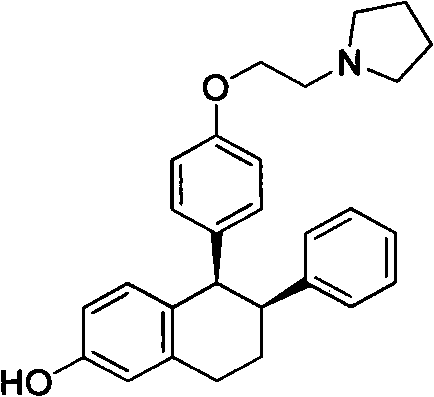
Method for preparing lasofoxifene intermediate
InactiveCN102311406AReduce manufacturing costShort routeOrganic chemistryPhenylboronic acidTetralone
The invention relates to a method for preparing a lasofoxifene intermediate (namely, a compound IV) or an inorganic acid salt thereof. The method comprises the following steps: after a compound I is converted into a Grignard reagent, reacting the Grignard reagent with 6-methoxy tetralone so as to obtain a compound II or an inorganic acid salt thereof; carrying out bromination reaction on the obtained compound II or the inorganic acid salt thereof so as to obtain a compound III or an inorganic acid salt thereof; and then, carrying out coupling reaction on the obtained compound III or the inorganic acid salt thereof so as to obtain the lasofoxifene intermediate (namely, the compound IV) or the inorganic acid salt thereof. The compound IV or inorganic acid salt thereof is a key intermediate for preparing a medicament lasofoxifene for treating osteoporosis. The method provided by the invention is low in production cost, good for environmental protection, and suitable for industrial production.
Owner:WUHAN QR PHARMA CO LTD +1
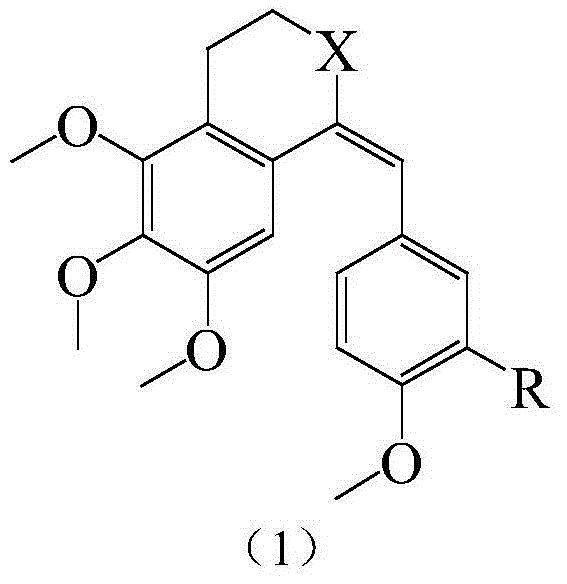
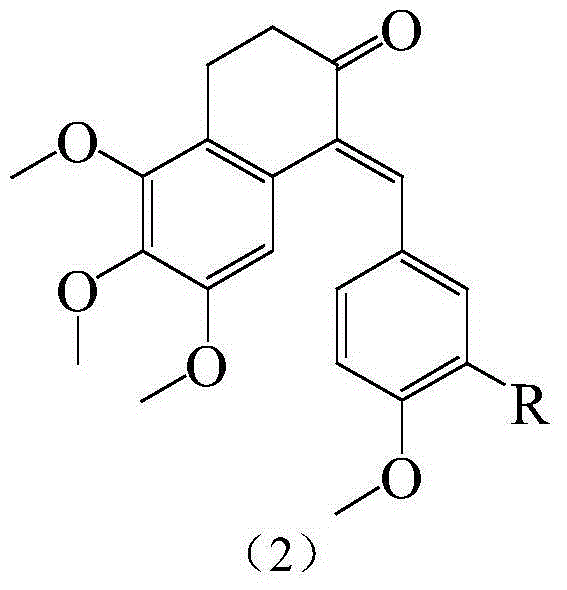
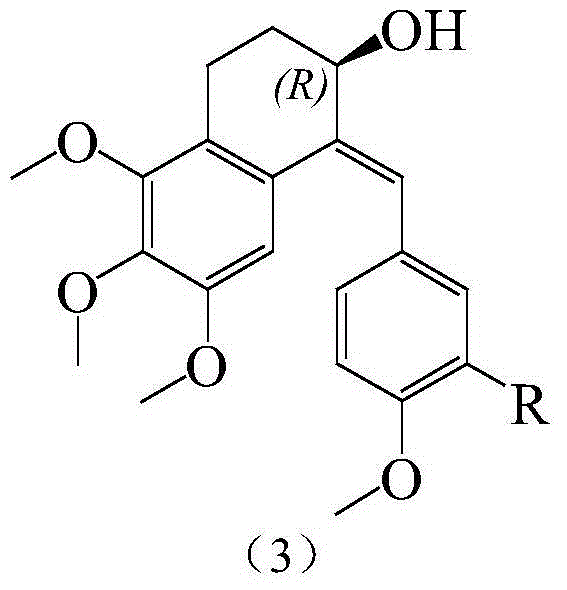
Substituted benzylidene tetralone derivatives and preparation method and applications
InactiveCN104649879AConvenient researchGood water solubilityAmino preparation from aminesCarboxylic acid nitrile preparationSolubilityTetralone
The invention relates to substituted benzylidene tetralone derivatives and a preparation method and application. The chemical structural formula of the substituted benzylidene tetralone derivatives is shown in the formula (1) in the specification, and in the formula (1), X represents CHOH or C=O, and R represents any one of H, OH, F, Cl, CN, CONH2, NO2, CH3, OCH3 and NH2. In the synthetic route, any substitution group can be introduced into two benzene rings, thus eliminating the limit on organic synthesis for finding out compounds with better activity. After water-soluble groups such as amino and the like are introduced, the water solubility can be greatly improved compared with a pilot compound CA-4, and the research of druggability is facilitated. In addition, after amino, hydroxyl and the like are introduced, not only can the bioactivity be improved, but also a prodrug can be prepared on the basis of the group, and the in-vivo activity study can be favorably conducted. The derivatives have the effects of treating ovarian cancer, colon cancer, thyroid cancer and leukemia.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
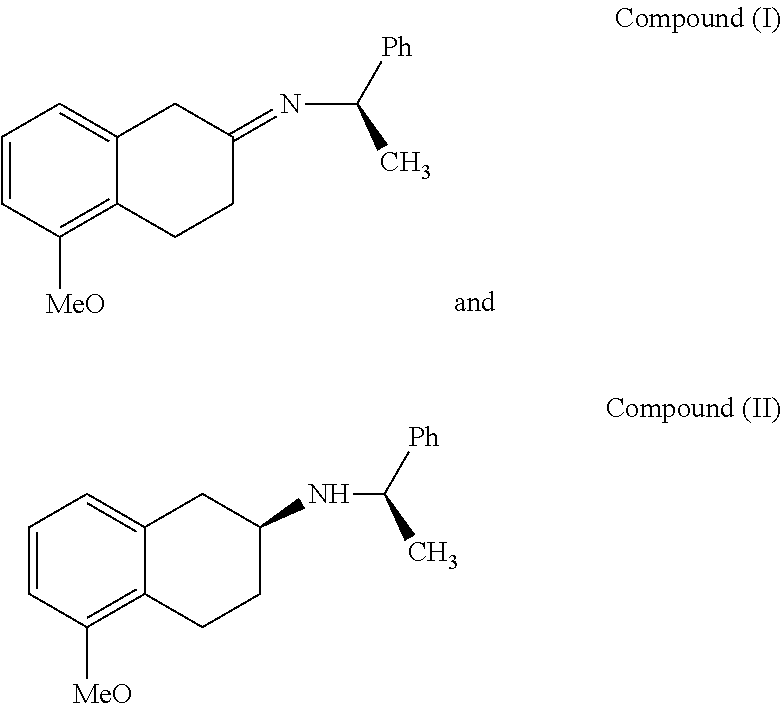
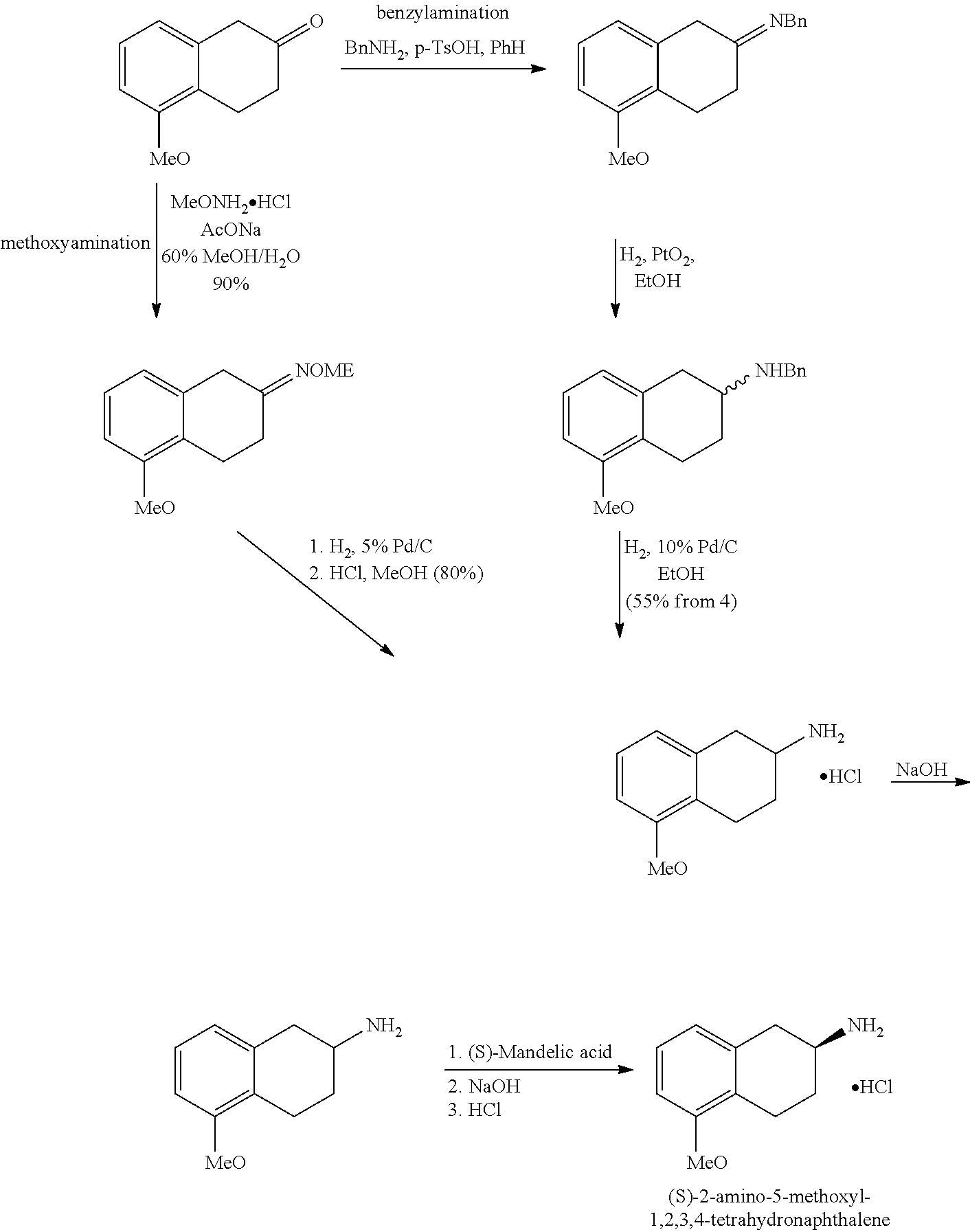
Method of Preparing (S)-2-amino-5-Methoxytetralin Hydrochloride
ActiveUS20140046095A1Increase productionLow yieldIsocyanic acid derivatives preparationOrganic compound preparationTetralin2-Tetralone
A method of preparing (S)-2-amino-5-methoxytetralin hydrochloride[(S)-2-amino-5-methoxyl-1,2,3,4-tetrahydronaphthalene hydrochloride], comprising the steps of: (1) producing a compound (I) by addition-elimination reaction of 5-methoxy-2-tetralone and R-(+)-a-phenylethylamine; (2) producing a compound (II) by reduction reaction of the compound (I) with a reducing agent; and (3) producing a compound (II) hydrochloride by reacting the compound (II) with a salt-forming agent, then carrying out reduction reaction with a palladium-carbon catalyst to produce (S)-2-amino-5-methoxytetralin hydrochloride. The method can significantly increase the yield of (S)-2-amino-5-methoxytetralin hydrochloride with short synthetic path, low preparation cost and less pollution, which is environmentally friendly and is suitable for medical industrialized production. The structural formulae of the compound (I) and the compound (II) are:resepectively.
