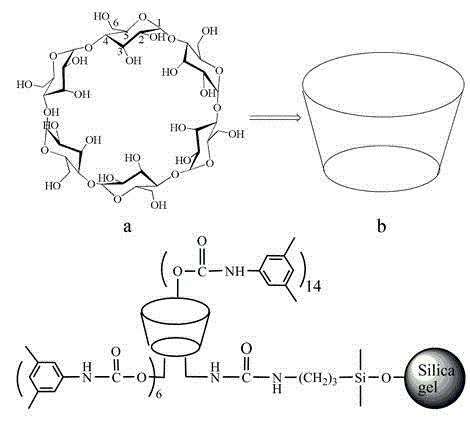
Application of bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral analysis and/or separation of sertraline hydrochloride intermediate (+/-)-Tetralone
A technology of dimethylaniline carbamoyl and chiral stationary phase, which is applied in the field of analysis and separation of chiral compounds, can solve the problems of limitations, coating-type chiral stationary phases are prone to swelling or dissolution, and achieve stable quality , good separation effect, high separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
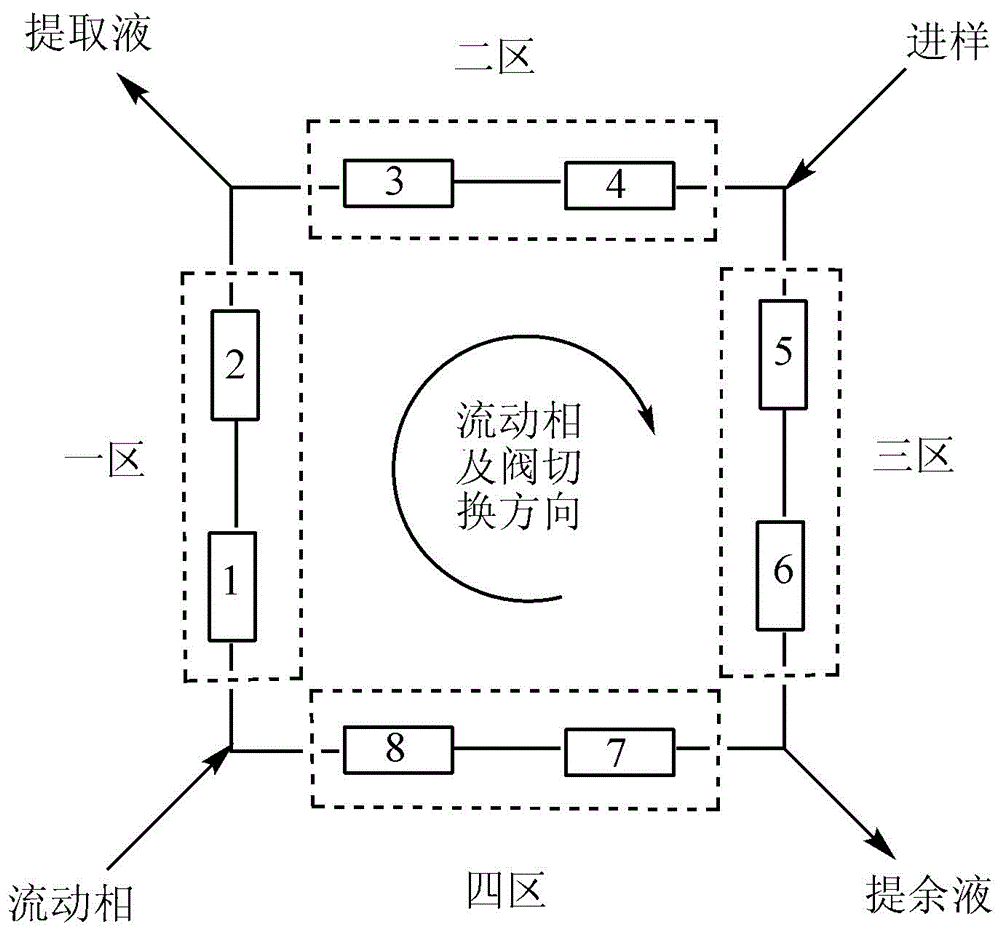
[0057] 1. Selection of mobile phase
[0058] The mobile phase composition affects the separation of (±)-Tetralone racemate and also affects the production efficiency of the simulated moving bed. As shown in Table 1, as the polarity of the mobile phase decreases (replacing ethanol with isopropanol, or reducing the alcohol content), the separation of (±)-Tetralone enantiomers on the chiral stationary phase increases, and the selectivity factor become larger, but the prolongation of the retention time will significantly reduce the production efficiency of the equipment and increase the solvent consumption, so follow-up work is carried out with n-hexane:alcohol=90:10 (v / v).
