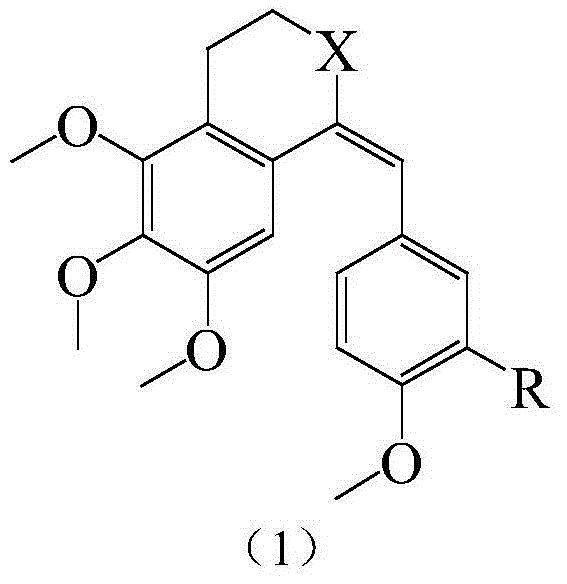
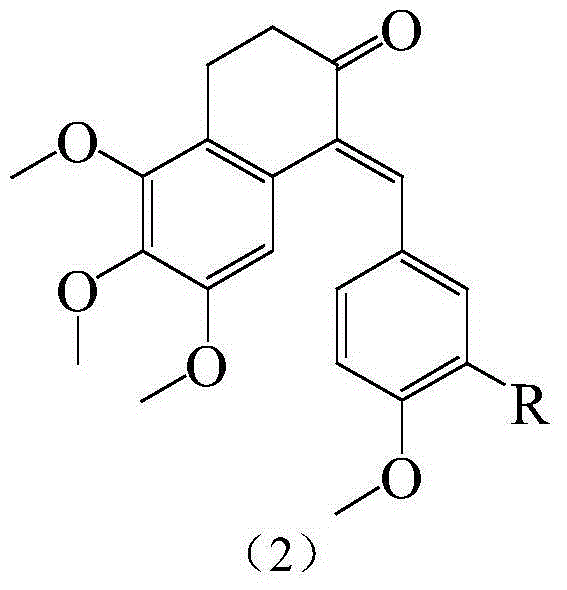
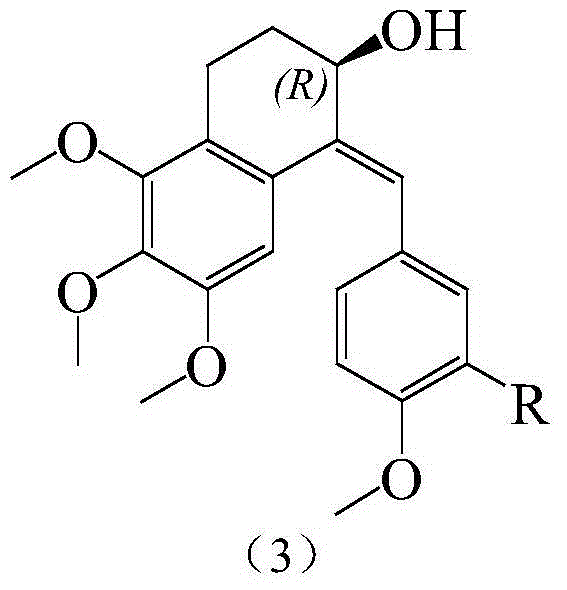
Substituted benzylidene tetralone derivatives and preparation method and applications
A technology of dihydronaphthalene and trimethoxy, which is applied in the field of substituted benzylidene tetralone derivatives and its preparation, can solve the problems that the application of substituted benzylidene tetralones has not been reported, and achieve an improvement Water-soluble, bioactivity-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: 2-(3,4,5-trimethoxyphenyl) ethyl acetate (1).
[0070] 2-(3,4,5-trimethoxyphenyl)acetic acid (90.5g, 0.4mol), absolute ethanol (120mL) and concentrated H 2 SO 4 (8mL) was added to toluene (500mL), refluxed overnight, and the water separator was removed. After the reaction was completed, it was concentrated under reduced pressure, extracted with EtOAc (400 mL), washed successively with 1N aqueous NaOH (50 mL) and brine, and Na 2 SO 4 Drying and concentration under reduced pressure gave compound 1 as light yellow oil (100.6 g, 99% yield). 1 H NMR (300MHz, CDCl 3 )δ6.52(s,2H),4.18(q,J=7.1Hz,2H),3.87(s,6H),3.85(s,3H),3.56(s,2H),1.29(t,J=7.1 Hz,3H).ESI-MS m / z=255.24[M+1] + .
Embodiment 2
[0071] Example 2: 2-(2-formyl-3,4,5-trimethoxyphenyl) ethyl acetate (2).
[0072] Put 70mL of freshly distilled POCl into a 1L three-neck flask 3 (0.76 mol) and N-methylformanilide (102.73 g, 0.76 mol) were vigorously stirred to produce a yellow solid. Then inject ethyl 2-(3,4,5-trimethoxyphenyl)acetate (1) (97.0g, 0.38mol) and stir at room temperature for 40 hours. The resulting red thick substance is slowly poured into 2000mL of crushed ice , a yellow crude product was precipitated, filtered, and recrystallized from acetone to obtain white needle crystals 2 (65.5g, 61% yield). 1 H NMR (300MHz, CDCl 3 )δ10.34(s,1H),6.52(s,1H),4.18(q,J=7.1Hz,2H),4.01(s,3H),3.93(s,3H),3.92(s,2H), 3.88(s,3H),1.29(t,J=7.1Hz,3H).ESI-MS m / z=283.14[M+1] + .
Embodiment 3
[0073] Example 3: Ethyl 3-(6-(2-ethoxy-2-carbonylethyl)-2,3,4-trimethoxyphenyl)acrylate (3).
[0074] Ethyl 2-(2-formyl-3,4,5-trimethoxyphenyl)acetate (2) (23.0g, 0.081mol) and ethoxyformylmethylenetriphenylphosphine (30.0g, 0.086mol) was dissolved in dry toluene (180mL), under nitrogen protection, and refluxed for 20 hours. Cool to room temperature and distill under reduced pressure to obtain a pale yellow solid, which was dissolved in diethyl ether (180 mL), heated to reflux for half an hour, and filtered to remove the solid. The filtrate was concentrated, passed through a neutral aluminum oxide chromatography column, and the mobile phase PE:EA=5:1 was washed to obtain the product 2 as a white solid (26.2 g, 92% yield). 1 H NMR (300MHz, CDCl 3 )δ7.76(d, J=16.1Hz, 1H), 6.63(s, 1H), 6.57(d, J=16.1Hz, 1H), 4.27(q, J=7.2Hz, 2H), 4.20(q, J=7.3Hz, 2H), 3.90(s, 3H), 3.89(s, 3H), 3.88(s, 3H), 3.72(s, 2H), 1.35(t, J=7.1Hz, 3H), 1.29( t,J=7.1Hz,3H).ESI-MS m / z=375.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com