Owner:ANHUI QINGYUN PHARMA & CHEM
Method for synthesizing tetralone by liquid-phase catalytic oxidation of tetralin
InactiveCN101337872AImprove conversion rateHigh selectivityMolecular sieve catalystsCarbonyl compound preparation by oxidationTetraloneHydrogen
The invention relates to a method for catalytically oxidizing tetrahydronaphthalene to tetralone in liquid phase. The invention relates to the synthesis method of ketone compounds, particularly the synthesis method of tetralone. The invention aims to develop a method for catalytically oxidizing tetrahydronaphthalene to tetralone in liquid phase at high conversion rate and high selectivity. The method selects a mesoporous material as a heterogeneous catalyst to catalytically oxidize tetrahydronaphthalene to tetralone under the condition of low temperature and liquid phase. The test result shows that the conversion rate of tetrahydronaphthalene is 97.6%, and the selectivity of tetralone is 75.5%.
Owner:YUNNAN UNIV
Synthesis method of 6-methoxy-1-tetralone
ActiveCN111333494ARaw materials are easy to getSave raw materialsOrganic compound preparationSulfonic acid esters preparationAluminium chlorideO-Phosphoric Acid
The invention discloses a synthesis method of 6-methoxy-1-tetralone, and belongs to the technical field of medicine synthesis. The method comprises the following steps: (1) anisole reacts with an acylating agent in Lewis acid and a solvent at -10 to 40 DEG C to generate an intermediate 1, with the molar ratio of Lewis acid to the acylating agent to the anisole being (1-10): (1-10): 1, and the Lewis acid being one or more of concentrated sulfuric acid, phosphoric acid, polyphosphoric acid, zinc chloride, aluminum trichloride, superacid and heteropolyacid; (2) the intermediate 1 does not need tobe separated, the temperature is increased to 70-120 DEG C, and the intermediate 1 continues to react to generate 6-methoxy-1-tetralone; and (3) the reaction product is cooled, water is added to terminate the reaction, extraction purification and desolvation are performed to obtain a 6-methoxy-1-tetralone crude product, and carrying out solvent refining on the crude product to obtain the high-purity product. The synthesis method adopts a one-pot method, reaction steps are shortened, and the yield is improved.
Owner:武汉海昕药物研究有限公司
Preparation technique of 5-methoxy-2-tetralone
The invention discloses technology for preparing 5-methoxyl-2-tetralone, which belongs to a method for preparing tetralone compounds and is technology for preparing the 5-methoxyl-2-tetralone by using sodium metals to reduce 1,6-dimethoxy benzene in an alcohol medium and an ammonia medium at a temperature of between 15 and 35 DEG C, wherein the weight ratio of anhydrous alcohol to the 1,6-dimethoxy benzene during reduction is 6.0-9.0:1; the weight ratio of an ammonia liquid to the 1,6-dimethoxy benzene is 0.05-0.4:1; the weight ratio of the sodium metals to the 1,6-dimethoxy benzene is 0.7-1.2:1; and the reduction temperature is between 15 and 35 DEG C, and the reduction time is between 35 and 48 hours. The technology for preparing the 5-methoxyl-2-tetralone has the advantages of easily obtained reaction raw materials, simple conditions, easy industrialization and high selectivity of the 5-methoxyl-2-tetralone. The 5-methoxyl-2-tetralone is important medical intermediate, and is mainly used for synthesizing medicines for treating Parkinson's disease.