[0059] Table 1 Selection of mobile phase
[0060]
[0061] Test conditions: Shimadzu LC-15C liquid chromatograph; column specification: I.D.4.6×250mm; filler is bonded 3,5-dimethylphenylcarbamoylated β-cyclodextrin chiral stationary phase (5 μm) ; Detection wavelength: 214nm; Flow rate: 1.0mL min -1 . ...
example 1
[0070] a. Operating conditions
[0071] Mobile phase: n-hexane: ethanol = 90:10 (v / v)
[0072] Injection concentration: 0.50mg·mL -1
[0073] Injection liquid flow rate: 0.05mL min -1
[0074] Eluent flow rate: 1.80mL min -1
[0075] Raffinate flow rate: 1.25mL min -1
[0076] Extraction flow rate: 0.60mL min -1
[0077] Switching time: 5min
[0078] System temperature: 25°C
[0079] b. Product analysis
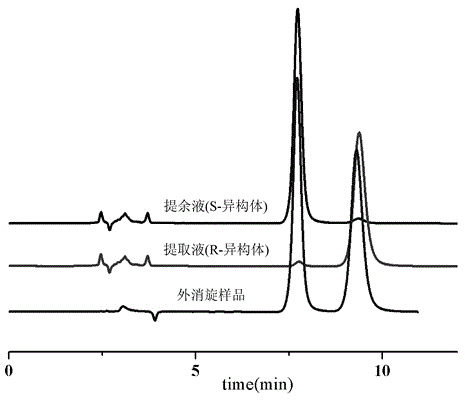
[0080] The simulated moving bed was operated continuously for 24 hours, the sample solution was collected at the raffinate outlet and the extraction outlet respectively, and then the product was analyzed. In the raffinate, the purity of (4S)-(+)-Tetralone was 95.64%; in the extract, the purity of (4R)-(-)-Tetralone was 95.44%. Each kilogram of chiral stationary phase can produce 0.50g of two isomer products each day, the consumption of mobile phase is 74L / g, and the recovery rate of the product is 94%.
example 2
[0082] a. Operating conditions
[0083] Mobile phase: n-hexane:isopropanol=90:10(v / v)
[0084] Injection concentration: 0.50mg·mL -1
[0085] Injection liquid flow rate: 0.10mL min -1
[0086] Eluent flow rate: 1.80mL min -1
[0087] Raffinate flow rate: 0.90mL min -1
[0088] Extraction flow rate: 1.00mL min -1
[0089] Switching time: 5min
[0090] System temperature: 25°C
[0091] b. Product analysis
[0092] The simulated moving bed was operated continuously for 24 hours, the sample solution was collected at the raffinate outlet and the extraction outlet respectively, and then the product was analyzed. In the raffinate, the purity of (4S)-(+)-Tetralone was 96.69%; in the extract, the purity of (4R)-(-)-Tetralone was 97.69%. Each kilogram of chiral stationary phase can produce 1.00g of two isomer products every day, the consumption of mobile phase is 37L / g, and the recovery rate of the product is 90.64%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com