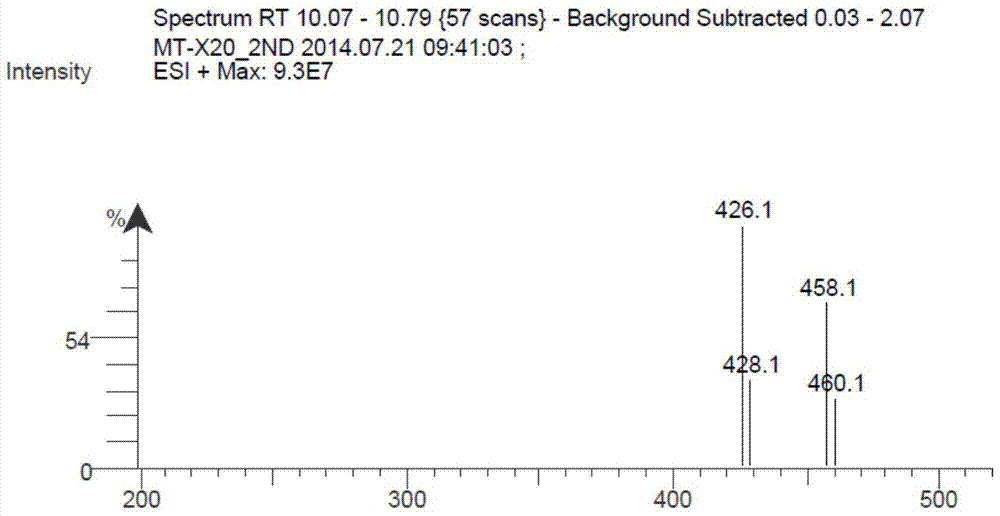
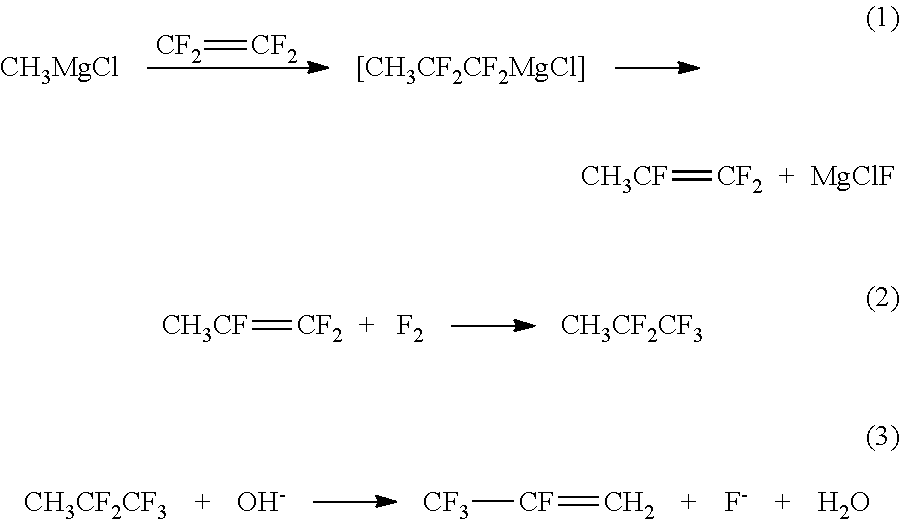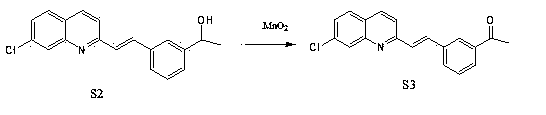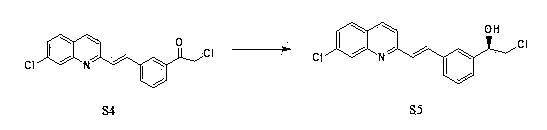Patents
Literature
45 results about "Methylmagnesium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