Owner:启东市滨化供水有限公司
Alpha, beta-unsaturated carbonyl tetralone derivative and application thereof
InactiveCN106278857AHas anti-Alzheimer's disease-related pharmacological activityPharmacologically activeNervous disorderOrganic chemistryDiseaseTetralone
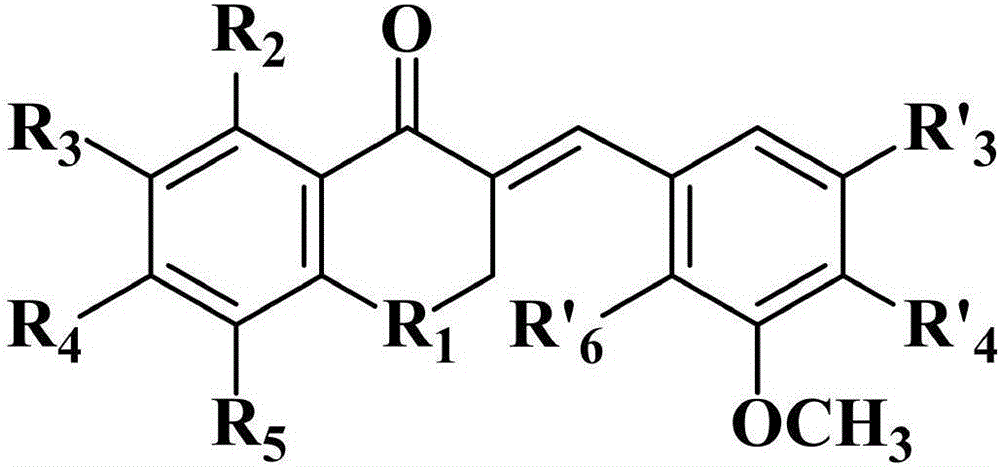
The invention discloses an alpha, beta-unsaturated carbonyl tetralone derivative and application thereof. The alpha, beta-unsaturated carbonyl tetralone derivative has the following structural formula, wherein R1 represents methylene or -HC-CH3; R2 represents hydrogen atoms or methoxyl; R3 represents hydrogen atoms, chlorine atoms, methoxyl, bromine atoms, fluorine atoms or nitryl; R4 represents hydrogen atoms or methoxyl; R5 represents hydrogen atoms, hydroxyl or methoxyl; R'3 and R'4 respectively represent hydrogen atoms or methoxyl; R'6 represents chlorine atoms or bromine atoms. The synthetic compound has pharmacological activities associated with anti-Alzheimer's disease, and is suitable for serving as a drug that can prevent, treat and diagnose Alzheimer's disease, and has a good potential application prospect. (Please see the formula in the description).
Owner:WUHAN UNIV OF TECH
Montelukast sodium preparation technology and intermediates
ActiveCN104293850AEfficient reuseReduce typesOrganic chemistryChemical recyclingTetralonePtru catalyst
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
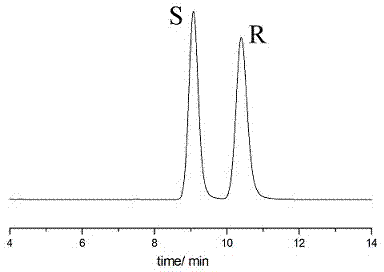
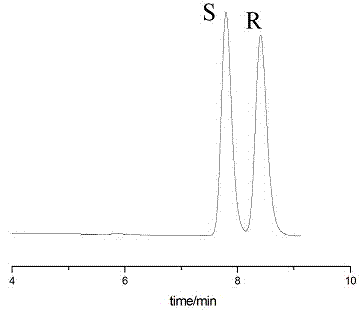
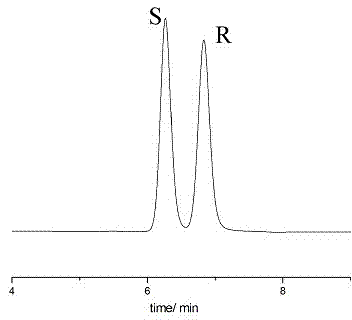
Sertraline hydrochloride intermediates (+/-)-tetralone and chiral chromatographic splitting method thereof
InactiveCN104122342AEfficient analysisEfficient separationComponent separationChromatographic separationStationary phase
The invention relates to a liquid chromatographic separation and analysis method, and particularly relates to sertraline hydrochloride intermediates (+ / -)-tetralone and a chiral chromatographic splitting method thereof. The sertraline hydrochloride intermediates (+ / -)-tetralone and the chiral chromatographic splitting method thereof are provided, wherein the method comprises the following steps: taking the sertraline hydrochloride intermediates (+ / -)-tetralone, and dissolving in an aliphatic hydrocarbon-alcohol mixed liquid; and providing a polysaccharide chiral stationary phase, taking the solution for sample introduction, and carrying out chiral chromatographic separation under a condition with the aliphatic hydrocarbon-alcohol mixed liquid as a mobile phase. A coating type polysaccharide chiral stationary phase material is adopted for effective separation and analysis on the sertraline hydrochloride intermediates and enantiomers thereof, impurities brought by preparation of sertraline hydrochloride with a new process are effectively controlled, and the quality of raw material drugs and preparations can be further controlled.
