Montelukast sodium preparation technology and intermediates
A kind of technology of montelukast sodium and preparation process, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 compound A2
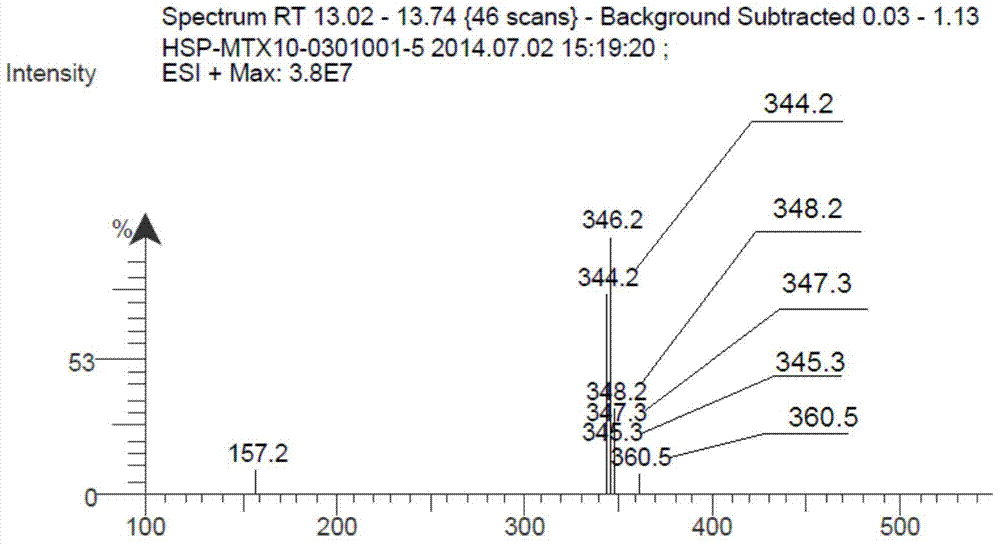
[0051] In a 1000ml three-necked flask, first add 300ml of toluene and 4.8ml of glacial acetic acid (0.084mol, 0.3eq), and under mechanical stirring, add 50g (0.28mol, 1eq) of 7-chloro-2-methylquinine, 3-bromobenzene Formaldehyde 77.7g (0.42mol, 1.5eq), after the feeding is complete, heat up to 70°C, add 45ml (0.48mol, 1.71eq) of acetic anhydride after the raw materials are completely dissolved, then heat up to toluene reflux, control the temperature at 120-125°C, reflux After 10 hours, TLC detected the reaction until the raw material point of 7-chloro-2-methylquinine disappeared, the reaction was completed, the heating was stopped, the reaction solution was cooled to room temperature, stirred for 2 hours, left to stand for 1 hour, suction filtered, and the filter cake was used for 30ml Wash with toluene and dry. The product compound A286.8g was obtained, the yield was 90%, MS m / z: 346 (M+1) + .
Embodiment 2
[0052] The preparation of embodiment 2 compound A2
[0053] In a 1000ml three-necked flask, first add 300ml of toluene and 8ml of glacial acetic acid (0.14mol, 0.5eq), under mechanical stirring, add 50g (0.28mol, 1eq) of 7-chloro-2-methylquinine, 3-bromobenzaldehyde 62.16g (0.34mol, 1.2eq), after the addition is complete, heat up to 70°C, add 45ml (0.48mol, 1.71eq) of acetic anhydride after all the raw materials are dissolved, then heat up to toluene reflux, control the temperature at 120-125°C, and reflux for 11 After 1 hour, TLC detects the reaction until the 7-chloro-2-methylquinine raw material point disappears, the reaction ends, the heating is stopped, the reaction solution is cooled to room temperature, and after stirring for 2 hours, it is left to stand for 1 hour, suction filtered, and the filter cake is washed with 30ml toluene Wash and dry. The product compound A282.9g was obtained, the yield was 86%, MS m / z: 346 (M+1) + .
Embodiment 3
[0054] The preparation of embodiment 3 compound A2
[0055] In a 1000ml three-neck flask, first add 350ml xylene and 4.8ml (0.084mol, 0.3eq) of glacial acetic acid, and under mechanical stirring, add 50g (0.28mol, 1eq) of 7-chloro-2-methylquinine, 3-bromo Benzaldehyde 62.16g (0.34mol, 1.2eq), after the feeding is complete, heat up to 70°C, add propionic anhydride 54.6ml (0.42mol, 1.5eq) after the raw materials are completely dissolved, then heat up to toluene reflux, control the temperature at 120-125°C , refluxed for 12 hours, TLC detected the reaction until the raw material point of 7-chloro-2-methylquinine disappeared, the reaction was completed, the heating was stopped, the reaction solution was cooled to room temperature, stirred for 2 hours, left to stand for 1 hour, suction filtered, and the filter cake was Wash with 30 ml of toluene and dry. Obtained product A284.4g, yield 87.5%, MS m / z:346(M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com