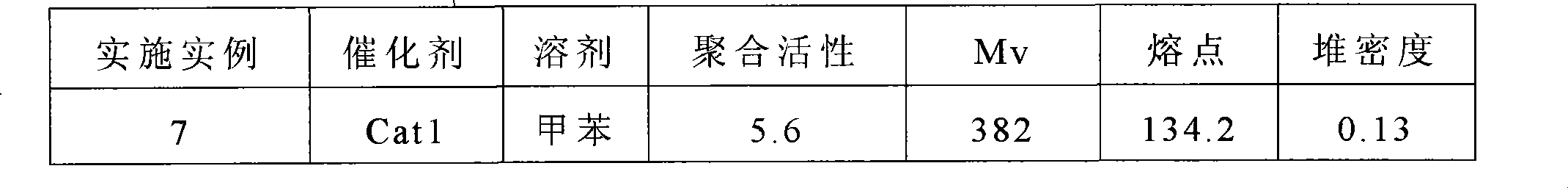
Application Domain
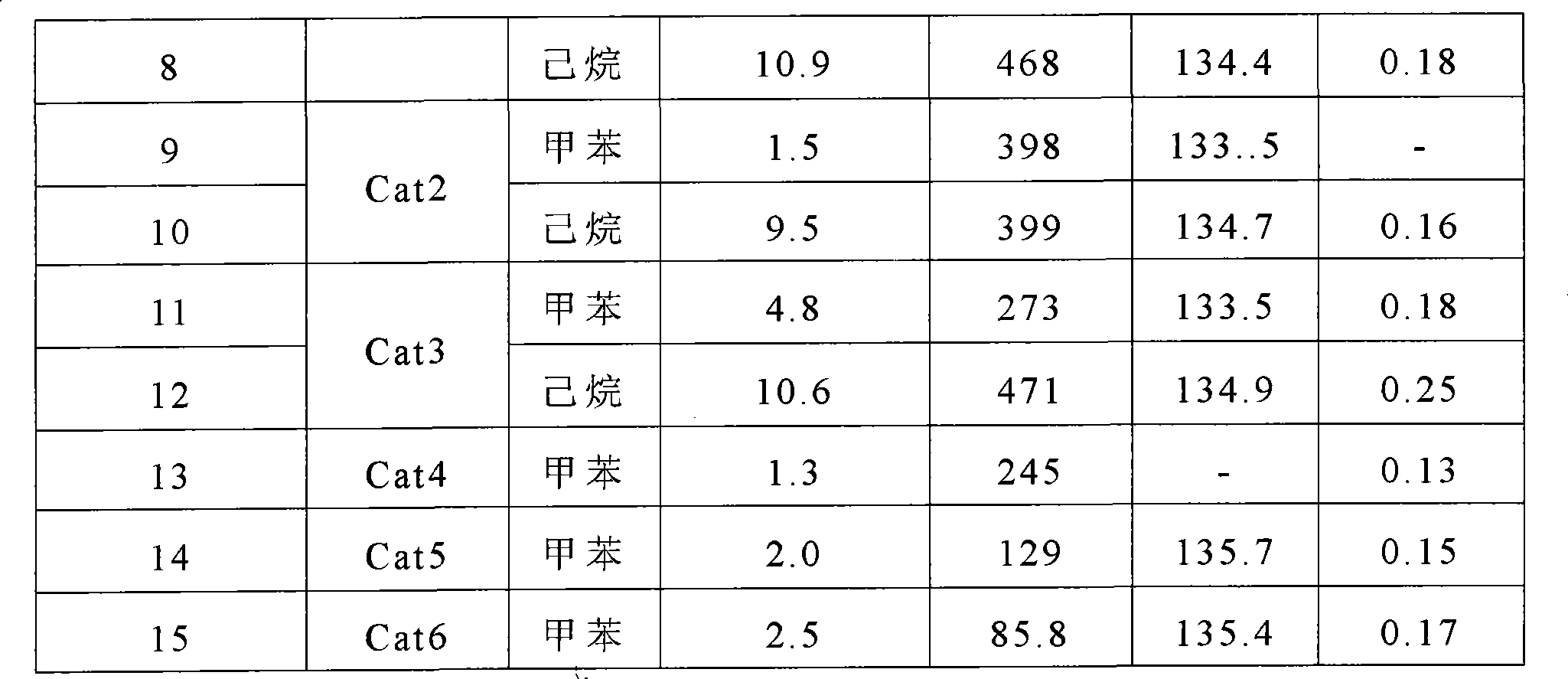
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methylmagnesium chloride is an organometallic compound with the general formula CH₃MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.