Owner:RAFFLES PHAMRMATECH CO LTD +1
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS20060052639A1Easy to handleOrganic compound preparationCarboxylic acid amides preparationTetraloneHydrogen
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Manganese-base catalyst for synthesizing alpha-tetralone from tetrahydronaphthalene and preparation method thereof
InactiveCN102886257AImprove low temperature catalytic activityLow costOrganic compound preparationCarbonyl compound preparationTetraloneOxygen
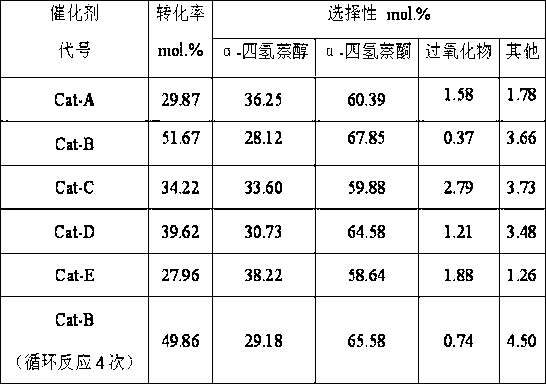
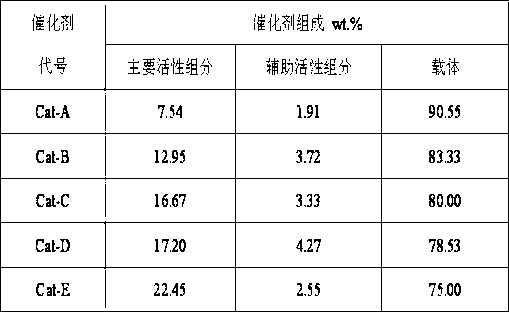
The invention provides a manganese-base catalyst for synthesizing alpha-tetralone from tetrahydronaphthalene and a preparation method thereof, belonging to the technical field of catalyst preparation. The manganese-base catalyst provided by the invention uses active aluminum oxide as a supporter; and Mn oxide is used as the main active component of the catalyst, and oxide of Zr, Co, Fe, Cu or Ce is used as the auxiliary active component of the manganese-base catalyst. The preparation method comprises the following steps: impregnating the active aluminum oxide supporter in a mixed solution of Mn nitrate and nitrate of Zr, Co, Fe, Cu or Ce, drying, and roasting to obtain the catalyst. The manganese-base catalyst provided by the invention has the outstanding advantage of high low-temperature activity, has the characteristic of low cost, can completely substitute toxic chrome-containing catalyst, and has favorable industrial application prospects. For example, after the reaction is carried out at 90 DEG C for 8 hours by using oxygen as an oxidizer, the conversion rate of tetrahydronaphthalene is up to 51.67%; and after the reaction is circulated four times, the activity of the catalyst is basically unchanged, and the peroxide content in the product is very low.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Preparation method of trifluoromethyl tetralone compound
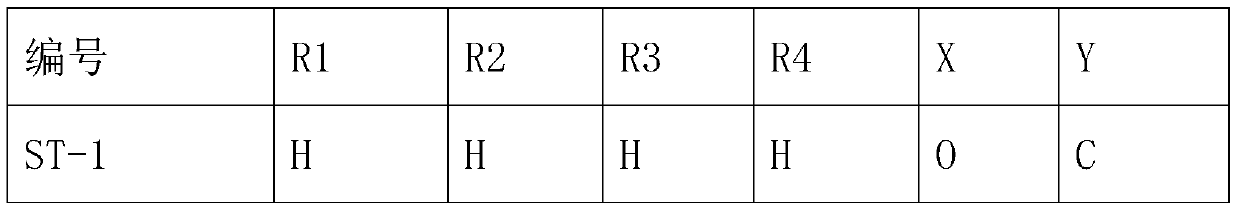
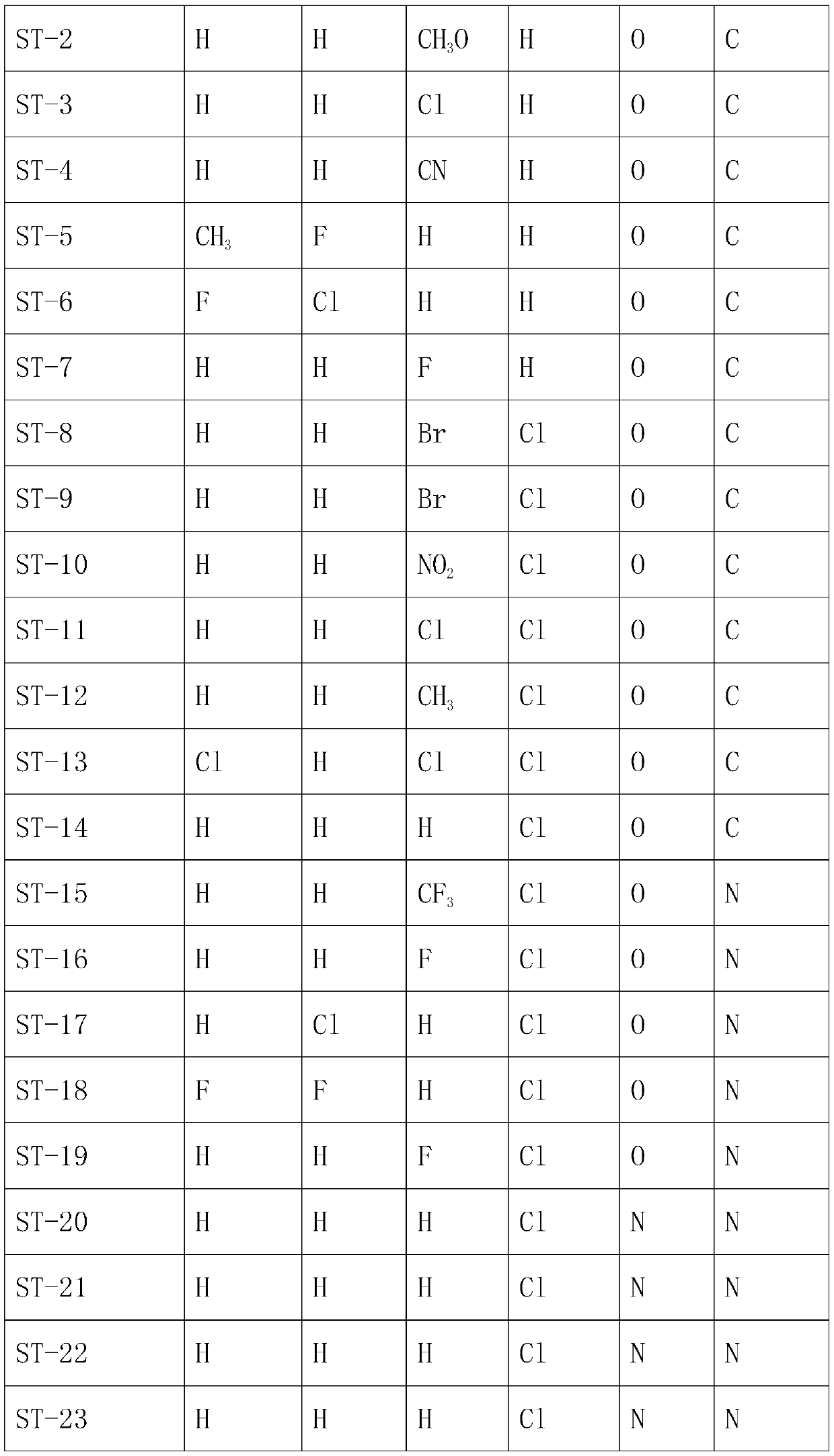
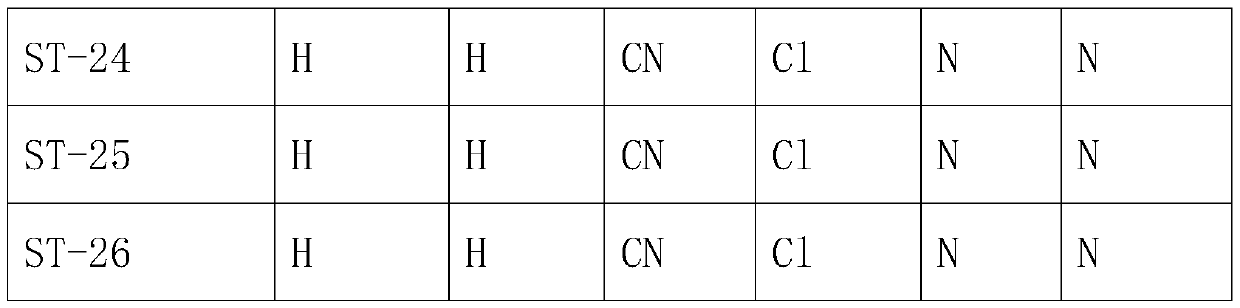
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of a trifluoromethyl tetralone compound. The problem that a large number of widely applied antibiotics have resistance so that serious side effects and heavy economic pressure are caused on patients due to excessive and excessive use of antibiotics in the prior art is solved. The structuralgeneral formula of the compound is shown in the specification, wherein R1, R2, R3, R4, X and Y are defined in the claim 1, and R1, R2, R3 and R4 are the same or different. The compound provided by theinvention has significant bactericidal and bacteriostatic effects, is suitable for the fields of medicines and farm chemicals, solves the problems of excessive use and excessive use of antibiotics caused by resistance of a large amount of antibiotics in the prior art, and is worthy of further development.
