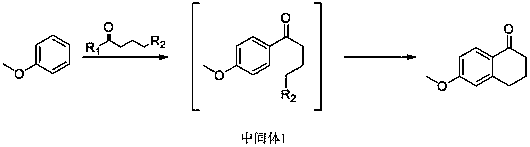
Synthesis method of 6-methoxy-1-tetralone
A synthetic method, methoxyl technology, applied to the preparation of carbon-based compounds, chemical instruments and methods, condensation to prepare carbonyl compounds, etc., can solve the problems of low overall yield, large pollution, and many reaction steps, and achieve easy raw materials The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 1000ml three-necked reaction flask, add 500ml of dichloroethane and 100g of anisole successively to cool down to about 0°C, slowly add 300g of aluminum trichloride, and stir for 30 minutes. Slowly add 150 g of 4-chlorobutyryl chloride dropwise, and the dropwise addition is completed in about 2-2.5 hours.
[0038] After the dropwise addition, the reaction was incubated for 1 hour. The temperature was raised again to 80-90° C. for 6-8 hours.
[0039] After the reaction was completed, the temperature was lowered to room temperature, and the reaction mixture was slowly poured into a beaker with 1000 ml of ice water, and stirred while pouring. The mixture was allowed to stand still, and the water layer was separated. The water layer was extracted once with 200ml of dichloroethane, and the dichloroethane layers were combined and analyzed once with 200ml of water. Dichloroethane was evaporated to dryness under reduced pressure, dissolved in 100ml of ethyl acetate, then ...
Embodiment 2
[0041] In a 1000ml three-necked reaction flask, add 500ml of dichloroethane and 100g of anisole successively to cool down to about 0°C, slowly add 300g of aluminum trichloride, and stir for 30 minutes. Add 150 g of 4-chlorobutyryl chloride directly, and complete the addition within about 10 minutes.
[0042] After the dropwise addition, the reaction was incubated for 1 hour. The temperature was raised again to 80-90° C. for 6-8 hours.
[0043] After the reaction was completed, the temperature was lowered to room temperature, and the reaction mixture was slowly poured into a beaker with 1000 ml of ice water, and stirred while pouring. The mixture was allowed to stand still, and the water layer was separated. The water layer was extracted once with 200ml of dichloroethane, and the dichloroethane layers were combined and analyzed once with 200ml of water. Dichloroethane was evaporated to dryness under reduced pressure, dissolved in 100ml of ethyl acetate, then 100ml of petroleu...
Embodiment 3
[0046] Add 500ml of dichloroethane and 100g of anisole successively to a 2000ml three-necked reaction flask and cool down to about 0°C, slowly add 300g of aluminum trichloride, and stir for 30 minutes. Slowly add 200 g of 4-bromobutyryl chloride dropwise, and the dropwise addition is completed in about 2-2.5 hours.
[0047] After the dropwise addition, the reaction was incubated for 1 hour. The temperature was raised again to 80-90° C. for 6-8 hours.
[0048] After the reaction was completed, the temperature was lowered to room temperature, and the reaction mixture was slowly poured into a beaker with 1000 ml of ice water, and stirred while pouring. The mixture was allowed to stand still, and the water layer was separated. The water layer was extracted once with 200ml of dichloroethane, and the dichloroethane layers were combined and analyzed once with 200ml of water. Dichloroethane was evaporated to dryness under reduced pressure, dissolved in 100ml of ethyl acetate, then 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com