Magnesium chloride/mesoporous molecular sieve bisupported Ziegler-Natta polyethylene catalyst, preparation and use
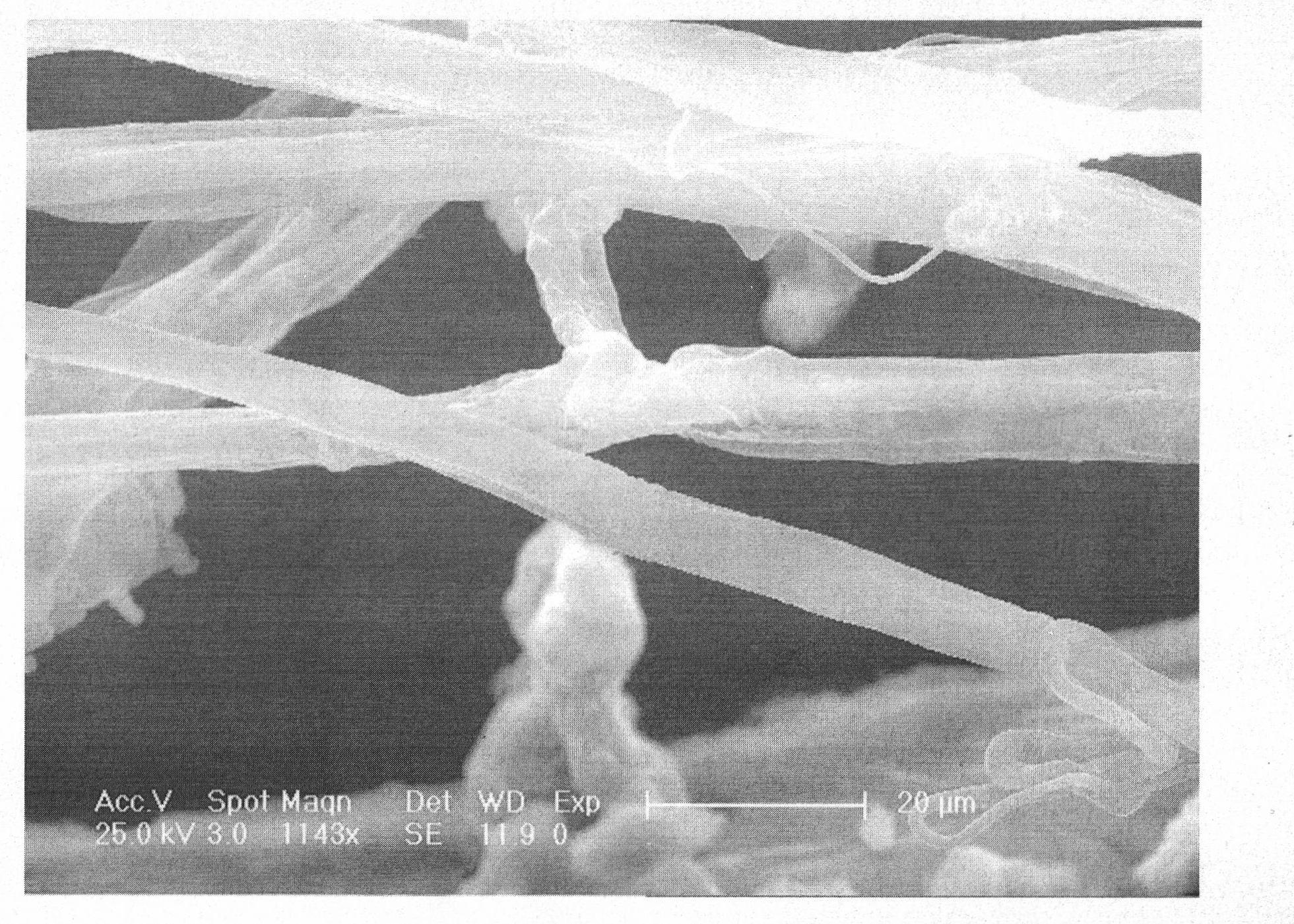
The invention relates to a Ziegler-Natta polyethylene catalyst loaded by double carriers of magnesium chloride / mesoporous molecular sieve, a preparation method and an application thereof. The catalyst is made up by taking titanium tetrachloride as a main catalyst of active components and organic aluminum as a cocatalyst. The double carriers of the magnesium chloride / mesoporous molecular sieve are taken as a carrier; an organic reagent of magnesium is used, by the breaking and creation of chemical bonds, Si-O-Mg bond is generated so as to obtain a complex carrier with clear structure. The dried mesoporous molecular sieve is suspended in toluene and added with tetrahydrofuran solvent of methyl magnesium chloride; the methyl magnesium chloride on a surface is reacted with silanol group to form the Si-O-Mg bond; and an obtained complex carrier has the frame of the mesoporous molecular sieve and the surface of the magnesium chloride, and can possess the advantages of the double carriers. The active titanium is loaded on the double carriers of chloride / mesoporous molecular sieve to catalyze the polymerization of ethane, thereby obtaining polyethylene with a super molecular weight of 600 thousand to 7 million.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for producing 2-methyl-4-methylamino-6-methoxyl-1,3,5-triazine