Owner:上海孜岚医药科技有限公司
Method for preparing benzothiazole quinazoline derivatives through catalysis
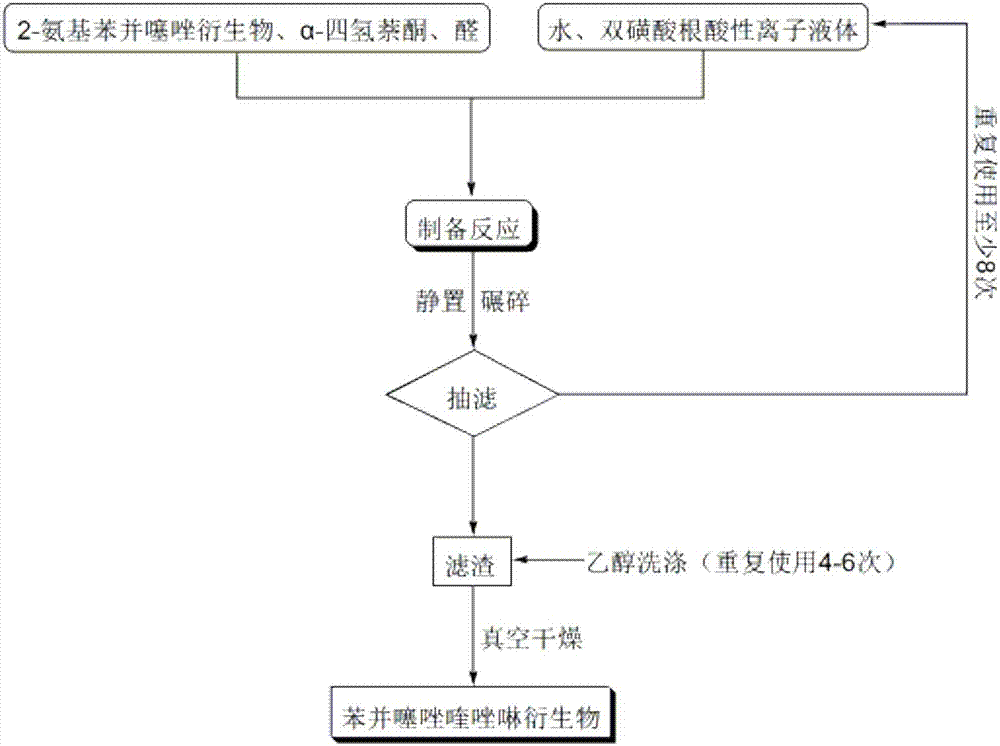
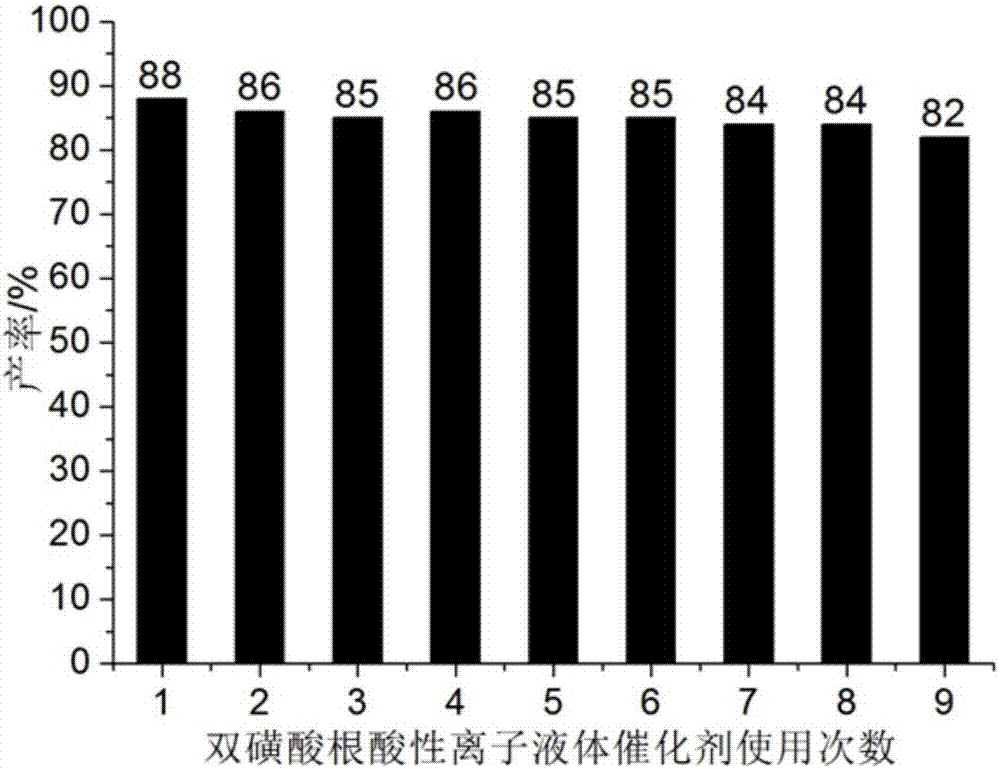
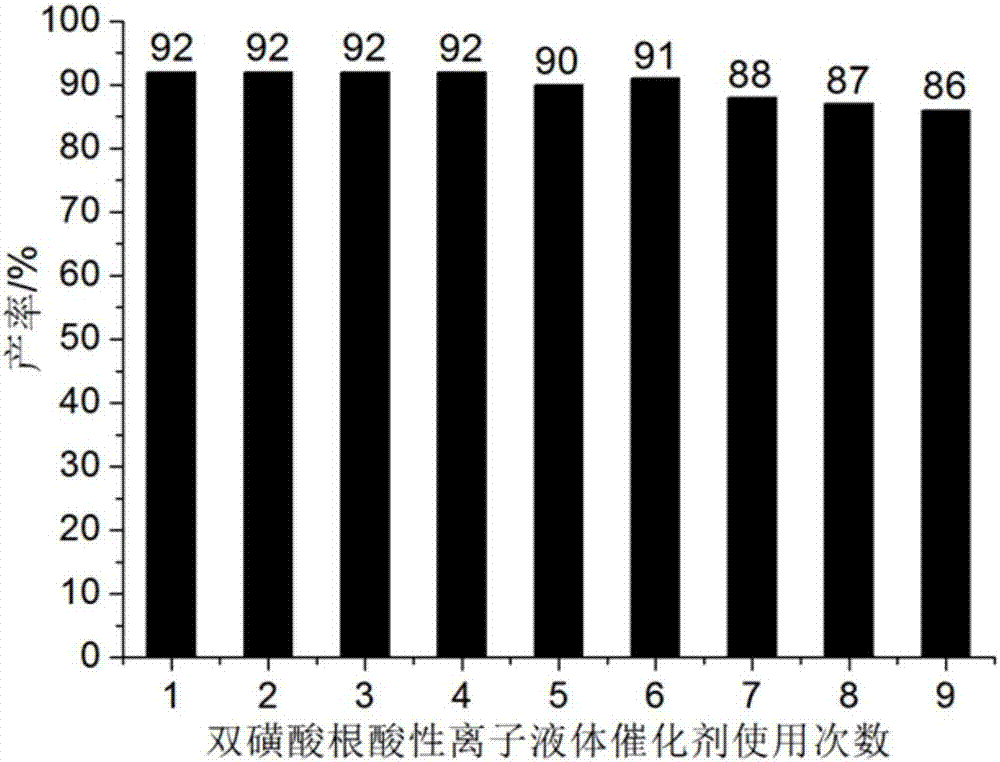
ActiveCN106967095AHigh catalytic activityReduce usageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsWater basedTetralone
The invention discloses a method for preparing benzothiazole quinazoline derivatives through catalysis and belongs to the technical field of ionic liquid catalysis. In the preparation reaction, the molar ratio of 2-aminobenzothiazole derivative to alpha-tetralone to aldehyde is 1:1:(1-1.1), the molar use amount of a double sulfonate radical acidic ionic liquid catalyst is 5 to 8 percent of the molar use amount of 2-aminobenzothiazole derivative, the volume amount of reaction solvent water based on milliliter is 5 to 7 times of the molar weight of the 2-aminobenzothiazole derivative based on millimole, the reaction temperature is 65 to 78 DEG C, and the reaction time is 24 to 57 minutes; after the reaction, cooling is conducted to room temperature, suction filtration is conducted, and the filter residue is washed with ethanol and vacuum-dried to obtain the benzothiazole quinazoline derivatives. Compared with the existing preparation method, the method for preparing the benzothiazole quinazoline derivatives through catalysis has the characteristics that the use amount of catalyst is small, the catalytic activity is stable, biodegradation is facilitated, the whole preparation process is simple and convenient to operate, and the greening degree is high; and industrialized large-scale production is realized easily.
Owner:东营睿港投资服务有限责任公司
Preparation method of 5,8-dimethoxy-2-tetralin ketone
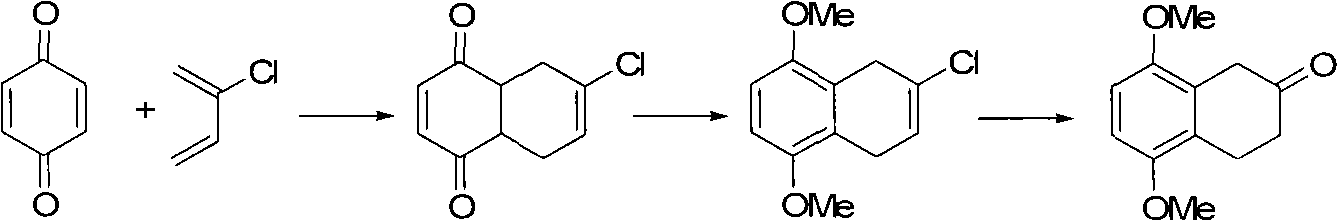
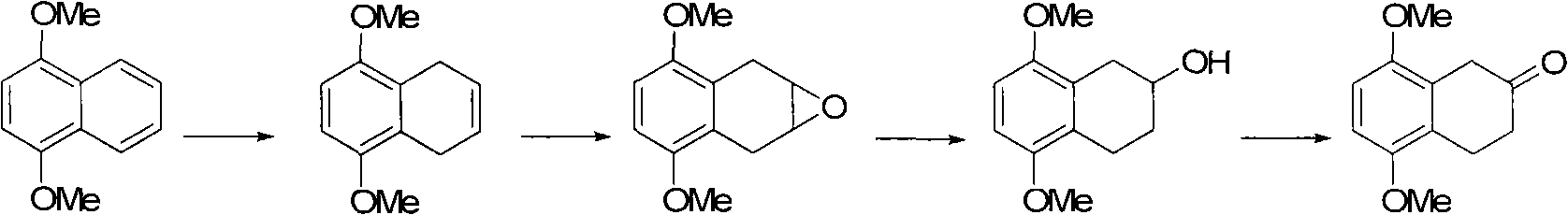
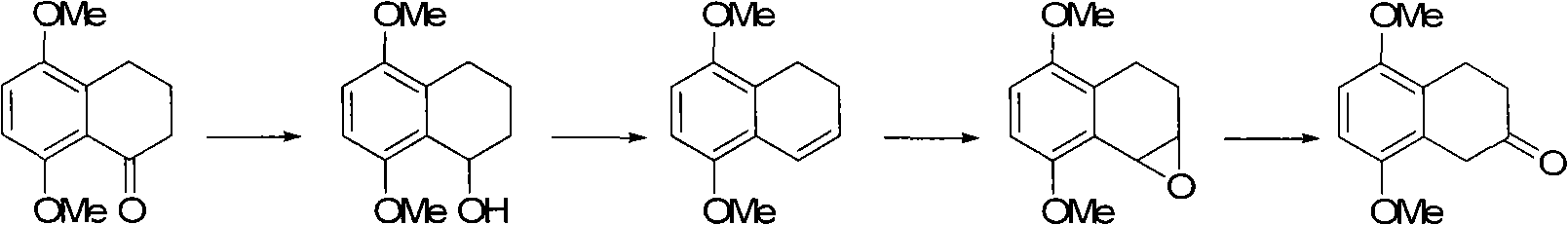
The invention discloses a preparation method of 5,8-dimethoxy-2-tetralin ketone as an organic compound. The method comprises the following steps of: generating 4a,5,8,8a-tetrahydro-1,4-naphthoquinone through a Diels-Aldel reaction by using benzoquinone and 1,3-butadiene as starting raw materials; then converting the 4a,5,8,8a-tetrahydro-1,4-naphthoquinone into 5,8-dimethoxy-1,4-dihydronaphthalene through enolization and etherification; converting the 5,8-dimethoxy-1,4-dihydronaphthalene into 5,8-dimethoxy-1,2-dihydronaphthalene in the presence of base catalysis and then carrying out epoxidation to obtain 5,8-dimethoxy-1,2-epoxy-3,4-dihydronaphthalene; and carrying out epoxy ring opening in the presence of acid catalysis to obtain the 5,8-dimethoxy-2-tetralin ketone.
