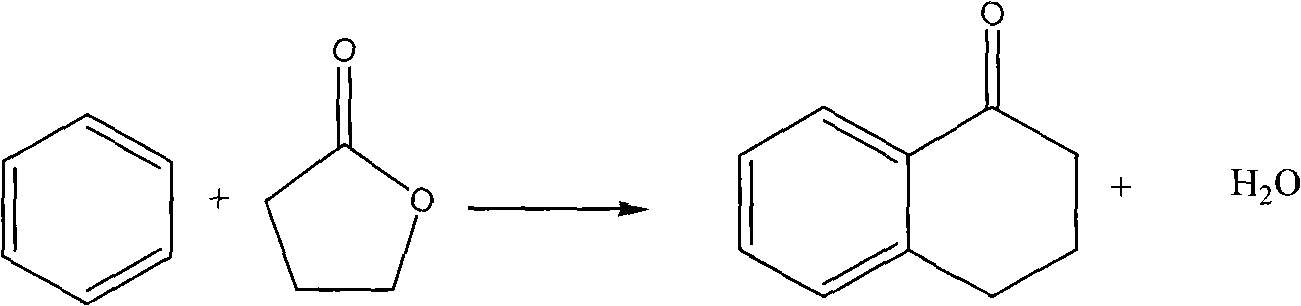
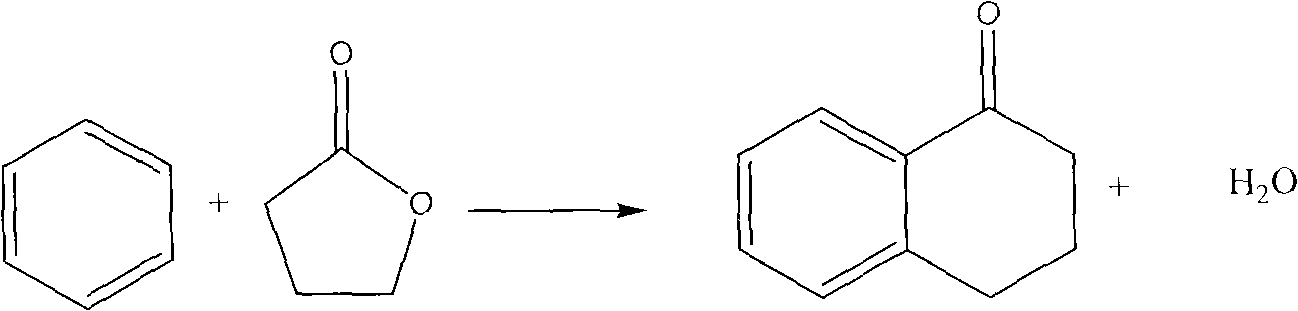
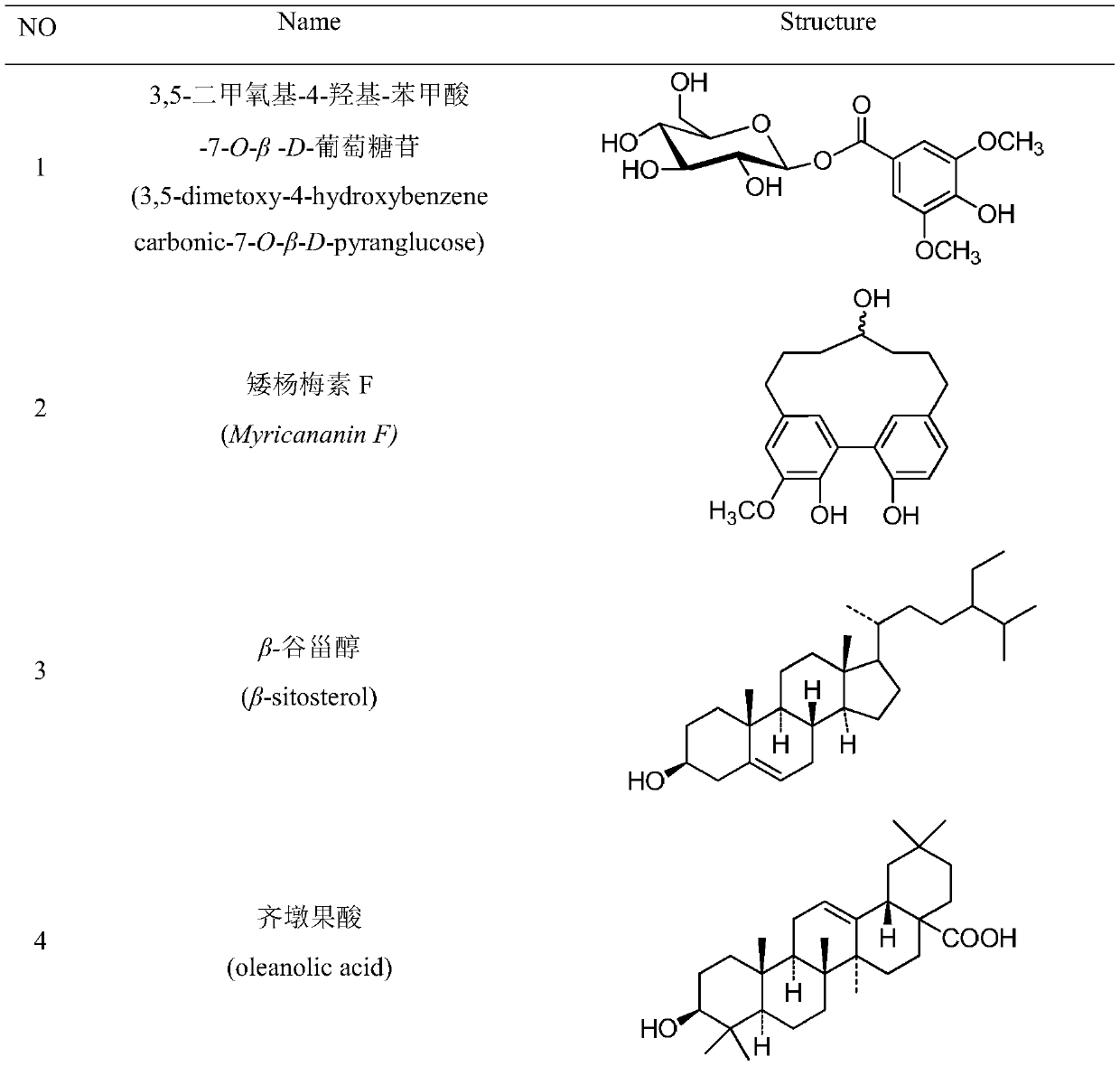
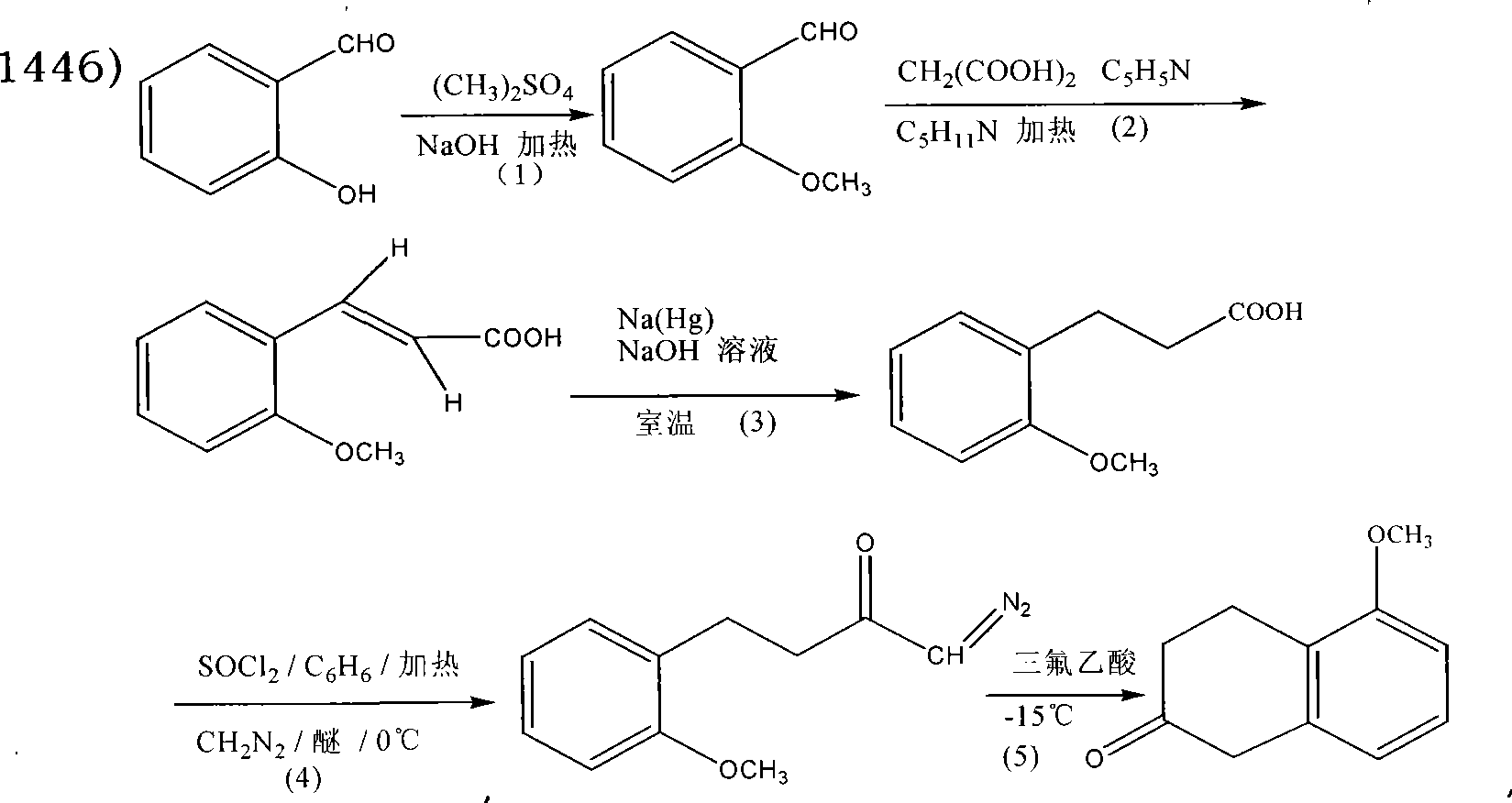
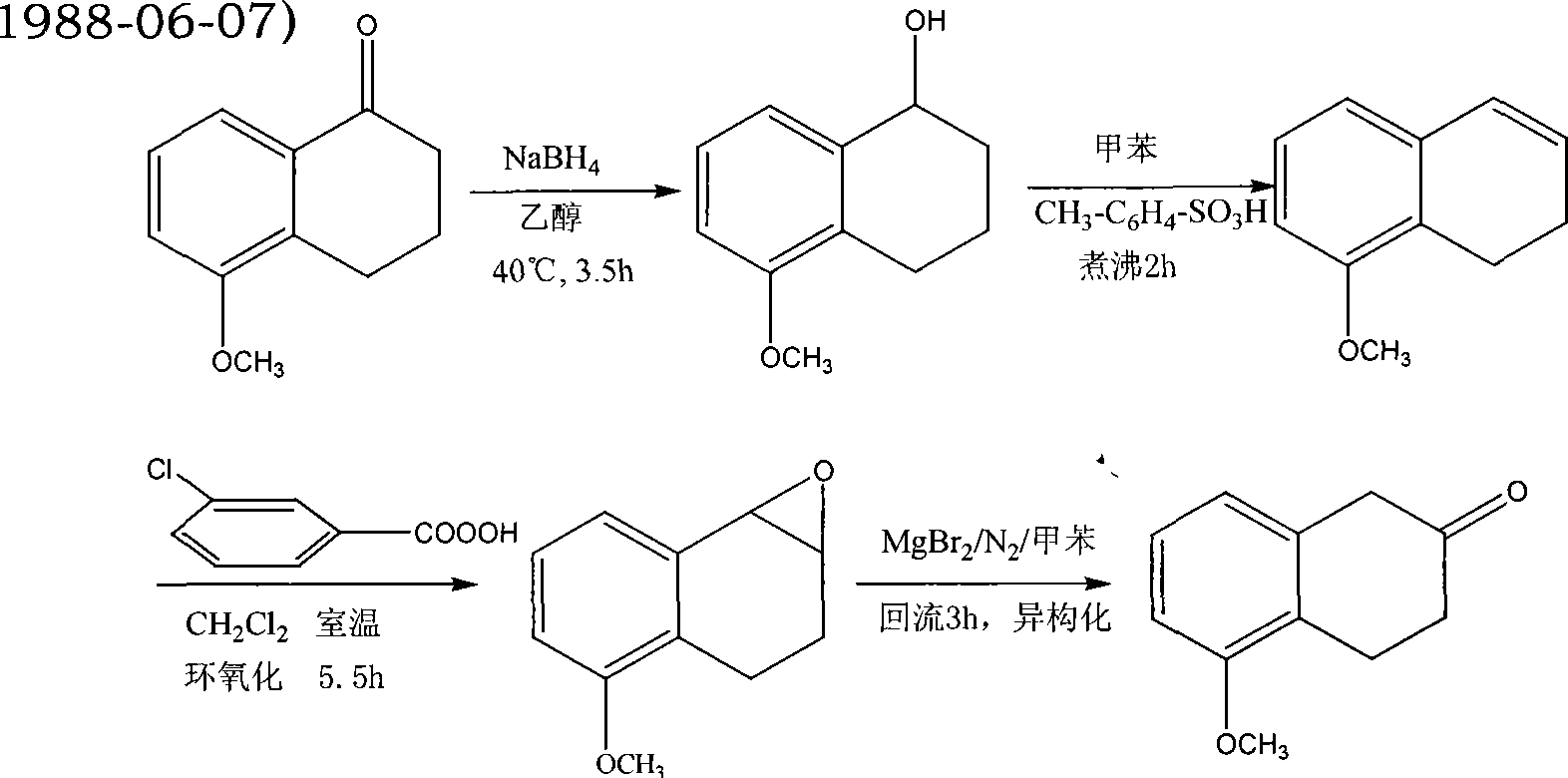
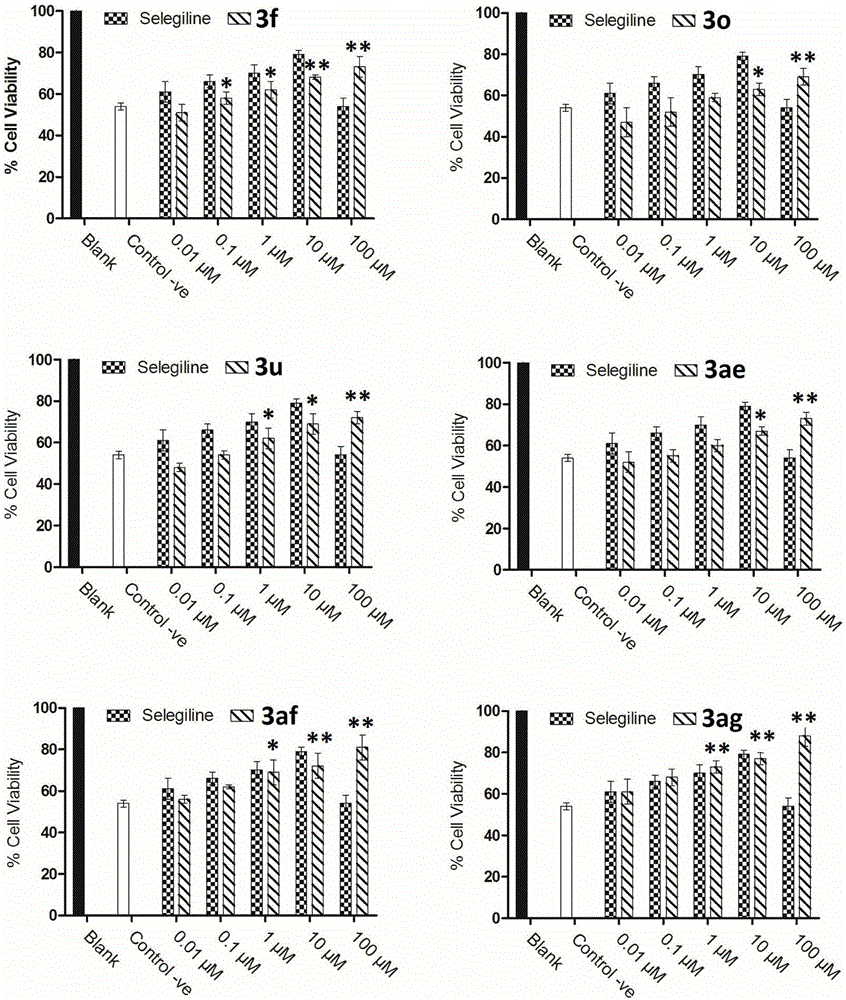
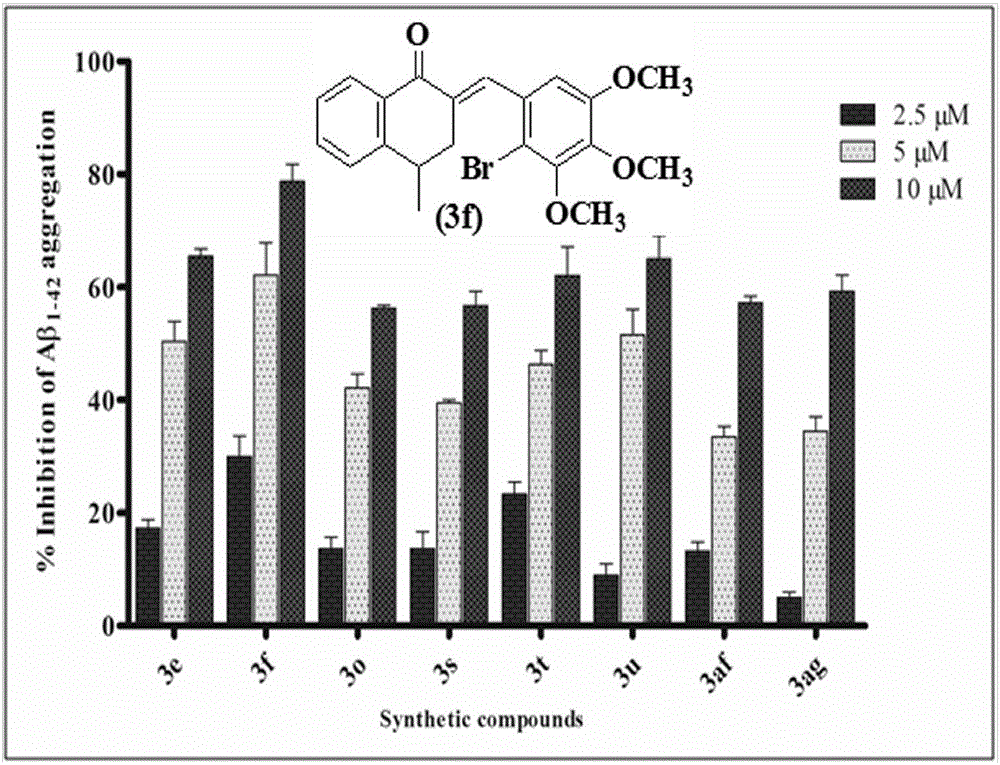
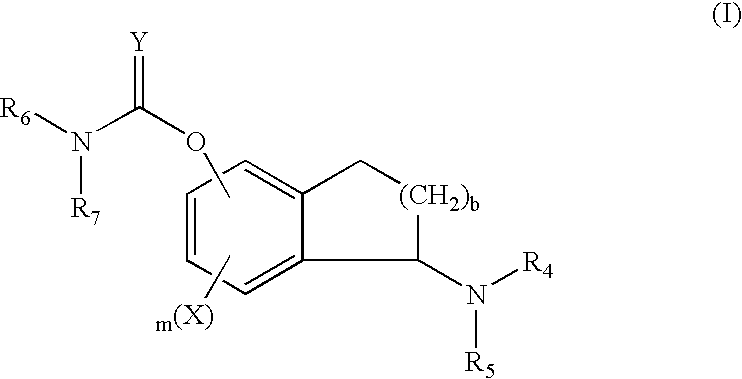
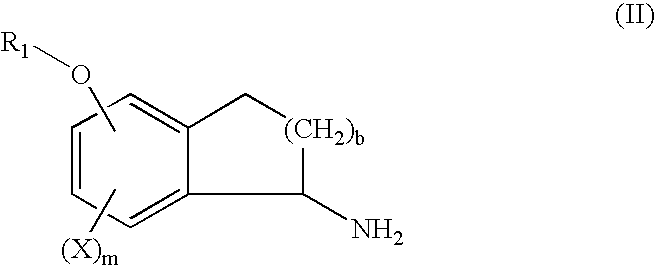
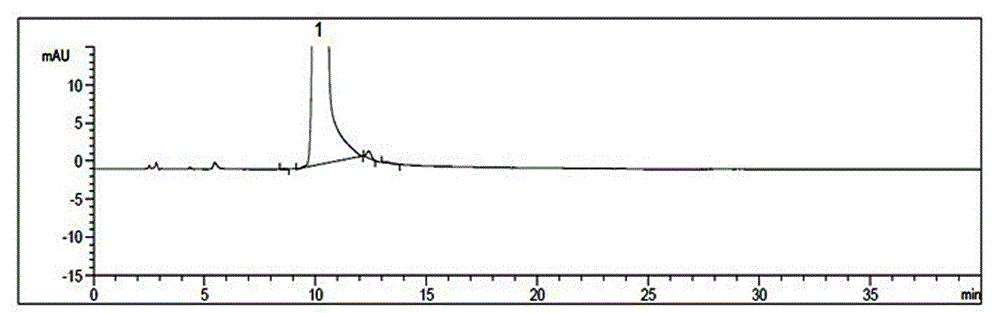
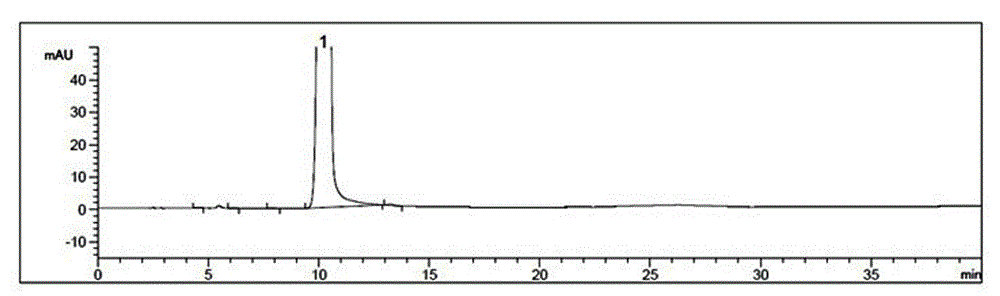
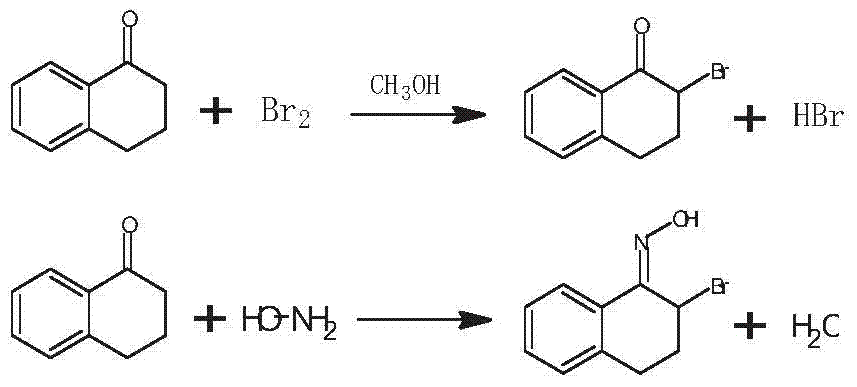
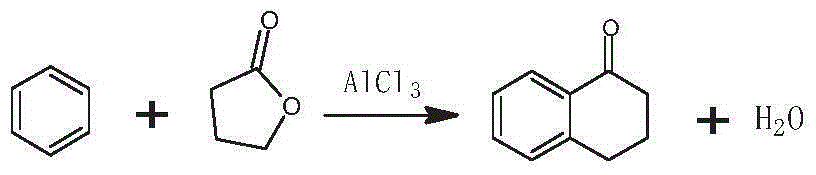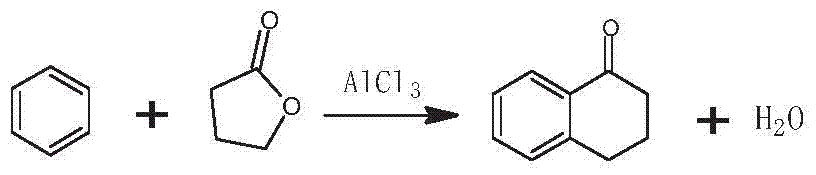Patents
Literature
77 results about "Tetralones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
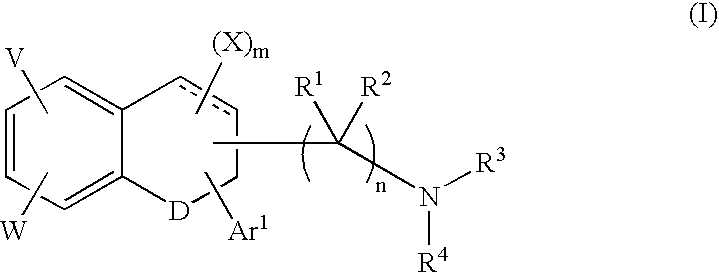
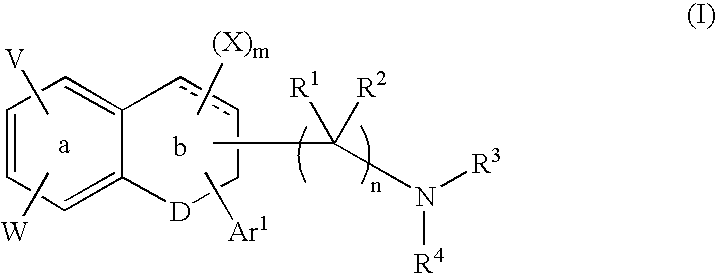
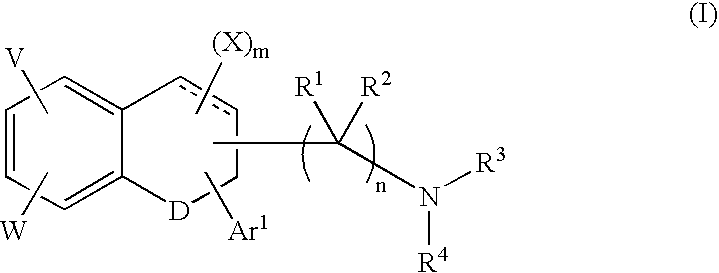
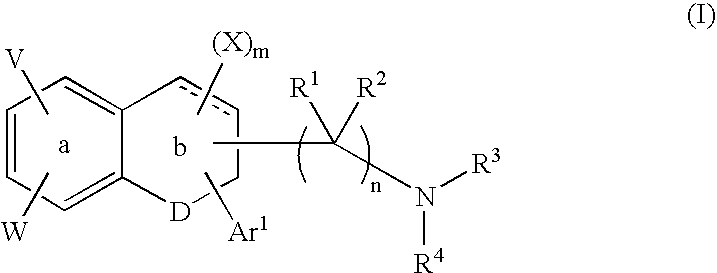
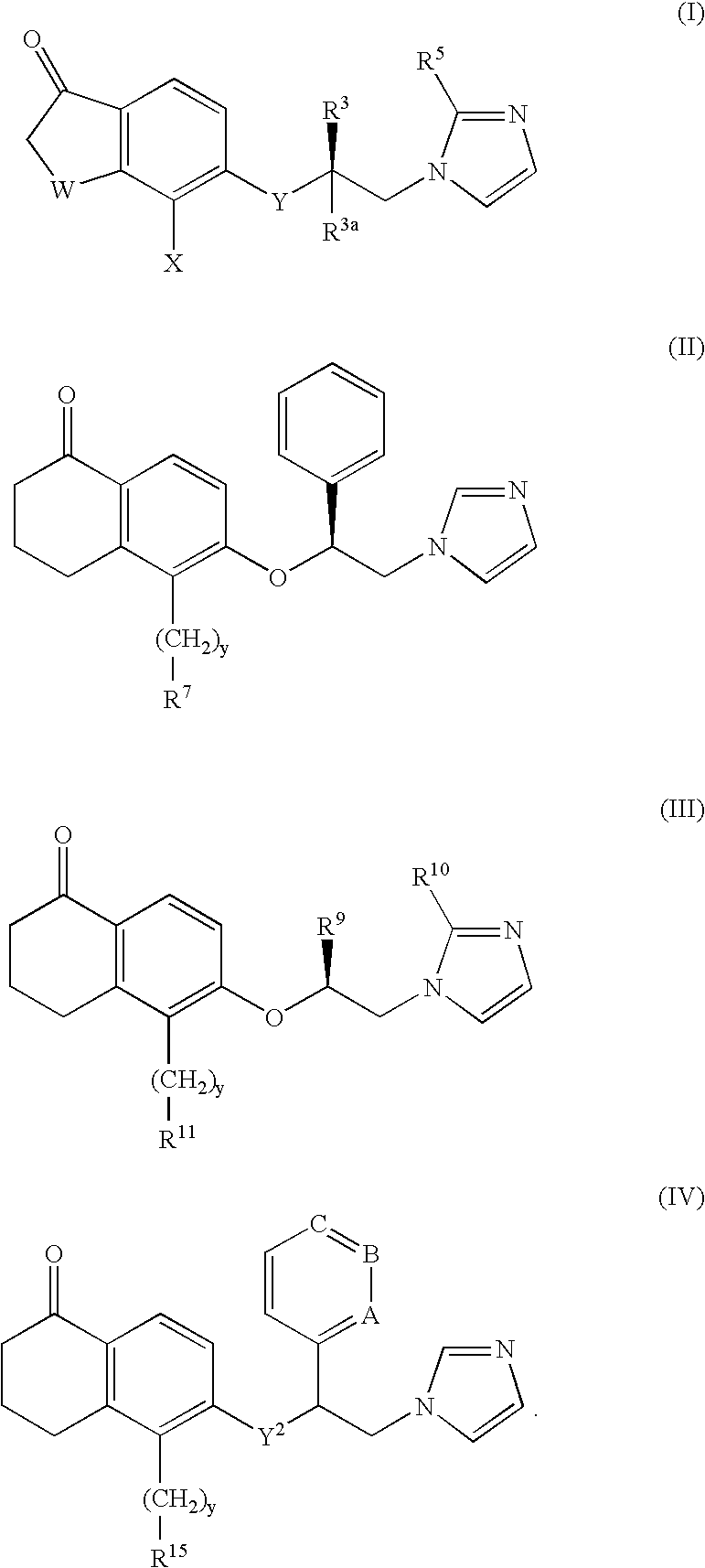
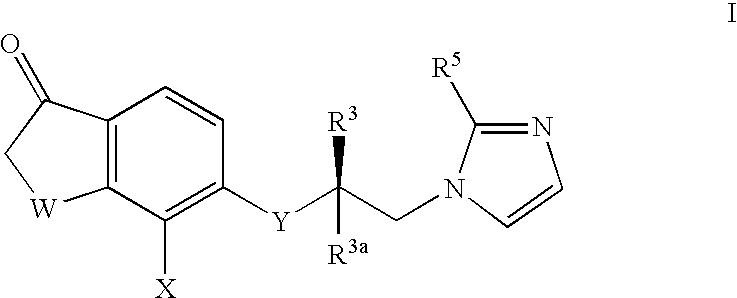
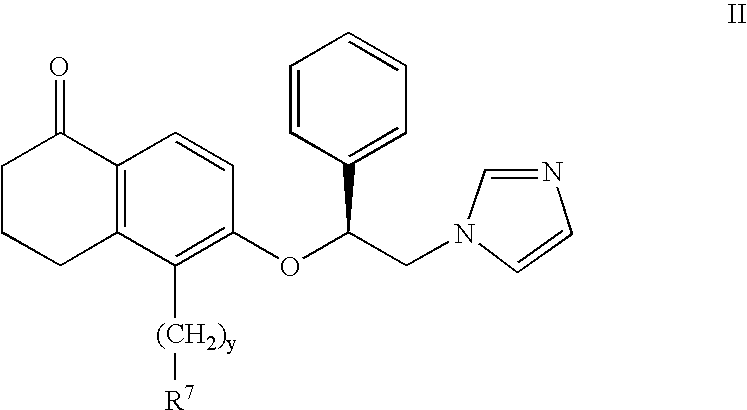
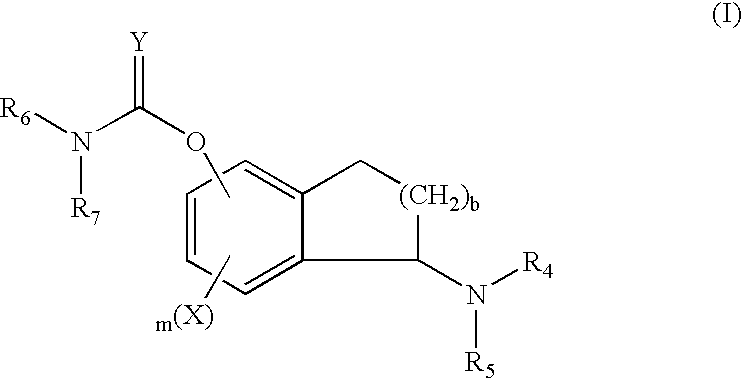
A group of TETRAHYDRONAPHTHALENES containing a keto oxygen.
Preparation method of meso-porous alumina and catalytic synthesis of alpha-tetralone
InactiveCN101337186AEasy to recycleImprove conversion rateCarbonyl compound preparation by oxidationMetal/metal-oxides/metal-hydroxide catalystsTetraloneHydrogen
The invention provides a method for preparing cobalt-doped mesoporous alumina by taking a rubber latex as a biological template, and a method for catalytically oxidizing tetralin into tetralone under liquid phase. The invention relates to the method for preparing the cobalt-doped mesoporous alumina by taking the rubber latex as the template, and the method for synthesizing tetralone. The invention aims to develop a method which is used for preparing the cobalt-doped mesoporous alumina by taking the rubber latex as the biological template and a high-conversion and high-selectivity method which is used for catalytically oxidizing tetralin into tetralone under the liquid phase, and comprises the following steps: a mesoporous material is taken as a heterogeneous catalyst, and tetralin is catalytically oxidized into tetralone under the low temperature liquid phase. The experimental results show that the conversion rate of the tetralin reaches 80.3 percent and the selectivity of tetralone reaches 74.5 percent.
Owner:YUNNAN UNIV
Tetralone-based monoamine reuptake inhibitors
ActiveUS8053603B2Increase synaptic availabilityImprove usabilityBiocideNervous disorderDiseaseSynaptic cleft