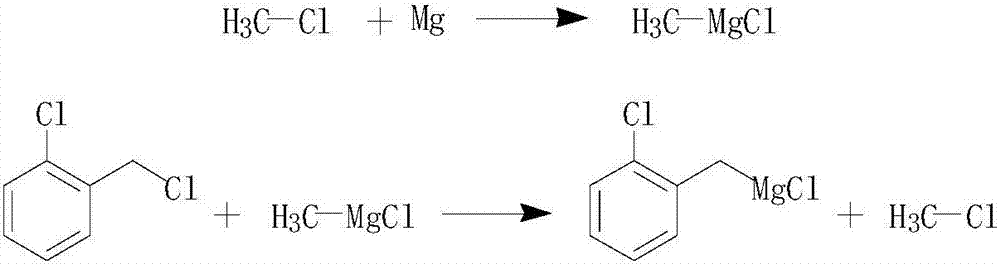
A process for preparing 2-methyl-4- methylamino-6- methoxyl-1, 3, 5- triazine, realized by reacting magnesium with methyl chloride in ether - aromatic hydrocarbon - alkane mixed solvent to obtain methylmagnesium chloride, then carrying out methylation with cyanuryl chloride, and proceeding methoxylation and methylaminolation with the action of quaternary ammonium salt phase transition catalyst.
Owner:NANJING UNIV OF TECH +1
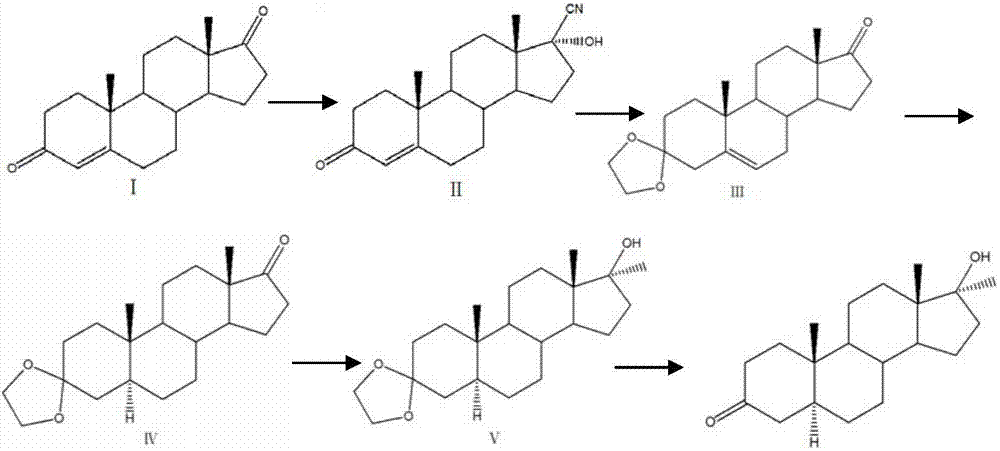
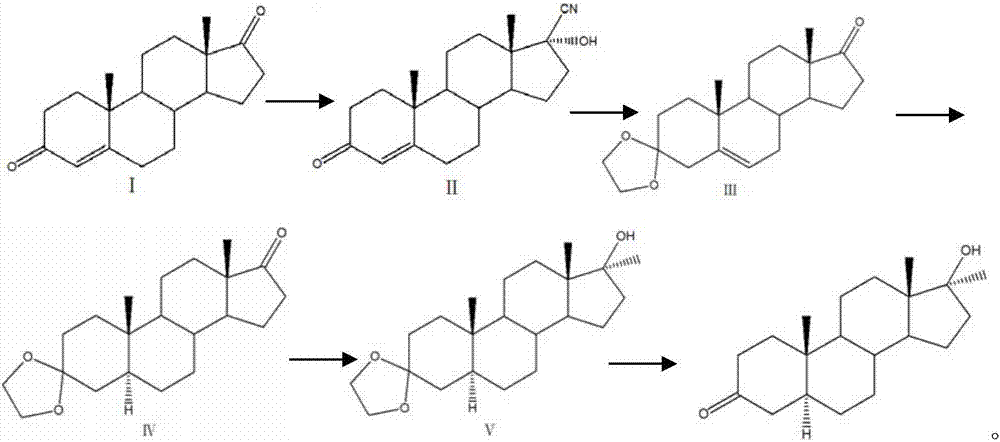
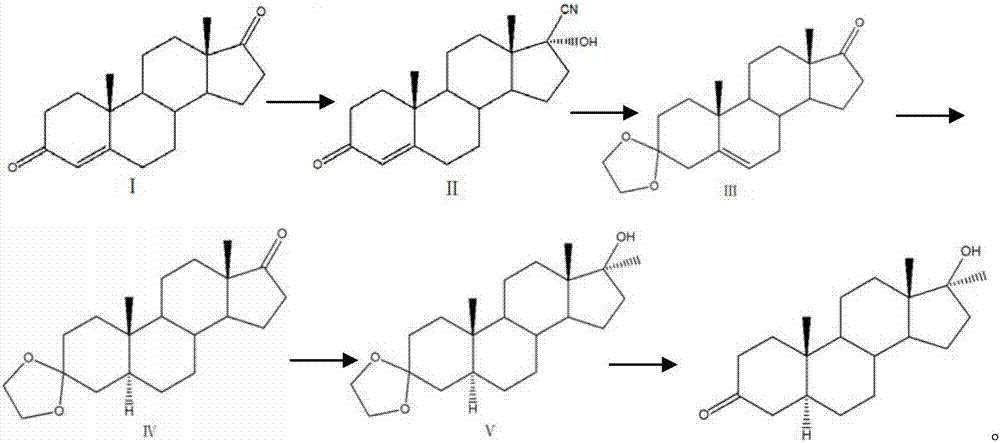
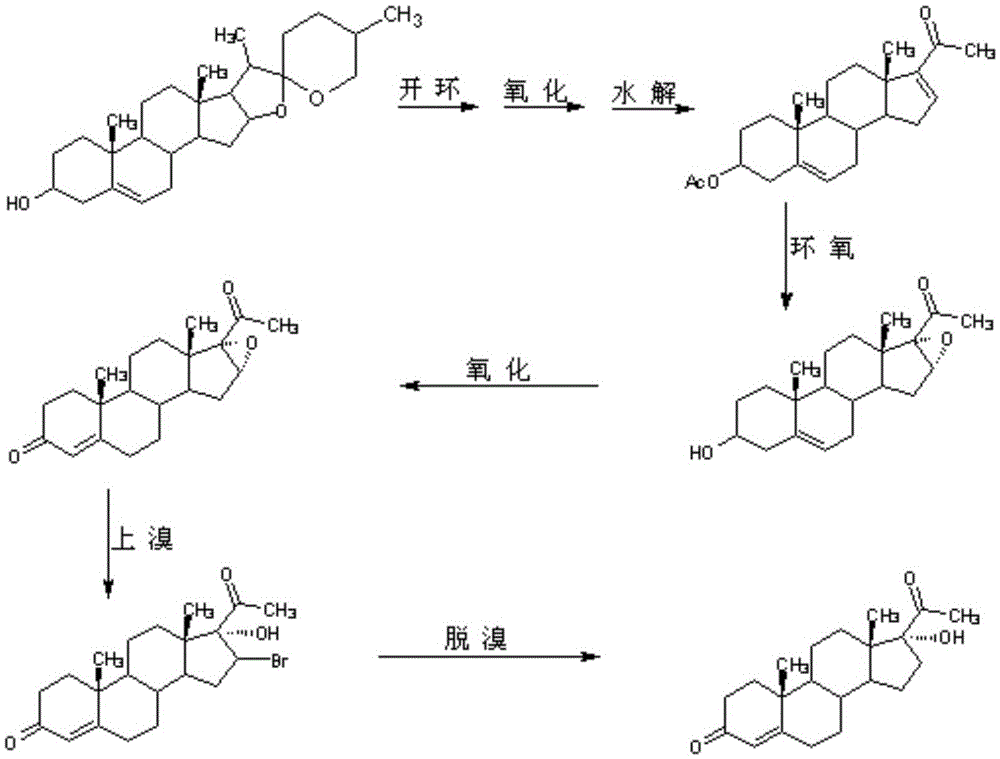
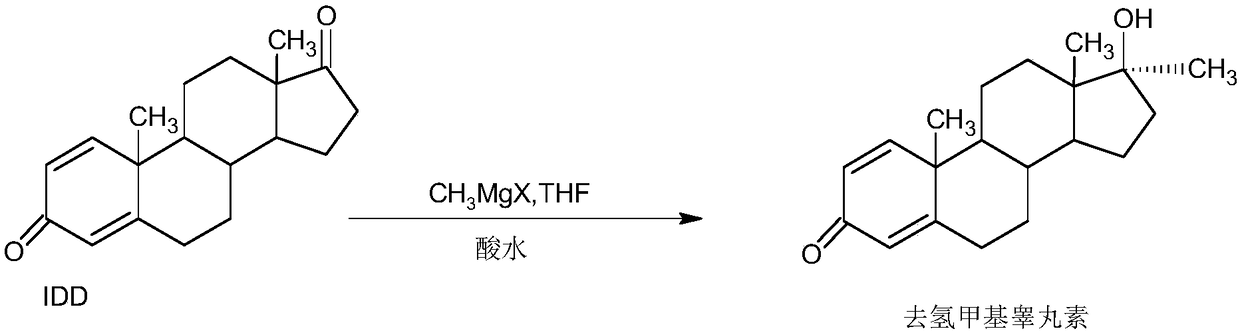
Preparation method of mestanolone
The invention discloses a preparation method of mestanolone. The structural formula of the mestanolone is as shown in the specification. The specific preparation method comprises the following steps of (1) generating a compound II with cyan from a compound I and acetone cyanohydrin or sodium cyanide; (2) adjusting a solution of the compound II into acidity, tracking ethylene glycol and triethyl orthoformate into ketal, adjusting the solution into strong basicity to form a