Owner:甘肃皓天科技股份有限公司
Cyclic amine derivative or salt thereof
InactiveCN101023055AHas NMDA receptor antagonistic effectNervous disorderOrganic chemistryFuranTetralone
Provided are compounds which are an NMDA antagonist having a broad safety margin and are useful as a treating agent or a preventing agent for Alzheimer's disease, cerebrovascular dementia, Parkinson's disease, ischemic apoplexy, pain, etc. Concretely provided are an amine derivative or its salt characterized in that the amine-containing structure A therein bonds to a 2- or 3-cyclic condensed ring (e.g., indane, tetralone, 4,5,6,7-tetrahydrobenzothiophene, 4,5,6,7-tetrahydrobenzofuran, 7,8-dihydro-6H-indeno[4,5-b]furan, 2,3-dihydro-1H-cyclopenta[1]naphthalene) via X 1 (bond or lower alkylene); and an NMDA antagonist containing it as an active ingredient thereof.A compound useful as an NMDA antagonist having a wide safety region which is a therapeutic agent for Alzheimer's disease, vascular dementia (vascular agnosia), Parkinson's disease, ischemic apoplexy, and pains or a preventive agent for these. The compound is an amine derivative or salt thereof characterized by comprising an amine-containing structure (A) and a di- or tricyclic fused ring (indane, tetralone, 4,5,6,7-tetrahydrobenzothiophene, 4,5,6,7-tetrahydrobenzofuran, 7,8-dihydro-6H-indeno[4,5-b]furan, 2,3-dihydro-1H-cyclopenta[a]naphthalene, etc.) bonded to the structure (A) through X<1> (a bond, lower alkylene, etc.). The NMDA antagonist contains the derivative or salt as an active ingredient.
Owner:ASTELLAS PHARMA INC
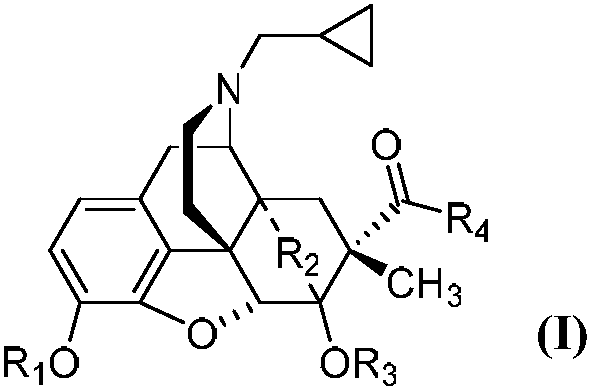
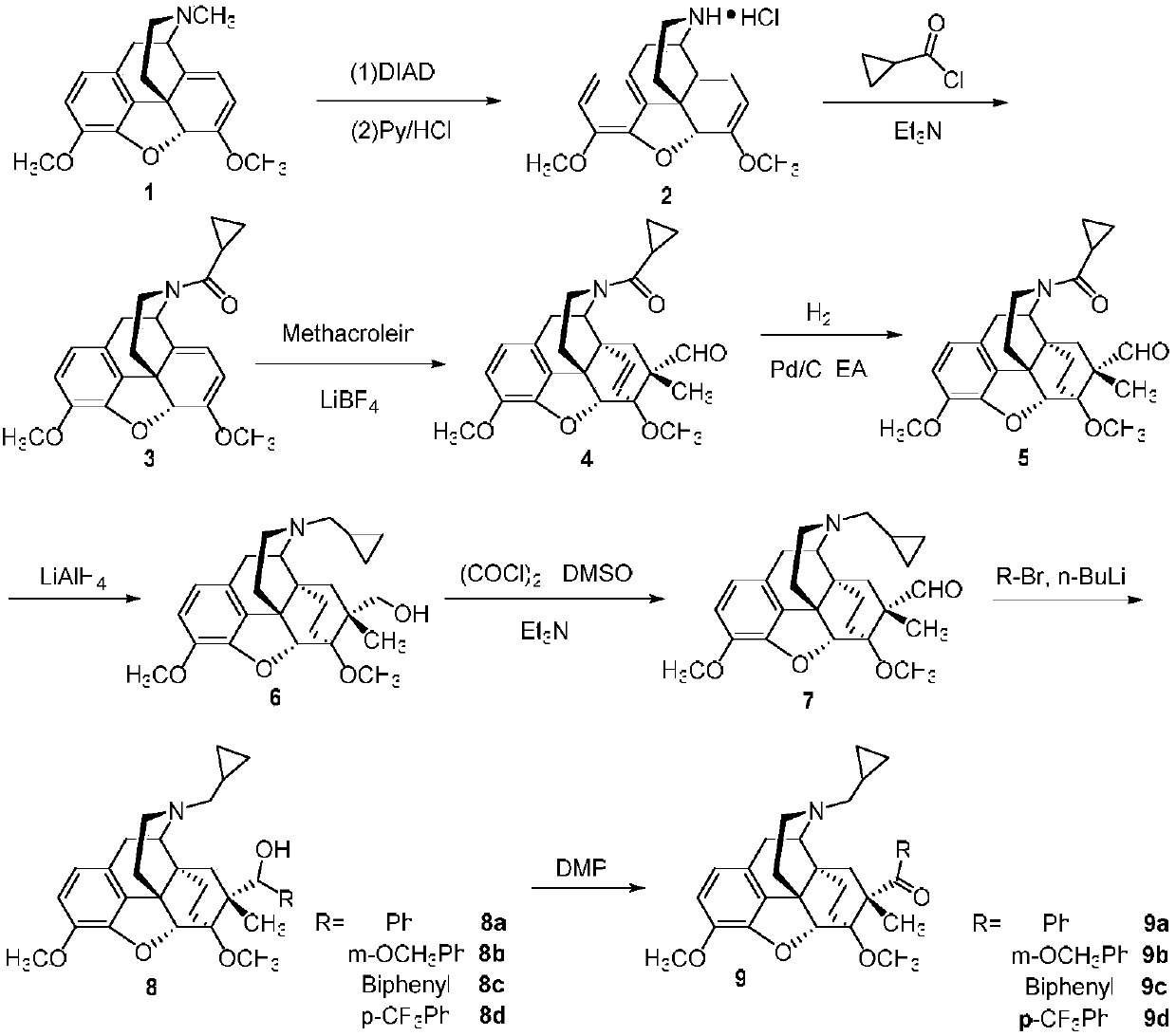
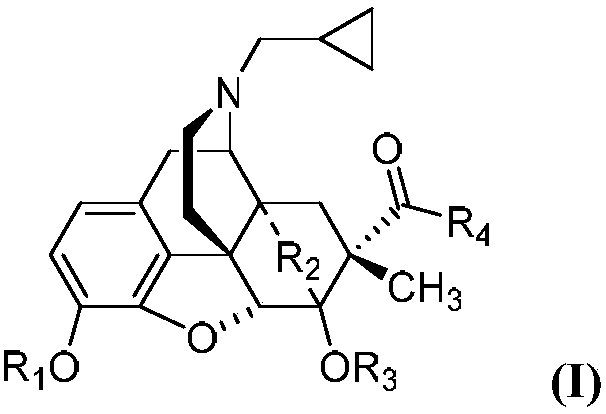
7Beta-methyl-tetralone derivative and preparation method and application thereof
The invention belongs to the field of chemical pharmacy and relates to a 7beta-methyl-tetralone derivative shown in a general formula (I) and a preparation method thereof. According to the compound shown in the general formula (I), with thebaine as a raw material, through related conversion methods such as N-demethylation, N-acylation, a Diels-Alder reaction and reduction, oxidation and Grignard reactions, the compound is synthesized. The compound shown in the general formula (I) belongs to an opioid receptor ligand, a radioactive receptor ligand combination experiment is adopted, the affinityand selectivity of the ligand to three subtypes of an opioid receptor are measured, and a [35S]GTPgammaS combination experiment is adopted for measuring the excitement inhibitory activity of the ligand; it is proved through a result that the compound has the functions of alleviating pain, resisting depression, withdrawing opioid addiction, relieving itching and the like and can be applied to preparation of an opioid receptor treatment medicine and applied to clinical analgesia or depression resistance or opioid addiction withdrawal treatment or itching relieving treatment.