The invention relates to novel tetralone based amines and their use in the treatment of central nervous system (CNS) disorders, such as depression, attention deficit hyperactivity disorder (ADHD) and Parkinson's disease. The invention further relates to pharmaceutical compositions containing the compounds and compositions of the invention as well as methods of inhibiting reuptake of one or more monoamine, such as such as dopamine and norepinephrine, from the synaptic cleft, and methods of modulating one or more monoamine transporter.
Owner:SUNOVION PHARMA INC
Tetralone-based monoamine reuptake inhibitors
ActiveUS20070197588A1Increase synaptic availabilityImprove usabilityBiocideNervous disorderSynaptic cleftTetralone
The invention relates to novel tetralone based amines and their use in the treatment of central nervous system (CNS) disorders, such as depression, attention deficit hyperactivity disorder (ADHD) and Parkinson's disease. The invention further relates to pharmaceutical compositions containing the compounds and compositions of the invention as well as methods of inhibiting reuptake of one or more monoamine, such as such as dopamine and norepinephrine, from the synaptic cleft, and methods of modulating one or more monoamine transporter.
Owner:SUNOVION PHARMA INC
Indanone and tetralone compounds for inhibiting cell proliferation
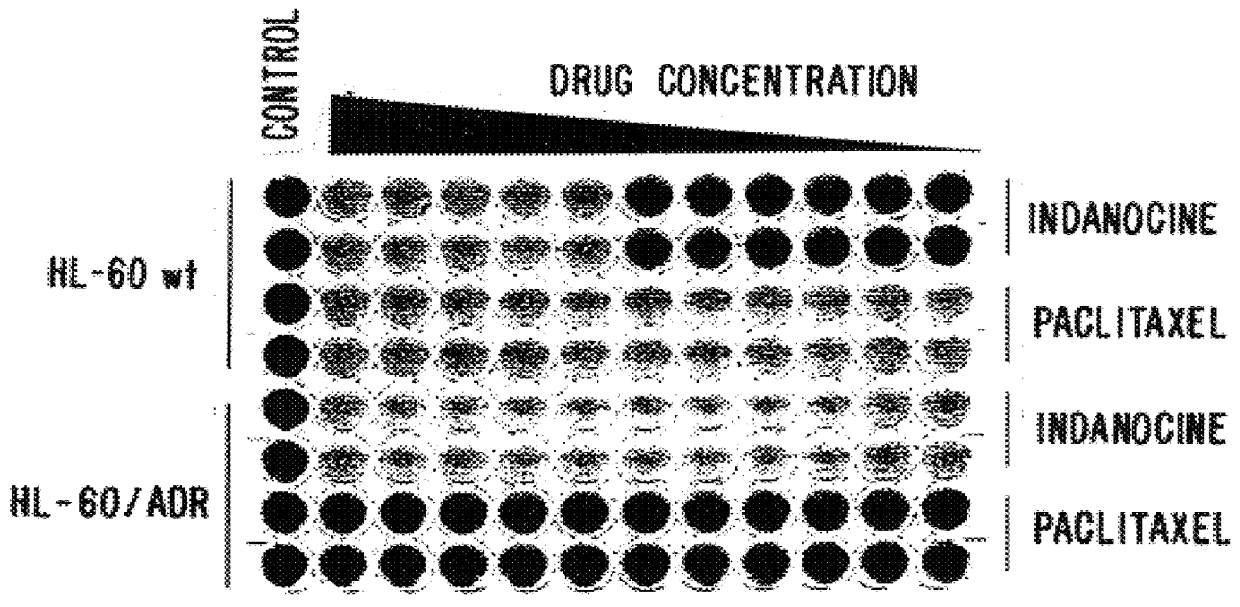
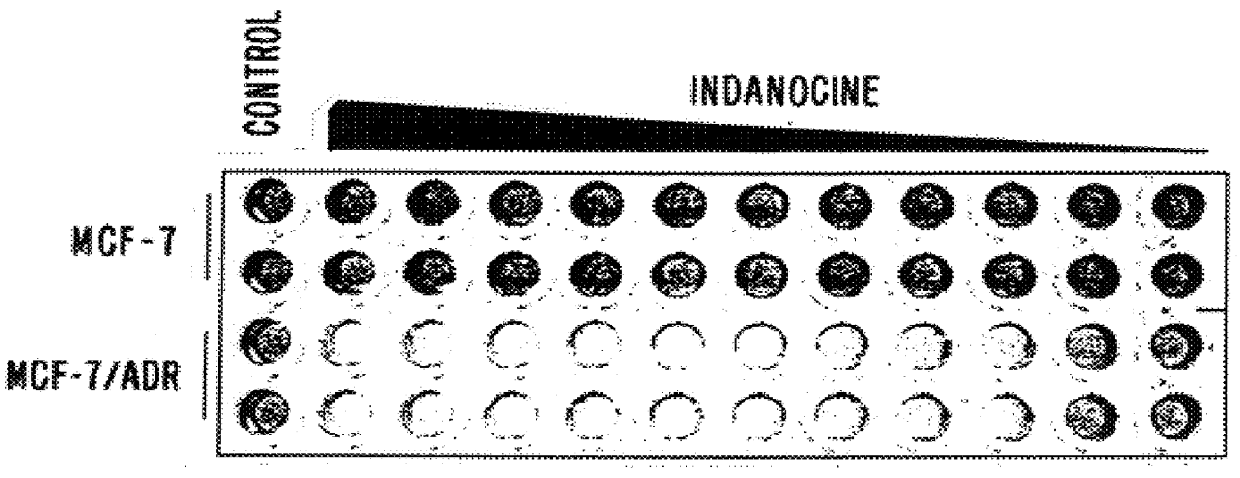
A new family of indanone and tetralone tubulin-binding compounds (TBs) is disclosed. Unlike classical TBs, which inhibit mitosis among affected dividing cells, the TBs of the invention possess two unique properties: (1) they induce apoptosis among stationary phase (non-dividing) malignant cells, yet do not impair the viability of normal nonproliferating cells; and, (2) they affect cells which have acquired MDR more powerfully than they affect cells without MDR. Thus, the TBs of the invention provide means to target malignant cells for chemotherapy, even after previous therapies have failed, without affecting normal cells and tissues in the host.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing alpha-tetralin ketone by catalyzed oxidation tetrahydronaphthalene
InactiveCN101177386AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetraloneTetralin
The invention relates to a method of preparing Alpha-tetralone by catalysis and oxidation of tetralin, comprising following steps: quinonyl compounds and N-hydroxyphthalimade are combined to be double-component non-metallic catalysis system; air or oxygen is used as oxygen source to prepare Alpha-tetralone through catalysis and oxidation of tetralin with high efficiency under a certain condition; the applied quinonyl compounds comprise organic compounds, such as benzoquinones, naphthoquinones and anthraquinones and substituted derivatives of the organic compounds. The invention has the advantages of mild preparation conditions for the method of preparing the Alpha-tetralone, high efficiency and environmental friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of substituted methylene benzocyclodecene ketoxime