Owner:FUDAN UNIV
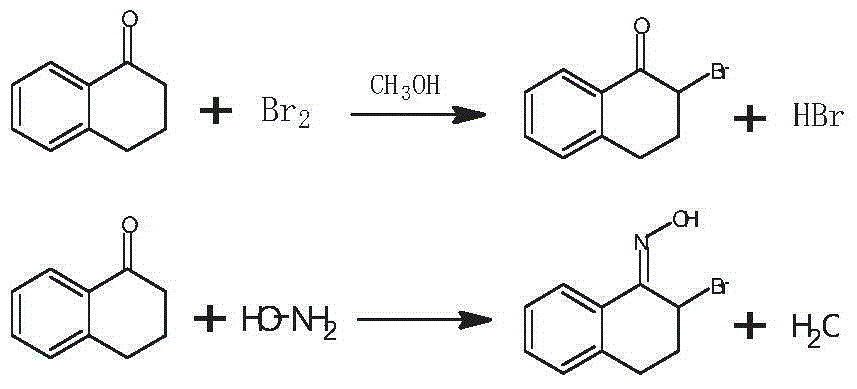
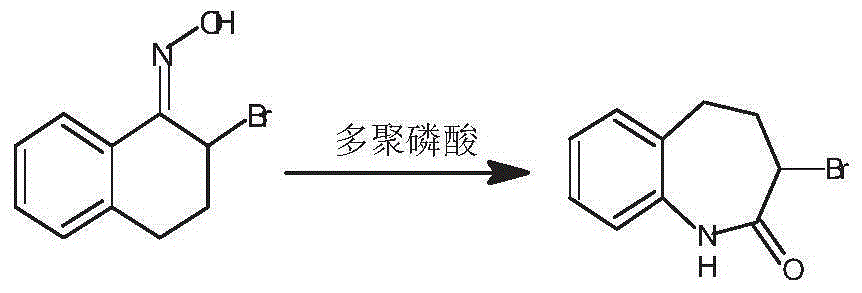
Preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-keto
The invention discloses a preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-benzazepine-2-keto. The preparation method comprises the following stages: alpha-tetralone preparation stage, 2-bromo-3,4-dihydro-N-hydroxy-(2H)-naphthalimide preparation stage and 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-keto, wherein the alpha-tetralone preparation stage comprises the following steps: after mixing gamma-butyrolactone, benzene and aluminum chloride anhydrous, raising the temperature to be 60-90 DEG C, and preserving the temperature for reaction for 5-30 hours; after the reaction is finished, performing hydrolysis, layering and washing on 25 parts of 6% hydrochloric acid solution until neutral; and then distilling to remove excessive benzene, and performing high vacuum rectification to obtain alpha-tetralone. Then, the finished product is finally prepared through a bromination reaction, an oximation reaction and a beckmann rearrangement reaction, the purity of the finished product is high, and the yield is as high as more than 90%. Moreover, the production technology is simple, the production cost is low, and the pollution is little.
Owner:ZHEJIANG BOJU NEW MATERIALS CO LTD
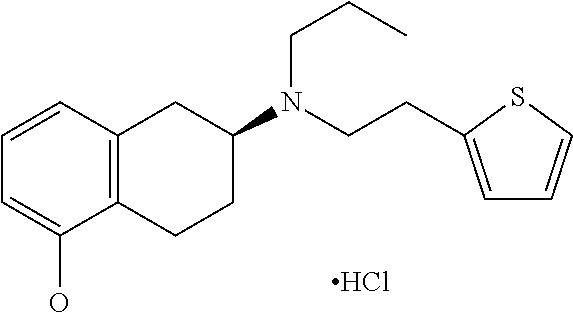
Preparation method of rotigotine
ActiveCN113234057AReduce lossHigh split yieldOrganic compound preparationOrganic chemistry methodsTetraloneMandelic acid
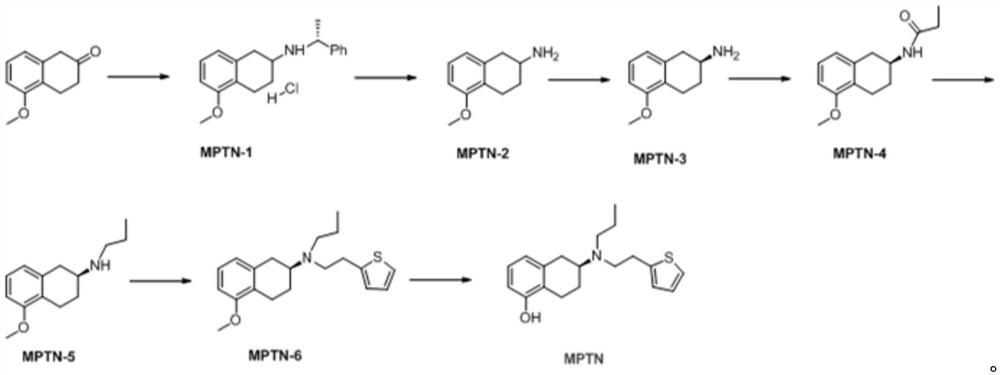
The invention relates to the technical field of medicine preparation, and discloses a preparation method of rotigotine, which comprises the following steps: by taking 5-methoxy-2-tetralone as an initial raw material, reacting with R-alpha-methylbenzylamine, performing debenzylation reduction and S-mandelic acid chiral resolution, then reacting with a propionyl chloride reagent to generate an amide compound, and then reducing by a sodium borohydride reagent to obtain the rotigotine; and finally, reacting with 2-(thiophene-2-yl) 2-nitric acid benzene sulfonic acid ethyl ester to obtain the rotigotine. The preparation process route is as follows: the rotigotine is mild in preparation condition, simple and convenient to operate, relatively high in yield of key intermediates, high in optical purity and easy for industrial large-scale production, and has a very good application prospect.
Owner:CHENGDU TECH UNIV +1
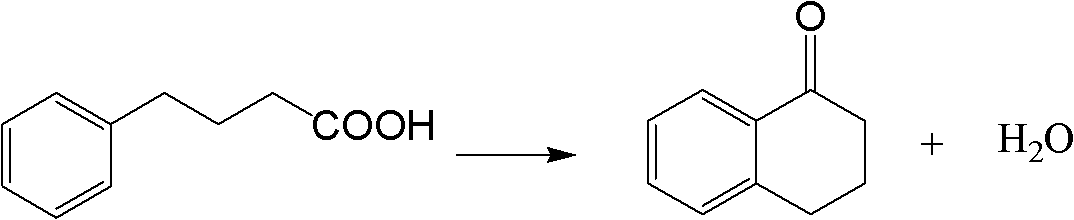
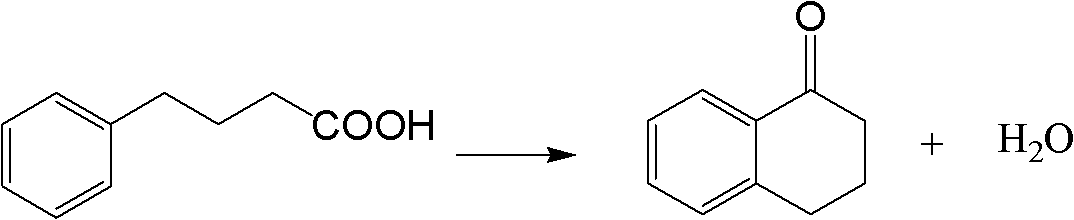
Method for synthesizing Alpha-tetralone through 4-phenylbutyric acid in catalytic way
InactiveCN102584556AOvercoming the problems of being easily deactivated and difficult to recycleContinuous operationCarbonyl compound preparation by condensationTetraloneSolid acid
The invention provides a method, which is characterized in that in a fixed bed device, solid acid is used as a catalyst, 4-phenylbutyric acid is used as a raw material and is dissolved into 1,2-dichlorobenzene according to a certain solid-to-liquid ratio, mixed liquid continuously flows through a catalyst layer after being gasified, the reaction is carried out on a catalyst, and the Alpha-tetralone can be continuously produced. The method solves the problem that in the existing intermittent liquid phase reaction system, the catalyst is easily inactivated and cannot be cyclically used. The gas-solid phase reaction has the advantages that the contact time between reactants and catalysts can be regulated so that the reaction condition is optimized, products generated in the reaction process are continuously separated in the reaction process, and the positive direction proceeding of the reaction is favorably realized. In addition, because the fixed bed catalytic mode is adopted, generated products leave the catalytic bed layer in time in the reaction process, the reaction can be continuously operated, the on-line regeneration can also be carried out when the catalyst is inactivated, and the production efficiency is greatly improved.