ActiveCN101671269AEasy to operateAvoid high vacuum distillationOximes preparationHydroxylamine HydrochlorideDiethyl ether
The invention discloses a preparation method which is characterized by using 7-methoxy-1-methyl-2-tetralone as an initial raw material to synthesize 5,6,7,8,9,10,11,12-octahydro-3-methoxy-5-methyl-5,11-methylene benzocyclodecene-13-ketoxime. In the method, 1,5-dibromopentane is taken as an alkylating agent in alkaline environment in the presence of methylbenzene and other solution as well as phase transfer catalysts to carry out alkylation reaction, forms ring in the presence of sodium hydride and then reacts with hydroxylamine hydrochloride. The preparation method of the invention features mild reaction conditions, avoids the defects of low temperature required originally, requirement of high vacuum distillation, use of diethyl ether for extraction, complex operation and the like, and has the advantages of simple operation, high safety, good product quality and low cost, etc.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for synthesizing alpha-tetralone by gas solid phase reaction
InactiveCN101633611AAvoid the problem of being unable to reuse and discharge large amounts of acidic wastewaterImprove automationMolecular sieve catalystsCatalyst regeneration/reactivationTetraloneFixed bed
The invention relates to a method for synthesizing alpha-tetralone by gas solid phase reaction. Benzene and gamma-butyrolactone are taken as raw materials; a molecular sieve is taken as a catalyst; the alpha-tetralone is continuously synthesized in a fixed bed reactor by the gas solid phase reaction; the reaction temperature is 210-300 DEG C; the reaction is carried out under the condition that the liquid space velocity is 1-6.0 h<-1>; the average conversion rate of gamma-butyrolactone is 25-79%; and the average mole yield of alpha-tetralone is 5-40%. The catalyst which is devitalized can be reproduced through oxygenation ignition to recover the catalyst performance. The method for synthesizing alpha-tetralone has the characteristics of avoiding the problems of not reusing the catalyst and discharging a great amount of acidic waste water. A serialization synthetic process is beneficial to automation production and production efficiency and enhances the production efficiency.
Owner:ZHEJIANG UNIV
Method for extracting, separating and purifying chemical components of walnut green seedcases and application of chemical components
InactiveCN109734758AStrong growth inhibitory activitySugar derivativesSteroidsBenzoic acidBeta-Carotene
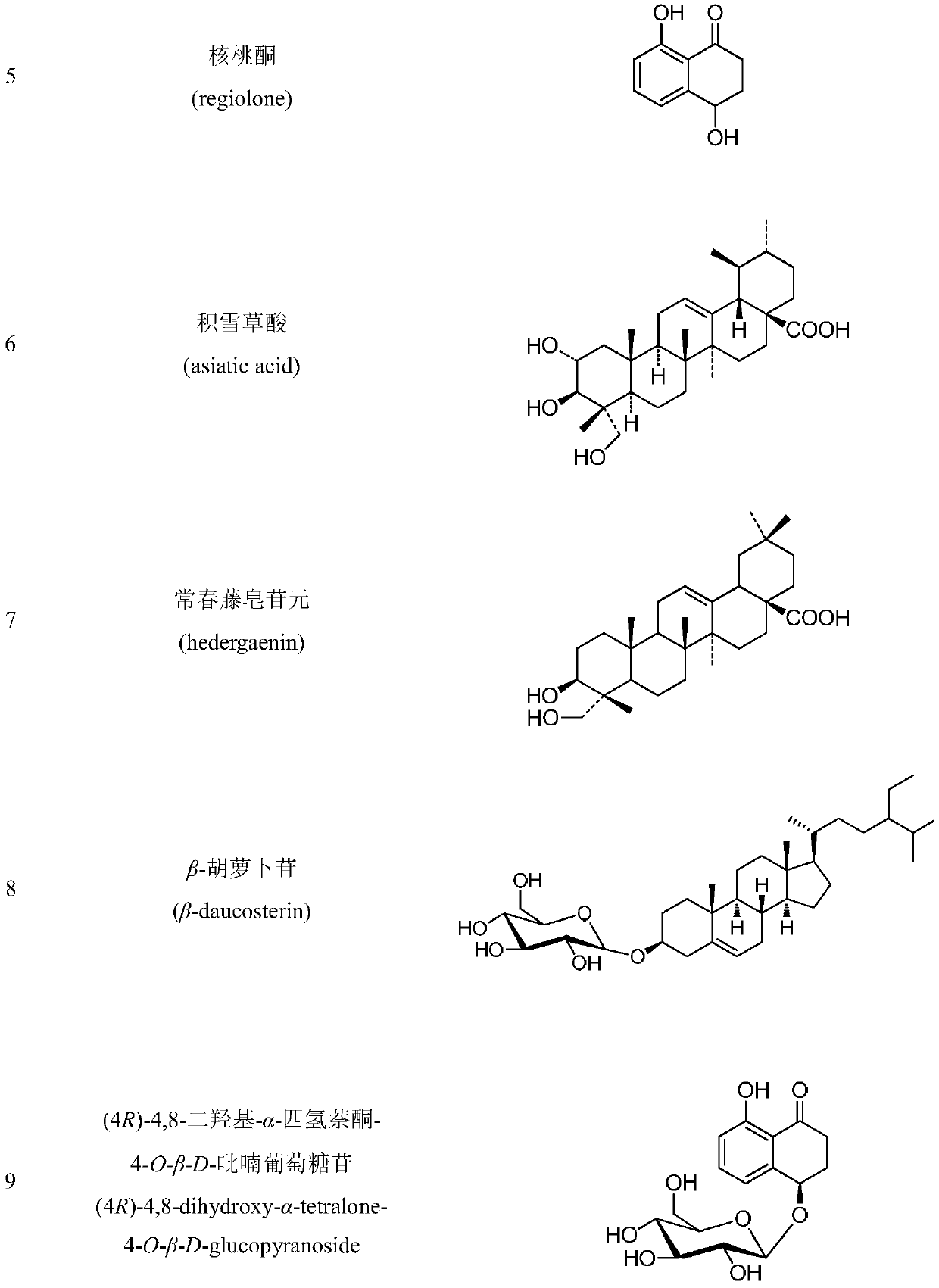
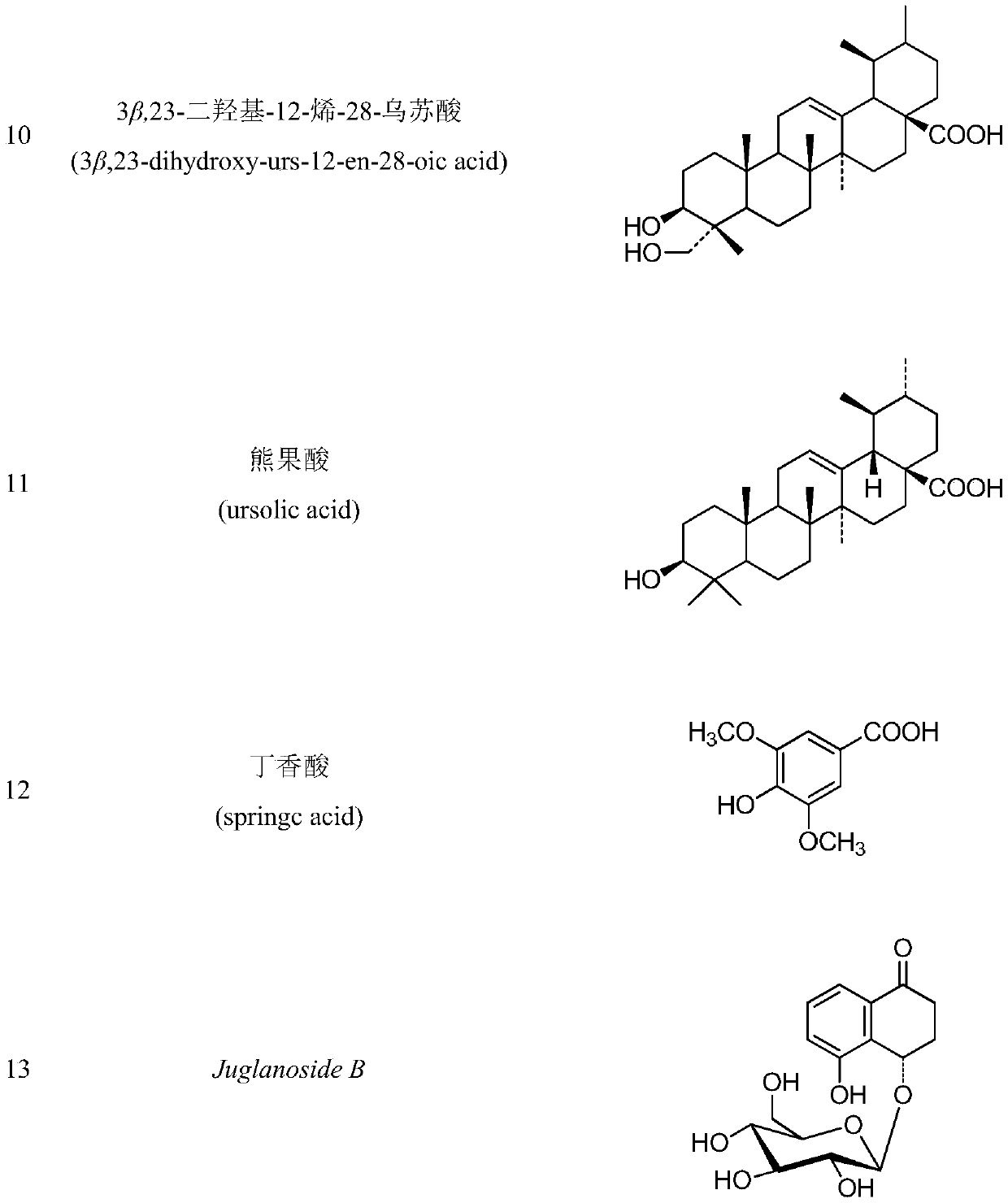
The invention discloses a method for extracting, separating and purifying chemical components of walnut green seedcases and application of the chemical components. According to the method, 13 compounds are separated from the walnut green seedcases and respectively identified as 3,5-dimethoxy-4-hydroxy-benzoic acid-7-O-beta-D-glucoside (1), myricananin F (2), beta-sitosterol (3), oleanolic acid (4), walnut ketone (5), asiatic acid (6), hederagenin (7), beta-carotene (8), (4R)-4,8-dihydroxy-alpha-tetralone-4-O-beta-D-glucopyranoside (9), 3beta,23-dihydroxy-12-olefin-28-ursolic acid (10), ursolicacid (11), syringic acid (12) and Juglanoside B (13). After screening of anti-tumor activity in vitro, it is found that some compounds have certain growth inhibitory activity against cancer cells andcan be further used for preparing anticancer drugs.
Owner:新乡医学院三全学院
Method for preparing lasofoxifene intermediate
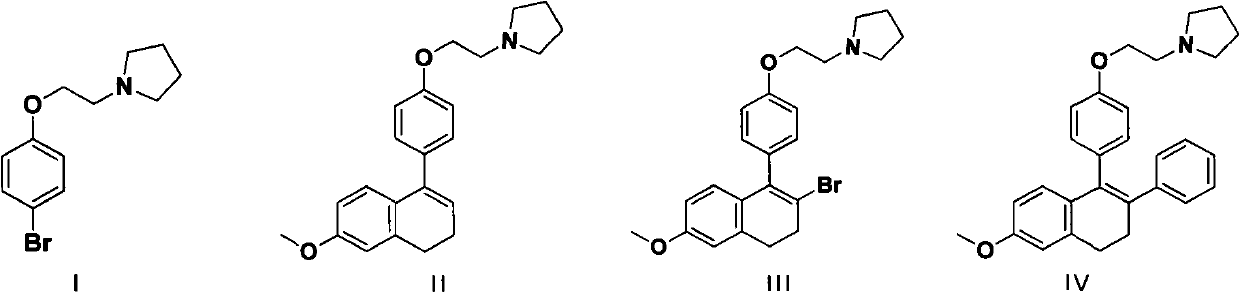
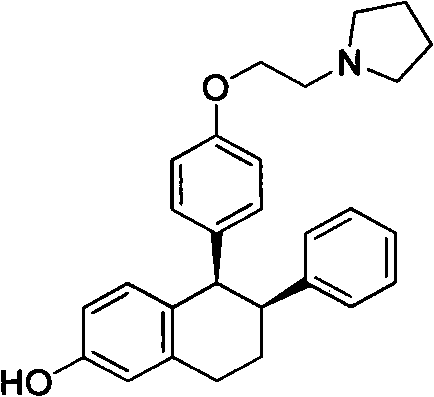
InactiveCN102311406AReduce manufacturing costShort routeOrganic chemistryPhenylboronic acidTetralone
The invention relates to a method for preparing a lasofoxifene intermediate (namely, a compound IV) or an inorganic acid salt thereof. The method comprises the following steps: after a compound I is converted into a Grignard reagent, reacting the Grignard reagent with 6-methoxy tetralone so as to obtain a compound II or an inorganic acid salt thereof; carrying out bromination reaction on the obtained compound II or the inorganic acid salt thereof so as to obtain a compound III or an inorganic acid salt thereof; and then, carrying out coupling reaction on the obtained compound III or the inorganic acid salt thereof so as to obtain the lasofoxifene intermediate (namely, the compound IV) or the inorganic acid salt thereof. The compound IV or inorganic acid salt thereof is a key intermediate for preparing a medicament lasofoxifene for treating osteoporosis. The method provided by the invention is low in production cost, good for environmental protection, and suitable for industrial production.