Owner:ZHEJIANG UNIV
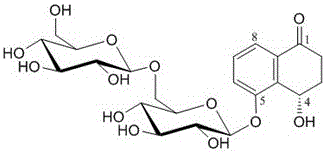
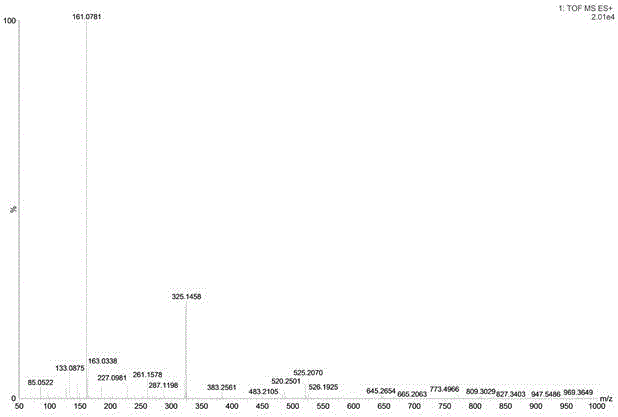
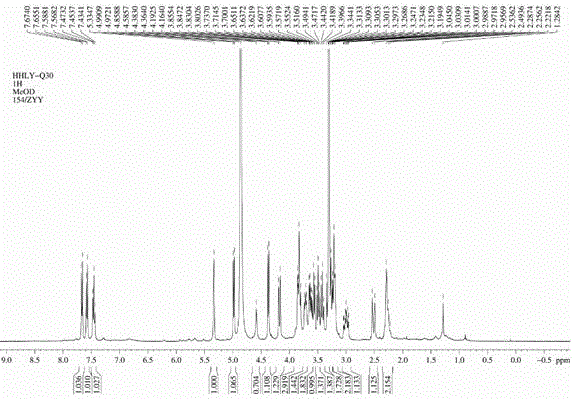
Compound 4(S)-4,5-dihydroxy-alpha-tetralone 5-O-beta-D-glucopyranose (1->6)-beta-D-glucopyranoside, and preparation method and application thereof
ActiveCN105801637AGood inhibition rateExpand sourceSugar derivativesRespiratory disorderD-GlucopyranoseTetralone
The invention discloses a compound with tumor inhibition activity, and a preparation method and application thereof. The compound is diglucoside; one position is simultaneously connected with two molecules of saccharide; a concrete structural formula is 4(S)-4,5-dihydroxy-alpha-tetralone 5-O-beta-D-glucopyranose (1->6)-beta-D-glucopyranoside. Experiments show that the compound provided by the invention has a better inhibition effect on human cervical carcinoma cells and lung cancer cells.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
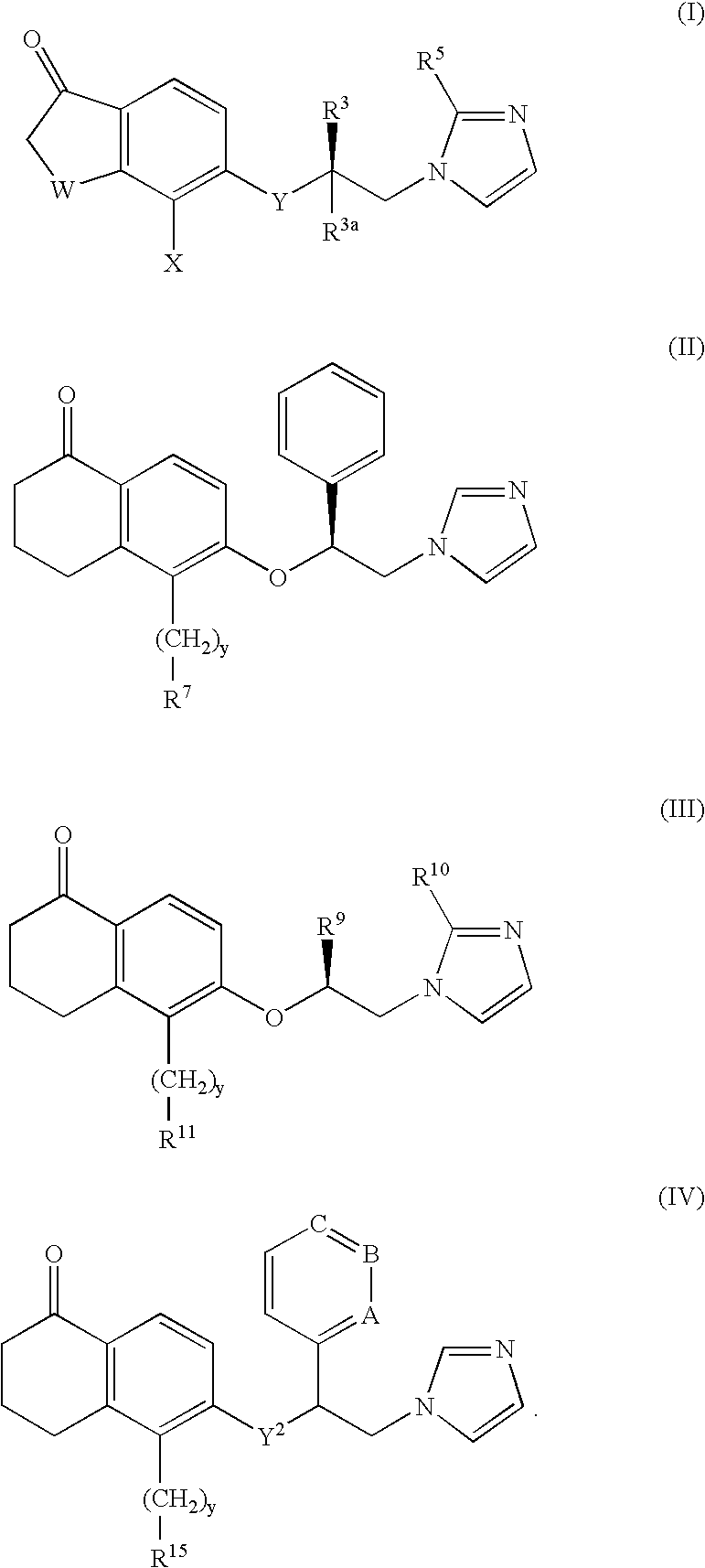
5-substituted tetralones as inhibitors of ras farnesyl transferase
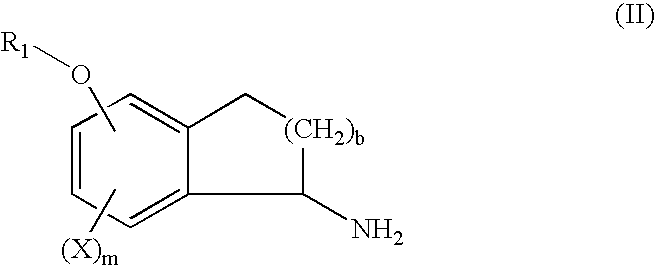
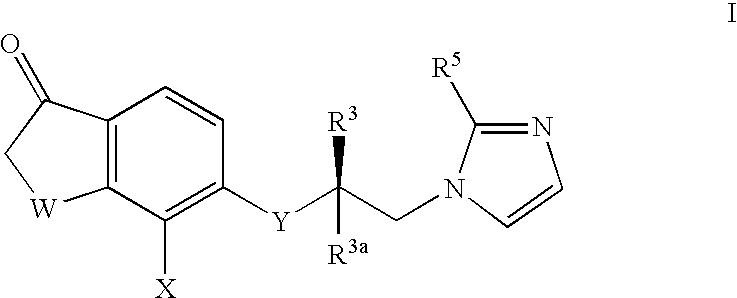
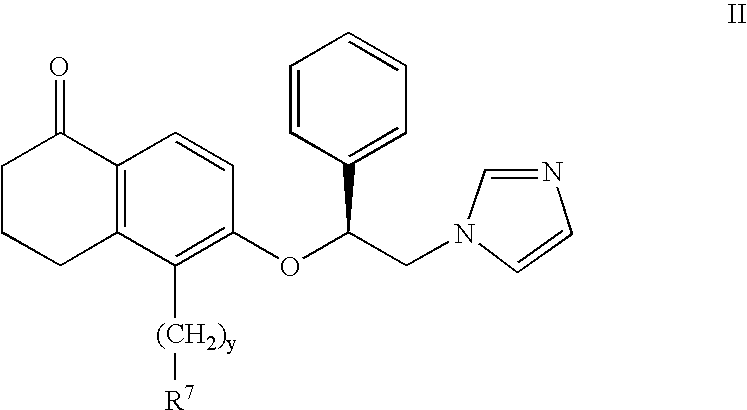
InactiveUS6943183B2Treating and preventing uncontrolled or abnormal proliferation of tissuesEasy to synthesizeBiocideSenses disorderTetralonePercent Diameter Stenosis
The present invention provides novel 5-substituted tetralones of Formulas I, II, III, and IV and pharmaceutically acceptable salts, esters, amides, and prodrugs thereof, which are useful for treating and preventing uncontrolled or abnormal proliferation of tissues, such as cancer, atherosclerosis, restenosis, and psoriasis. Specifically, the present invention relates to compounds that inhibit the farnesyl transferase enzyme
Owner:WARNER LAMBERT CO LLC
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS7262326B2Organic compound preparationCarboxylic acid amides preparationTetraloneHydrolysis
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com