Owner:WUHAN QR PHARMA CO LTD +1
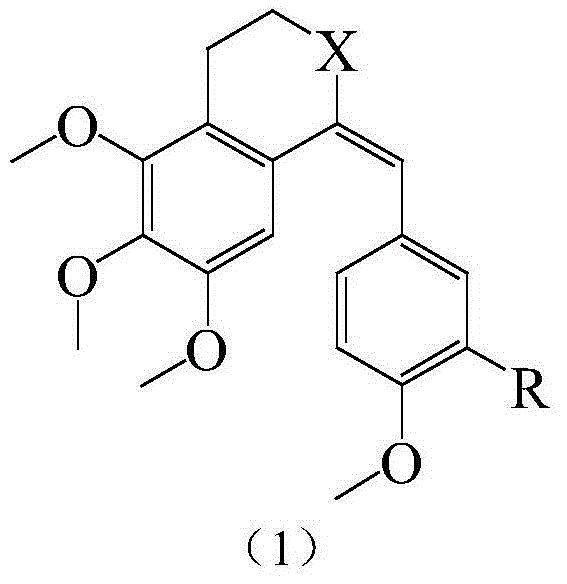
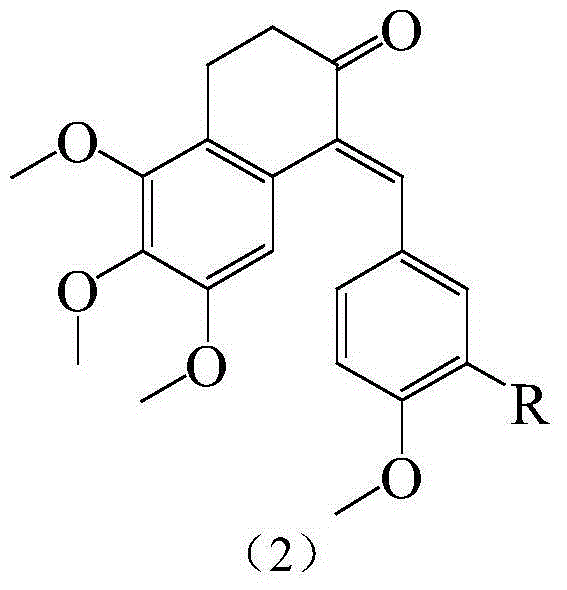
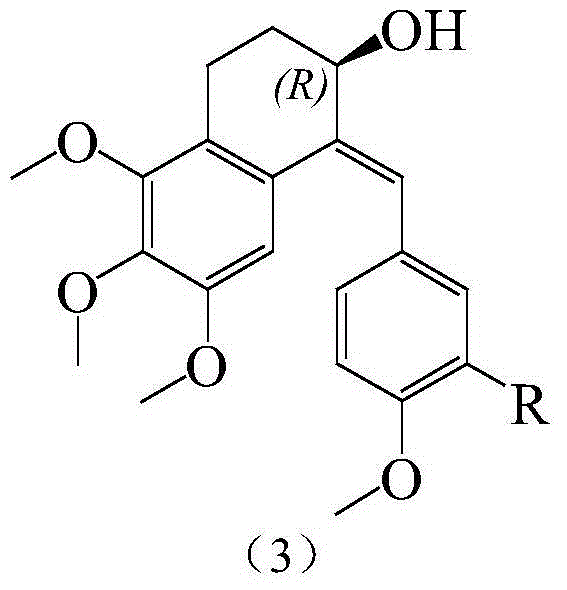
Substituted benzylidene tetralone derivatives and preparation method and applications
InactiveCN104649879AConvenient researchGood water solubilityAmino preparation from aminesCarboxylic acid nitrile preparationSolubilityTetralone
The invention relates to substituted benzylidene tetralone derivatives and a preparation method and application. The chemical structural formula of the substituted benzylidene tetralone derivatives is shown in the formula (1) in the specification, and in the formula (1), X represents CHOH or C=O, and R represents any one of H, OH, F, Cl, CN, CONH2, NO2, CH3, OCH3 and NH2. In the synthetic route, any substitution group can be introduced into two benzene rings, thus eliminating the limit on organic synthesis for finding out compounds with better activity. After water-soluble groups such as amino and the like are introduced, the water solubility can be greatly improved compared with a pilot compound CA-4, and the research of druggability is facilitated. In addition, after amino, hydroxyl and the like are introduced, not only can the bioactivity be improved, but also a prodrug can be prepared on the basis of the group, and the in-vivo activity study can be favorably conducted. The derivatives have the effects of treating ovarian cancer, colon cancer, thyroid cancer and leukemia.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for synthesizing tetralone by liquid-phase catalytic oxidation of tetralin
InactiveCN101337872AImprove conversion rateHigh selectivityMolecular sieve catalystsCarbonyl compound preparation by oxidationTetraloneHydrogen
The invention relates to a method for catalytically oxidizing tetrahydronaphthalene to tetralone in liquid phase. The invention relates to the synthesis method of ketone compounds, particularly the synthesis method of tetralone. The invention aims to develop a method for catalytically oxidizing tetrahydronaphthalene to tetralone in liquid phase at high conversion rate and high selectivity. The method selects a mesoporous material as a heterogeneous catalyst to catalytically oxidize tetrahydronaphthalene to tetralone under the condition of low temperature and liquid phase. The test result shows that the conversion rate of tetrahydronaphthalene is 97.6%, and the selectivity of tetralone is 75.5%.
Owner:YUNNAN UNIV
Preparation technique of 5-methoxy-2-tetralone
The invention discloses technology for preparing 5-methoxyl-2-tetralone, which belongs to a method for preparing tetralone compounds and is technology for preparing the 5-methoxyl-2-tetralone by using sodium metals to reduce 1,6-dimethoxy benzene in an alcohol medium and an ammonia medium at a temperature of between 15 and 35 DEG C, wherein the weight ratio of anhydrous alcohol to the 1,6-dimethoxy benzene during reduction is 6.0-9.0:1; the weight ratio of an ammonia liquid to the 1,6-dimethoxy benzene is 0.05-0.4:1; the weight ratio of the sodium metals to the 1,6-dimethoxy benzene is 0.7-1.2:1; and the reduction temperature is between 15 and 35 DEG C, and the reduction time is between 35 and 48 hours. The technology for preparing the 5-methoxyl-2-tetralone has the advantages of easily obtained reaction raw materials, simple conditions, easy industrialization and high selectivity of the 5-methoxyl-2-tetralone. The 5-methoxyl-2-tetralone is important medical intermediate, and is mainly used for synthesizing medicines for treating Parkinson's disease.
Owner:启东市滨化供水有限公司
Alpha, beta-unsaturated carbonyl tetralone derivative and application thereof
InactiveCN106278857AHas anti-Alzheimer's disease-related pharmacological activityPharmacologically activeNervous disorderOrganic chemistryDiseaseTetralone
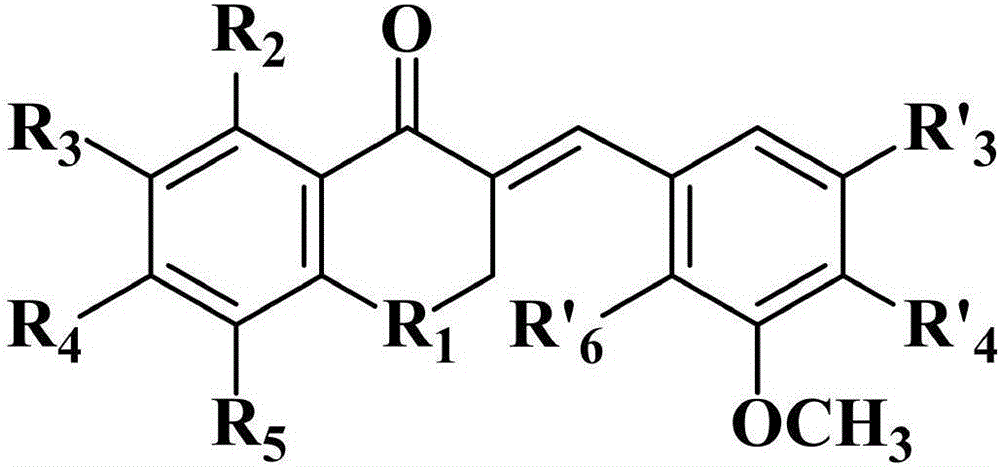
The invention discloses an alpha, beta-unsaturated carbonyl tetralone derivative and application thereof. The alpha, beta-unsaturated carbonyl tetralone derivative has the following structural formula, wherein R1 represents methylene or -HC-CH3; R2 represents hydrogen atoms or methoxyl; R3 represents hydrogen atoms, chlorine atoms, methoxyl, bromine atoms, fluorine atoms or nitryl; R4 represents hydrogen atoms or methoxyl; R5 represents hydrogen atoms, hydroxyl or methoxyl; R'3 and R'4 respectively represent hydrogen atoms or methoxyl; R'6 represents chlorine atoms or bromine atoms. The synthetic compound has pharmacological activities associated with anti-Alzheimer's disease, and is suitable for serving as a drug that can prevent, treat and diagnose Alzheimer's disease, and has a good potential application prospect. (Please see the formula in the description).
Owner:WUHAN UNIV OF TECH
Montelukast sodium preparation technology and intermediates
ActiveCN104293850AEfficient reuseReduce typesOrganic chemistryChemical recyclingTetralonePtru catalyst
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS20060052639A1Easy to handleOrganic compound preparationCarboxylic acid amides preparationTetraloneHydrogen
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Manganese-base catalyst for synthesizing alpha-tetralone from tetrahydronaphthalene and preparation method thereof
InactiveCN102886257AImprove low temperature catalytic activityLow costOrganic compound preparationCarbonyl compound preparationTetraloneOxygen
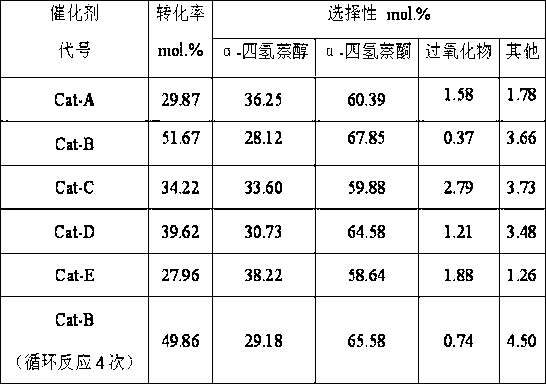
The invention provides a manganese-base catalyst for synthesizing alpha-tetralone from tetrahydronaphthalene and a preparation method thereof, belonging to the technical field of catalyst preparation. The manganese-base catalyst provided by the invention uses active aluminum oxide as a supporter; and Mn oxide is used as the main active component of the catalyst, and oxide of Zr, Co, Fe, Cu or Ce is used as the auxiliary active component of the manganese-base catalyst. The preparation method comprises the following steps: impregnating the active aluminum oxide supporter in a mixed solution of Mn nitrate and nitrate of Zr, Co, Fe, Cu or Ce, drying, and roasting to obtain the catalyst. The manganese-base catalyst provided by the invention has the outstanding advantage of high low-temperature activity, has the characteristic of low cost, can completely substitute toxic chrome-containing catalyst, and has favorable industrial application prospects. For example, after the reaction is carried out at 90 DEG C for 8 hours by using oxygen as an oxidizer, the conversion rate of tetrahydronaphthalene is up to 51.67%; and after the reaction is circulated four times, the activity of the catalyst is basically unchanged, and the peroxide content in the product is very low.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
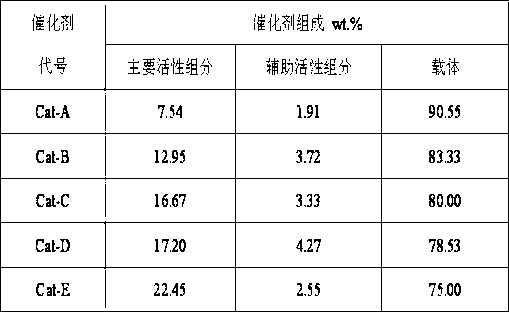
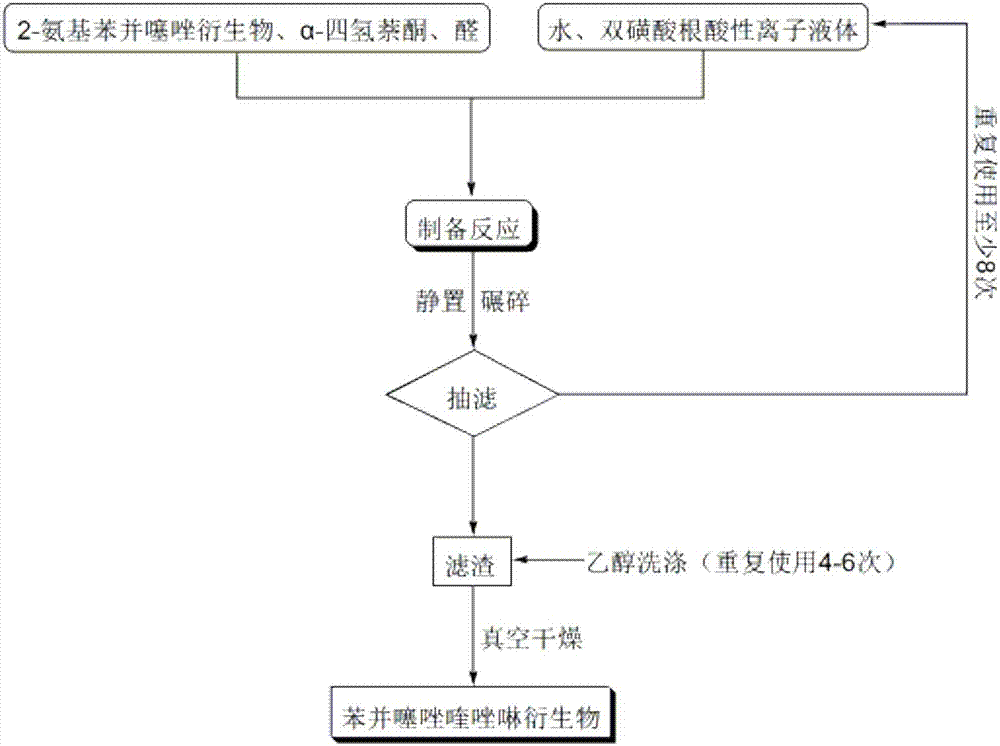
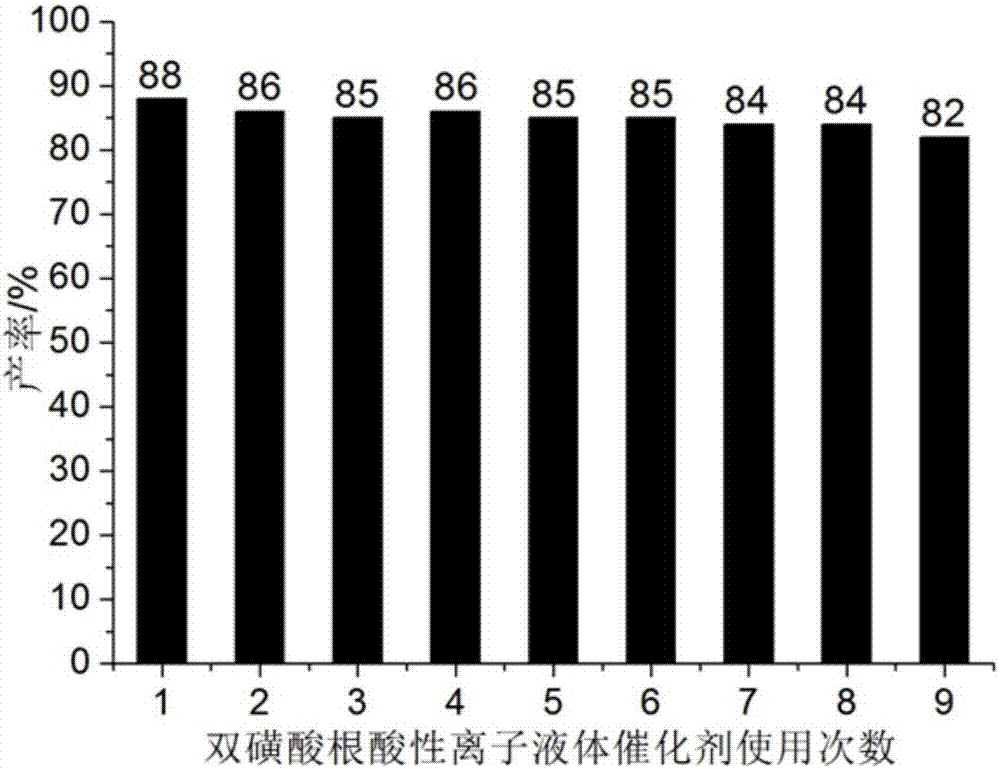
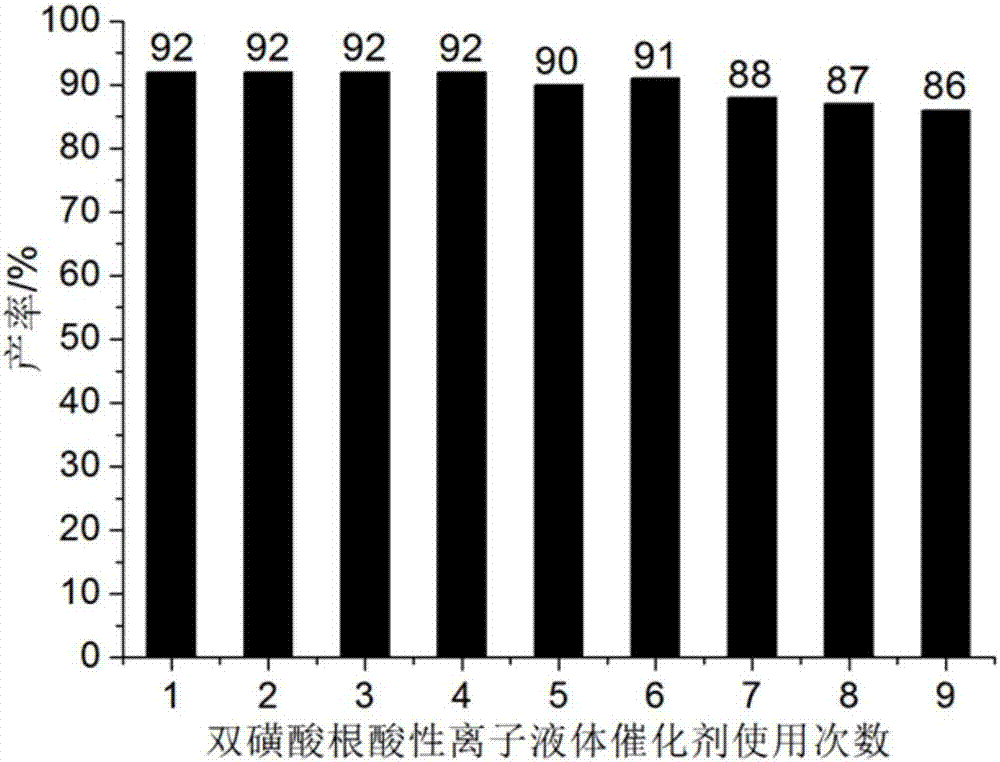
Method for preparing benzothiazole quinazoline derivatives through catalysis
ActiveCN106967095AHigh catalytic activityReduce usageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsWater basedTetralone
The invention discloses a method for preparing benzothiazole quinazoline derivatives through catalysis and belongs to the technical field of ionic liquid catalysis. In the preparation reaction, the molar ratio of 2-aminobenzothiazole derivative to alpha-tetralone to aldehyde is 1:1:(1-1.1), the molar use amount of a double sulfonate radical acidic ionic liquid catalyst is 5 to 8 percent of the molar use amount of 2-aminobenzothiazole derivative, the volume amount of reaction solvent water based on milliliter is 5 to 7 times of the molar weight of the 2-aminobenzothiazole derivative based on millimole, the reaction temperature is 65 to 78 DEG C, and the reaction time is 24 to 57 minutes; after the reaction, cooling is conducted to room temperature, suction filtration is conducted, and the filter residue is washed with ethanol and vacuum-dried to obtain the benzothiazole quinazoline derivatives. Compared with the existing preparation method, the method for preparing the benzothiazole quinazoline derivatives through catalysis has the characteristics that the use amount of catalyst is small, the catalytic activity is stable, biodegradation is facilitated, the whole preparation process is simple and convenient to operate, and the greening degree is high; and industrialized large-scale production is realized easily.
Owner:东营睿港投资服务有限责任公司
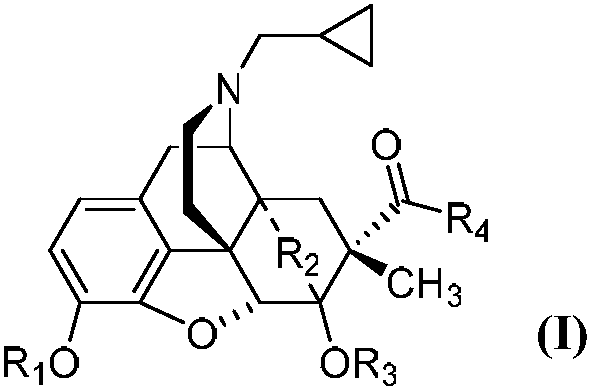
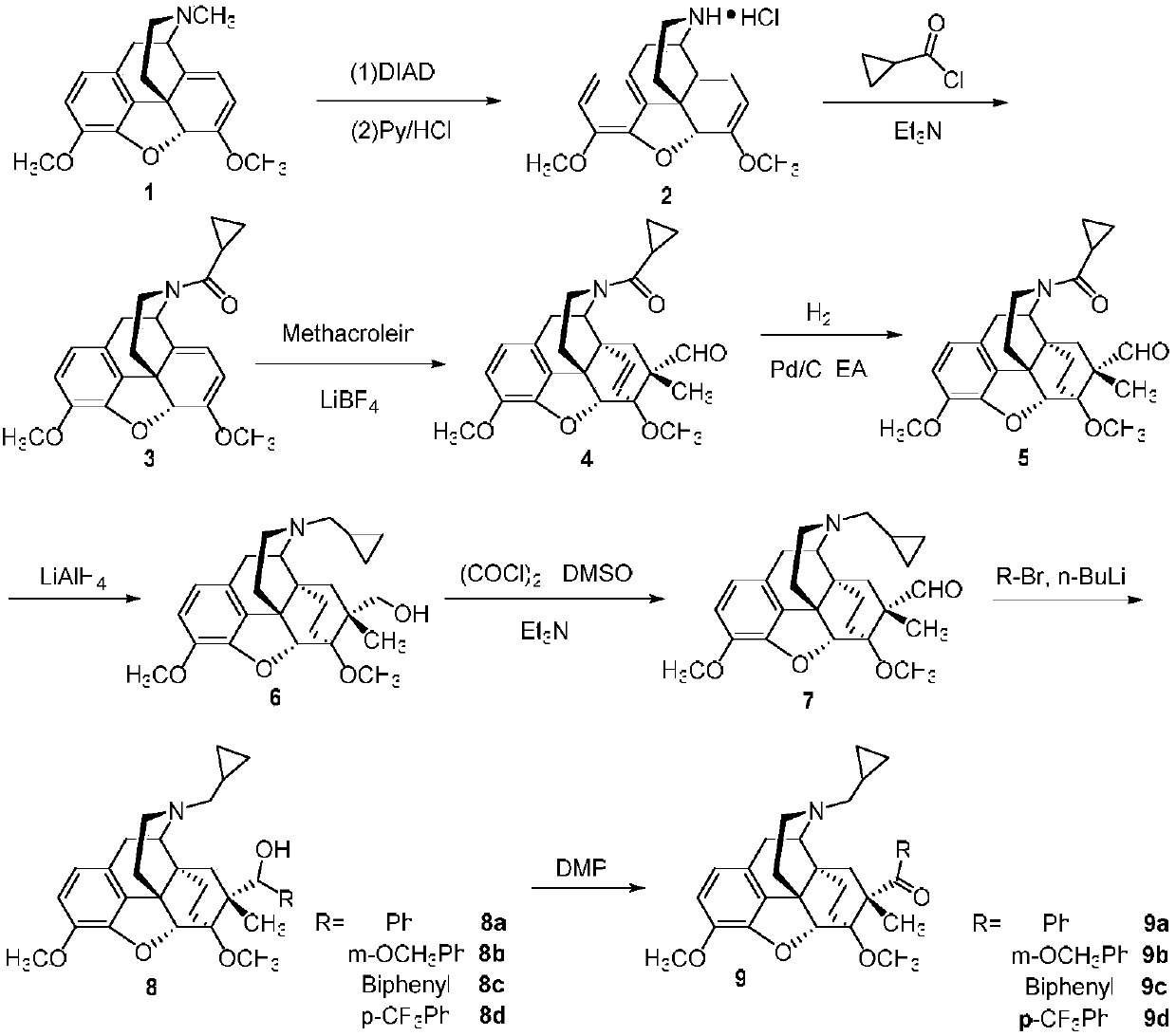
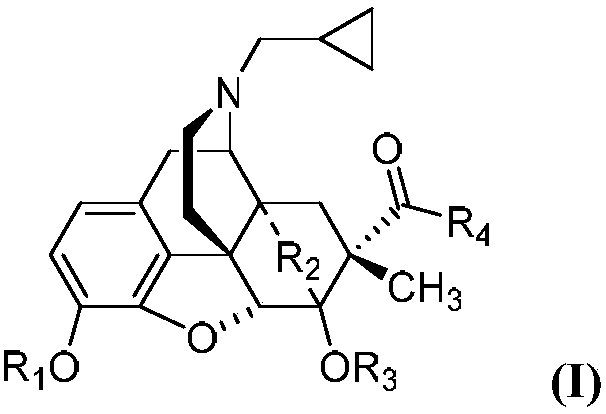
7Beta-methyl-tetralone derivative and preparation method and application thereof
The invention belongs to the field of chemical pharmacy and relates to a 7beta-methyl-tetralone derivative shown in a general formula (I) and a preparation method thereof. According to the compound shown in the general formula (I), with thebaine as a raw material, through related conversion methods such as N-demethylation, N-acylation, a Diels-Alder reaction and reduction, oxidation and Grignard reactions, the compound is synthesized. The compound shown in the general formula (I) belongs to an opioid receptor ligand, a radioactive receptor ligand combination experiment is adopted, the affinityand selectivity of the ligand to three subtypes of an opioid receptor are measured, and a [35S]GTPgammaS combination experiment is adopted for measuring the excitement inhibitory activity of the ligand; it is proved through a result that the compound has the functions of alleviating pain, resisting depression, withdrawing opioid addiction, relieving itching and the like and can be applied to preparation of an opioid receptor treatment medicine and applied to clinical analgesia or depression resistance or opioid addiction withdrawal treatment or itching relieving treatment.
Owner:FUDAN UNIV
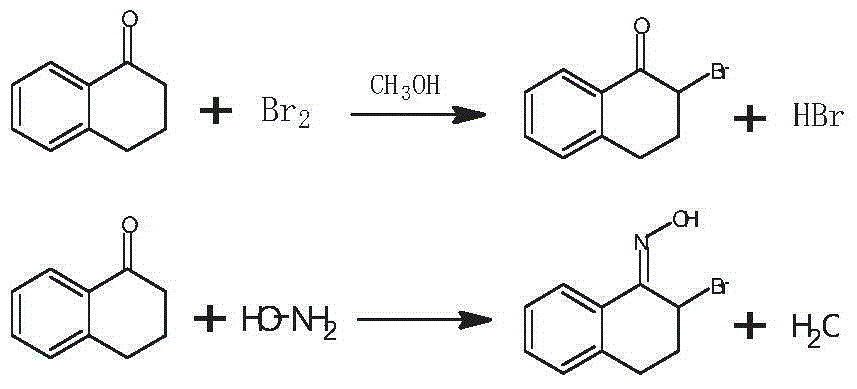
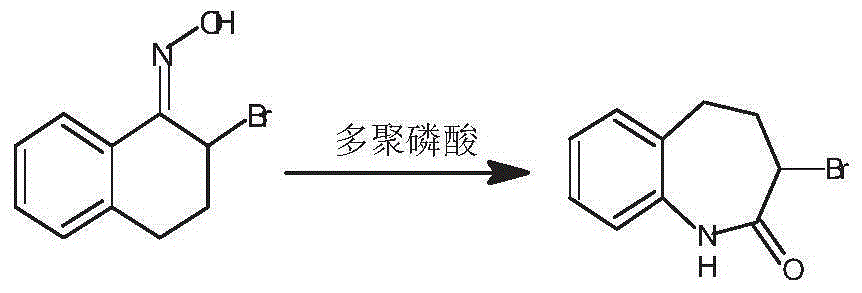
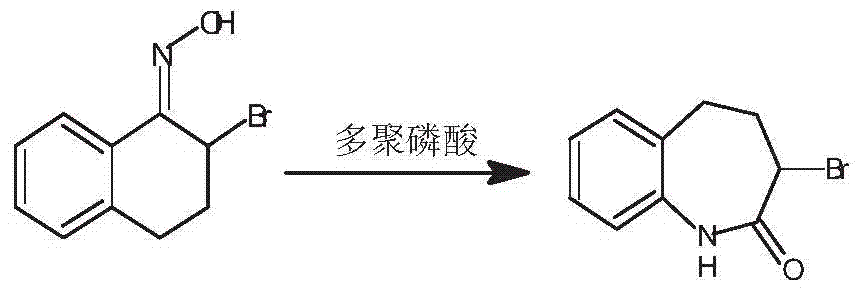
Preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-keto
The invention discloses a preparation method of 3-bromo-1,3,4,5-tetrahydro-2H-benzazepine-2-keto. The preparation method comprises the following stages: alpha-tetralone preparation stage, 2-bromo-3,4-dihydro-N-hydroxy-(2H)-naphthalimide preparation stage and 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepine-2-keto, wherein the alpha-tetralone preparation stage comprises the following steps: after mixing gamma-butyrolactone, benzene and aluminum chloride anhydrous, raising the temperature to be 60-90 DEG C, and preserving the temperature for reaction for 5-30 hours; after the reaction is finished, performing hydrolysis, layering and washing on 25 parts of 6% hydrochloric acid solution until neutral; and then distilling to remove excessive benzene, and performing high vacuum rectification to obtain alpha-tetralone. Then, the finished product is finally prepared through a bromination reaction, an oximation reaction and a beckmann rearrangement reaction, the purity of the finished product is high, and the yield is as high as more than 90%. Moreover, the production technology is simple, the production cost is low, and the pollution is little.
Owner:ZHEJIANG BOJU NEW MATERIALS CO LTD
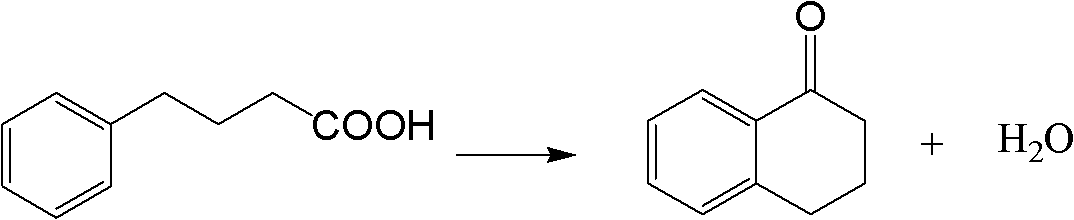
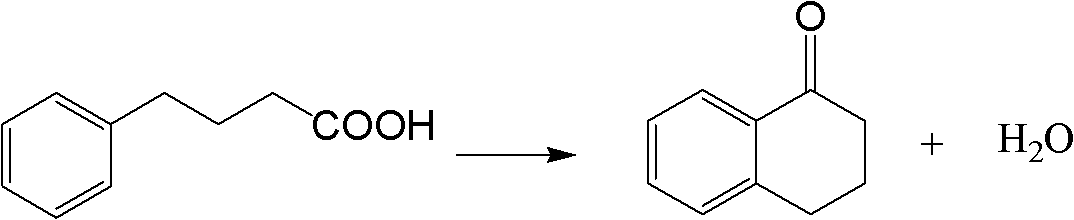
Method for synthesizing Alpha-tetralone through 4-phenylbutyric acid in catalytic way
InactiveCN102584556AOvercoming the problems of being easily deactivated and difficult to recycleContinuous operationCarbonyl compound preparation by condensationTetraloneSolid acid
The invention provides a method, which is characterized in that in a fixed bed device, solid acid is used as a catalyst, 4-phenylbutyric acid is used as a raw material and is dissolved into 1,2-dichlorobenzene according to a certain solid-to-liquid ratio, mixed liquid continuously flows through a catalyst layer after being gasified, the reaction is carried out on a catalyst, and the Alpha-tetralone can be continuously produced. The method solves the problem that in the existing intermittent liquid phase reaction system, the catalyst is easily inactivated and cannot be cyclically used. The gas-solid phase reaction has the advantages that the contact time between reactants and catalysts can be regulated so that the reaction condition is optimized, products generated in the reaction process are continuously separated in the reaction process, and the positive direction proceeding of the reaction is favorably realized. In addition, because the fixed bed catalytic mode is adopted, generated products leave the catalytic bed layer in time in the reaction process, the reaction can be continuously operated, the on-line regeneration can also be carried out when the catalyst is inactivated, and the production efficiency is greatly improved.
Owner:ZHEJIANG UNIV
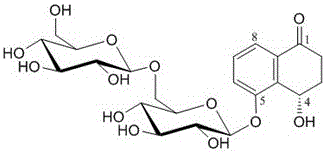
Compound 4(S)-4,5-dihydroxy-alpha-tetralone 5-O-beta-D-glucopyranose (1->6)-beta-D-glucopyranoside, and preparation method and application thereof
ActiveCN105801637AGood inhibition rateExpand sourceSugar derivativesRespiratory disorderD-GlucopyranoseTetralone
The invention discloses a compound with tumor inhibition activity, and a preparation method and application thereof. The compound is diglucoside; one position is simultaneously connected with two molecules of saccharide; a concrete structural formula is 4(S)-4,5-dihydroxy-alpha-tetralone 5-O-beta-D-glucopyranose (1->6)-beta-D-glucopyranoside. Experiments show that the compound provided by the invention has a better inhibition effect on human cervical carcinoma cells and lung cancer cells.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
5-substituted tetralones as inhibitors of ras farnesyl transferase
InactiveUS6943183B2Treating and preventing uncontrolled or abnormal proliferation of tissuesEasy to synthesizeBiocideSenses disorderTetralonePercent Diameter Stenosis
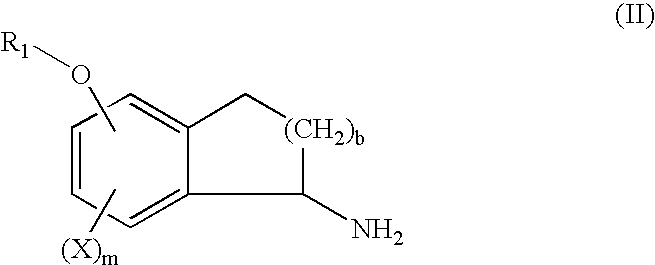
The present invention provides novel 5-substituted tetralones of Formulas I, II, III, and IV and pharmaceutically acceptable salts, esters, amides, and prodrugs thereof, which are useful for treating and preventing uncontrolled or abnormal proliferation of tissues, such as cancer, atherosclerosis, restenosis, and psoriasis. Specifically, the present invention relates to compounds that inhibit the farnesyl transferase enzyme
Owner:WARNER LAMBERT CO LLC
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS7262326B2Organic compound preparationCarboxylic acid amides preparationTetraloneHydrolysis
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Preparation of alpha-tetralone by two-step catalysis method
InactiveCN101544554AReduce dosageHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetraloneCatalytic decomposition
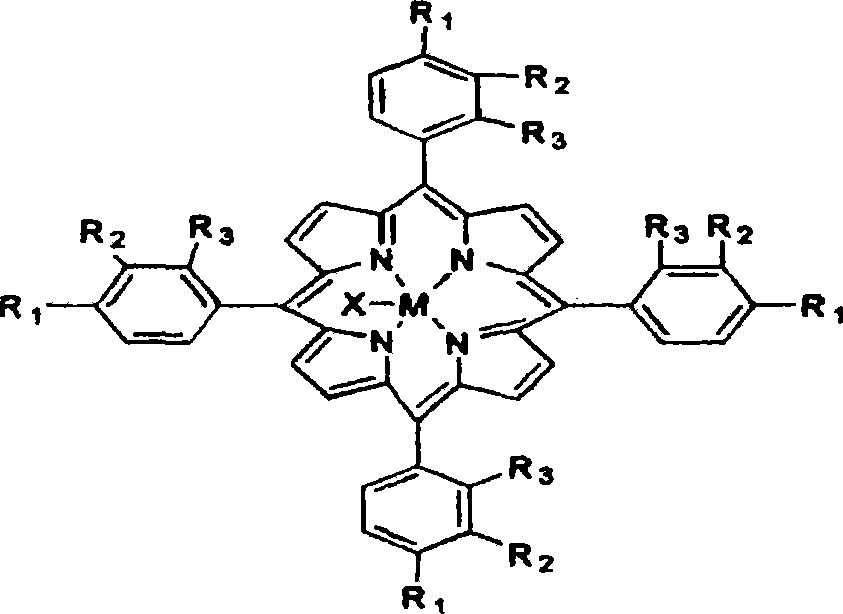
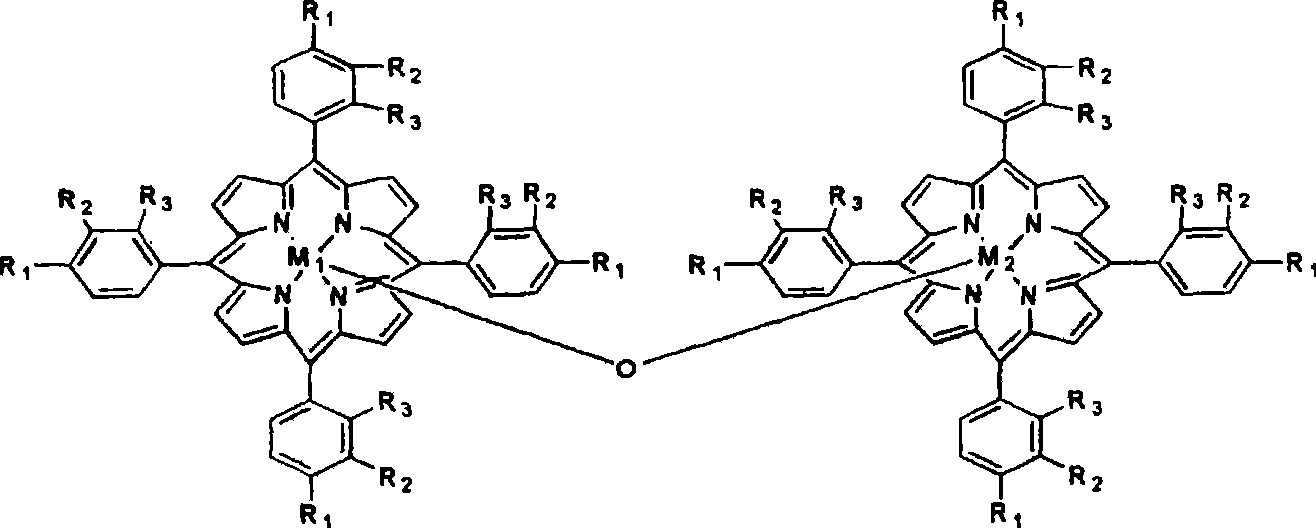
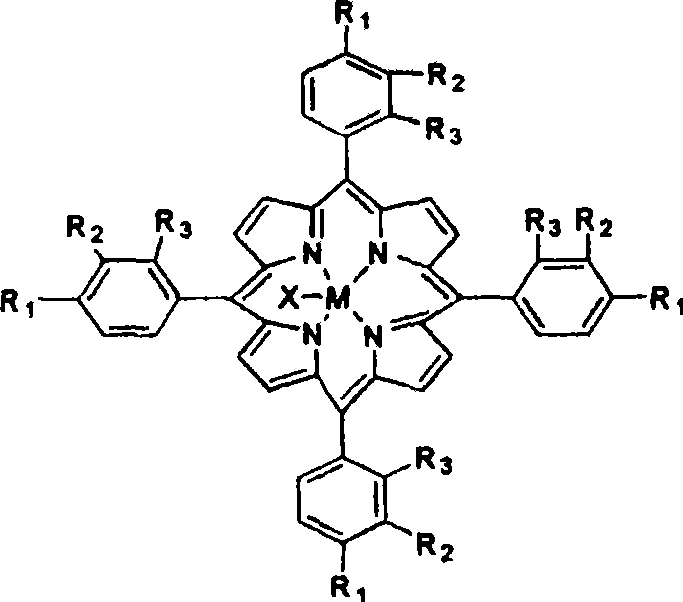
The invention discloses a novel method for preparing alpha-tetralone by using tetrahydronaphthalene as a raw material. The method comprises the following two steps: a first step, under the condition without solvent and assistant, 1 to 20 atm (absolute pressure) of air or oxygen or mixed gas of oxygen and inert gases is introduced, the reaction temperature is between 60 and 150 DEG C, monometallic porphyrin of general formula (I) or (II) and mu-O-bimetallic porphyrin or solid carriers thereof are used as a catalyst, the concentration of the catalyst is 1 to 80 ppm, the reaction time is 1 to 12 hours, and oxidized mixed solution containing high alpha-tetrahydronaphthalene peroxide and alpha-tetralone can be selectively obtained; and a second step, the alpha-tetrahydronaphthalene peroxide in the oxidized mixed solution obtained in the first step is high selectively and directionally decomposed into the alpha-tetralone by using transition metal salt as a catalyst. In the step of catalytic decomposition, the molar ratio of transition metal irons to the alpha-tetrahydronaphthalene peroxide is 1:1-100, and the reaction temperature is between 0 and 100 DEG C. The method has the advantages of short reaction time, mild reaction conditions, little using amount of the catalyst, high conversion rate and good selectivity of a target product; and the used catalyst is environment-friendly and has no corrosion to equipment.
Owner:HUNAN UNIV
Synthesis method of 2-chloro-4-phenylbenzoquinazoline
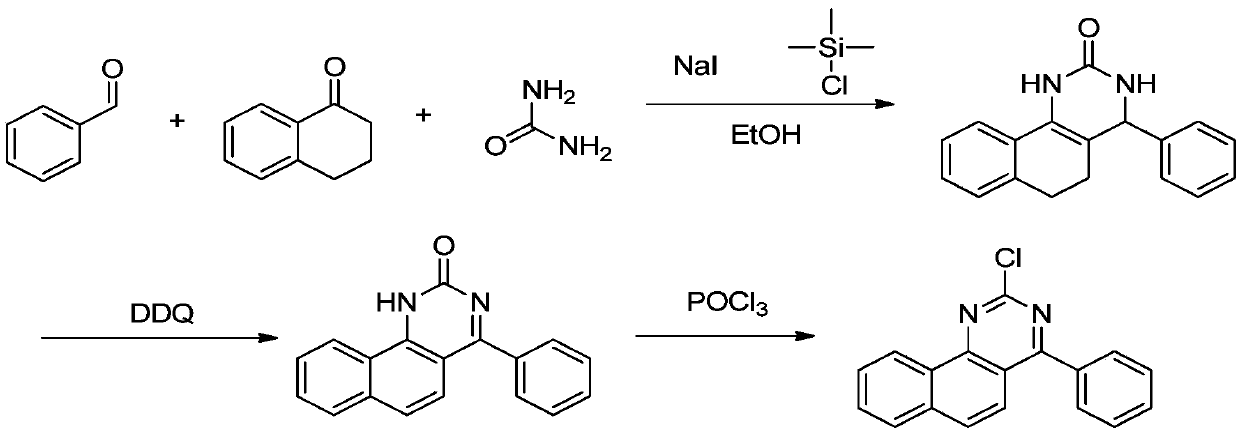
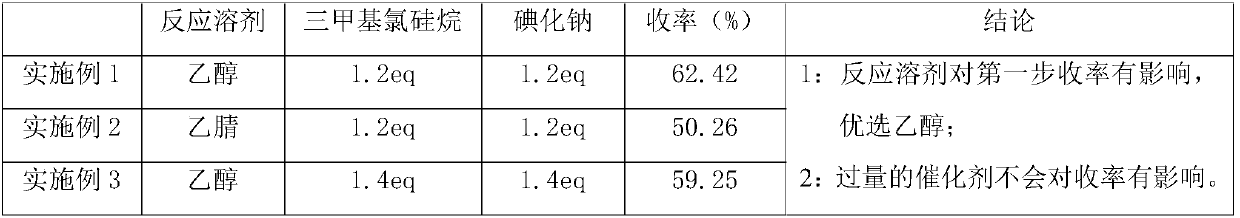
InactiveCN111377872APromote crystallizationMild reaction conditionsOrganic chemistryChemical synthesisTetralone
The invention discloses a synthesis method of 2-chloro-4-phenylbenzoquinazoline, and belongs to the field of organic chemical synthesis. In the synthesis method, 1-tetralone, benzaldehyde and urea areused as raw materials, and thereby the 2-chloro-4-phenylbenzoquinazoline is obtained through three steps of reaction of bigeminy reaction ring closing, oxidation and chlorination. The method has theadvantages of low requirements on equipment and reaction conditions, accessible raw materials, low cost and short process period, and can greatly enhance the synthesis efficiency and economic benefit.
Owner:GUANGDONG AGLAIA OPTOELECTRONICS MATERIALS
Method for preparing agomelatine
ActiveCN102875408AHigh purityMild responseOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideTetralone
The invention discloses a method for preparing agomelatine represented by structural formula 1. The method includes that 7-methoxy-tetralone represented by structural formula 5 is reacted with dimethyl cyanomethylphosphonate in an aprotic polar solvent under the sodium hydride catalytic action to generate (7-methoxyl-3,4-dihydro-2H-naphthalene-1-subunit) acetonitrile represented by structural formula 4, under the presence of an aromatization reagent, the (7-methoxyl-3,4-dihydro-2H-naphthalene-1-subunit) acetonitrile represented by the structural formula 4 is reacted with an organic acid to generate 1-cyano-7-methoxyl-1-naphthyl alcohol ester represented by structural formula 3, hydrogenation reduction is performed in an ammonia containing alcohol solvent under the catalysis of raney nickel to generate 2-(7- methoxyl-1-naphthyl) ethylamine represented by structural formula 2, finally the 2-(7- methoxyl-1-naphthyl) ethylamine represented by the structural formula 2 is reacted with acetic anhydride to generate the agomelatine represented by structural formula 1, and the agomelatine is separated out in a solid form. The method for preparing the agomelatine represented by the structural formula 1 has the advantages that the synthetic route is short, the reaction conditions are simple and mild, the raw materials are cheap and easy to obtain, the method is environment-friendly, the product yield and purity are high and the like, and the method is suitable for mass industrial production.
Owner:JIANGXI SYNERGY PHARMA
A kind of preparation method of 3-bromo-1,3,4,5-tetrahydro-2h-1-benzazepine-2-one
Owner:ZHEJIANG BOJU NEW MATERIALS CO LTD
Biocatalysis preparation method of chiral tetrahydro-2-naphthol compound
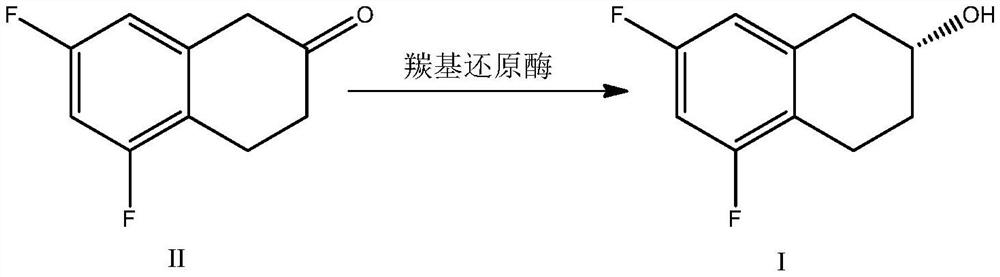
PendingCN113981013AShort reaction stepsMild reaction conditionsBacteriaMicroorganism based processesTetraloneCarbonyl Reductase
The invention discloses a biocatalysis preparation method of a chiral tetrahydro-2-naphthol compound. The biocatalysis preparation method comprises the following steps: (a) with 5,7-difluoro-2-tetralone as a substrate, carrying out asymmetric reduction reaction in the presence of coenzyme under the catalysis of carbonyl reductase in a liquid reaction system to form (R)-5,7-difluoro-1,2,3,4-tetrahydro-2-naphthol; and (b) optionally separating (R)-5,7-difluoro-1,2,3,4-tetrahydro-2-naphthol from the reaction system after the reaction in the step (a). The invention also discloses carbonyl reductase prepared by using the method, a coding gene of the carbonyl reductase, a vector and a bioengineering strain. The method has the advantages of short steps, mild conditions, remarkably improved yield and high optical purity of the product.
Owner:默沃智造(上海)生物技术有限公司
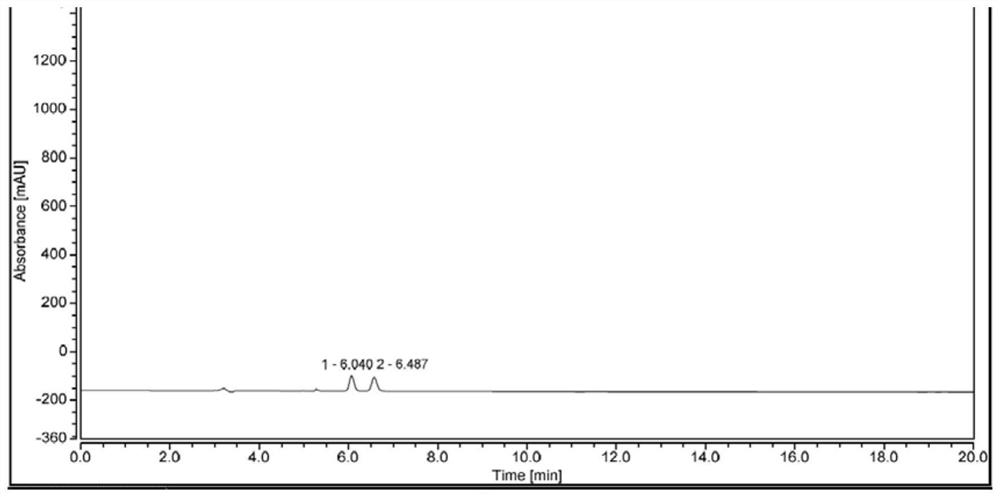
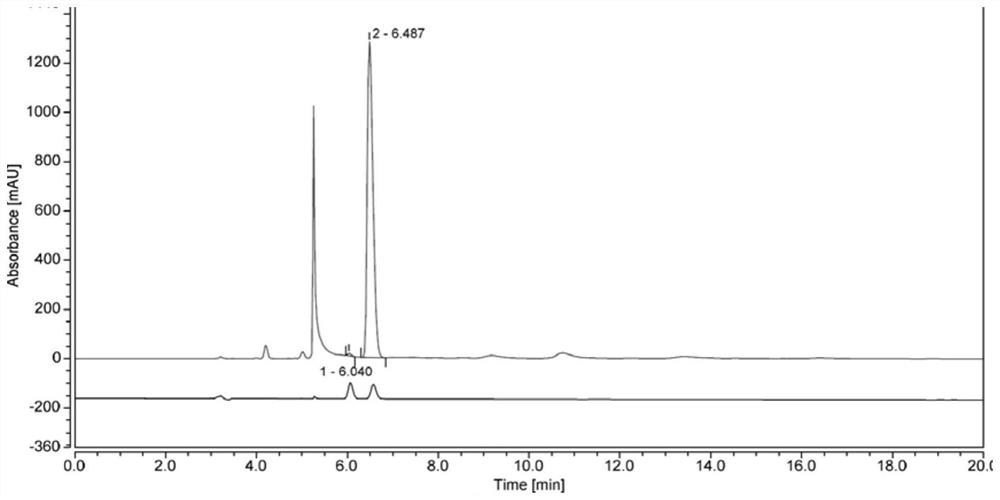
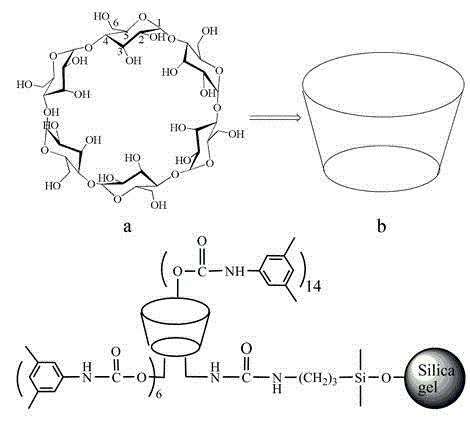
Application of bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral analysis and/or separation of sertraline hydrochloride intermediate (+/-)-Tetralone
InactiveCN104826619AEasy to separateSimple post-processingComponent separationOther chemical processesChromatographic separationTetralone
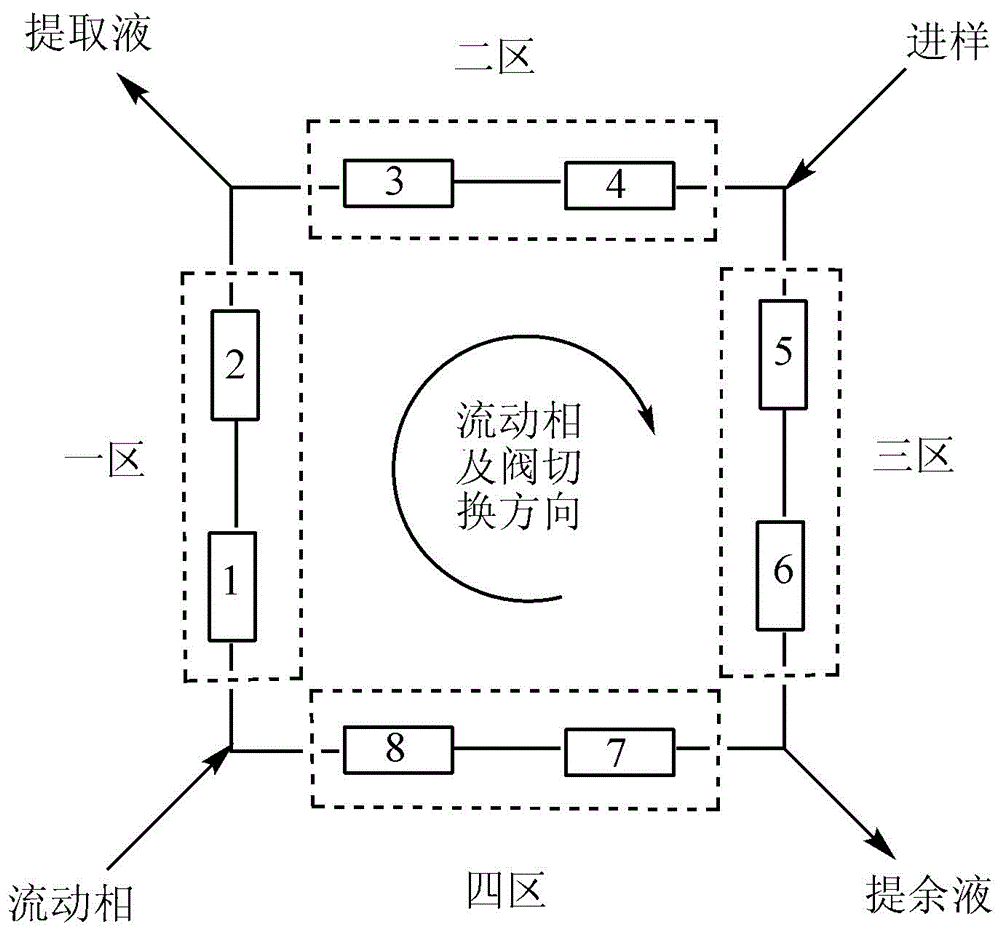
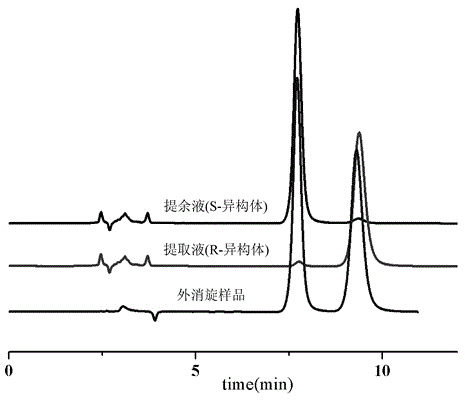
The invention discloses application of a bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral analysis and / or separation of a sertraline hydrochloride intermediate (+ / -)-Tetralone. The invention also discloses application of the bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral separation of a sertraline hydrochloride intermediate (+ / -)-Tetralone by simulated moving bed chromatography. The cyclodextrin chiral stationary phase can have better separating effects than the existing chiral stationary phases under identical conditions. The separation method, which adopts a four-region simulated moving bed system and uses a bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase as a chiral column filler and an n-hexane-fatty alcohol mixed solution as a mobile phase, has the advantages of continuous sampling, high automation degree, high product purity and low mobile phase consumption, is suitable for industrialized chromatographic separation on (+ / -)-Tetralone to obtain the optically Tetralone isomer product, and widens the application range of the cyclodextrin chiral stationary phase in the preparative chromatography system.
Owner:广州研创生物技术发展有限公司
Preparation process of montelukast sodium and its intermediate product
ActiveCN104293850BEfficient reuseReduce typesOrganic chemistryChemical recyclingMethylmagnesium chlorideCoupling
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com