compound III; (3) adding a catalyst and hydrogen to the solution of the compound III to generate a compound IV; (4) putting the compound IV into a container and dissolving by using tetrahydrofuran, dropping a Grignard reagent of methylmagnesium chloride or methyl magnesium bromide to obtain a compound V; and (5) elutriating the compound V in the same container under an acid condition to obtain 3-ketal, namely mestanolone. Chemical production of the mestanolone is achieved by adopting the simplest process, the required raw materials are few, the pollutants are few, meanwhile, the produced mestanolone is high in purity and the relative yield is increased.
Owner:ZHEJIANG PURUI PHARMA
Montelukast sodium preparation technology and intermediates
ActiveCN104293850AEfficient reuseReduce typesOrganic chemistryChemical recyclingTetralonePtru catalyst
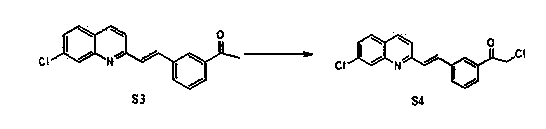
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
Preparation method of trimethyl gallium
ActiveCN106749354AReduce manufacturing costImprove use valueGroup 3/13 element organic compoundsOrganic solventHigh energy
Owner:CHNA ENERGY INVESTMENT CORP LTD +1
Preparation method of 2-dimethyl-2-octanol
InactiveCN103058823AStable in natureEasy to storeOrganic compound preparationHydroxy compound preparationGrignard reagentMethylmagnesium chloride
The invention discloses a preparation method of 2-dimethyl-2-octanol. Methyl Grignard reagent is prepared by using methyl magnesium chloride or methyl magnesium bromide as an initiator. The 2-dimethyl-2-octanol is prepared by carrying out reaction of the methyl Grignard reagent and 2-octanone. The preparation method of the 2-dimethyl-2-octanol is suitable for continuous production, iodine is used for initiation for the first time, halogenated methane liquid state is added in the iodine in a dropwise manner to prepare an methyl magnesium chloride initiator and an methyl magnesium bromide initiator, and the methyl Grignard reagent produced in the previous production is completely adopted as the initiator in subsequent production to carry out the continuous production. The properties of the initiators of the preparation method are relatively stable. The initiator is easy to store, compared with a common initiator, the initiator has the advantages of being fast in initiation speed, strong in generality, low in usage, convenient to store and low in toxicity and the like, and is especially suitable for the preparation for the Grignard reagent in mass production. According to the preparation method of the 2-dimethyl-2-octanol, preparation safety is good, and production yield is high.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
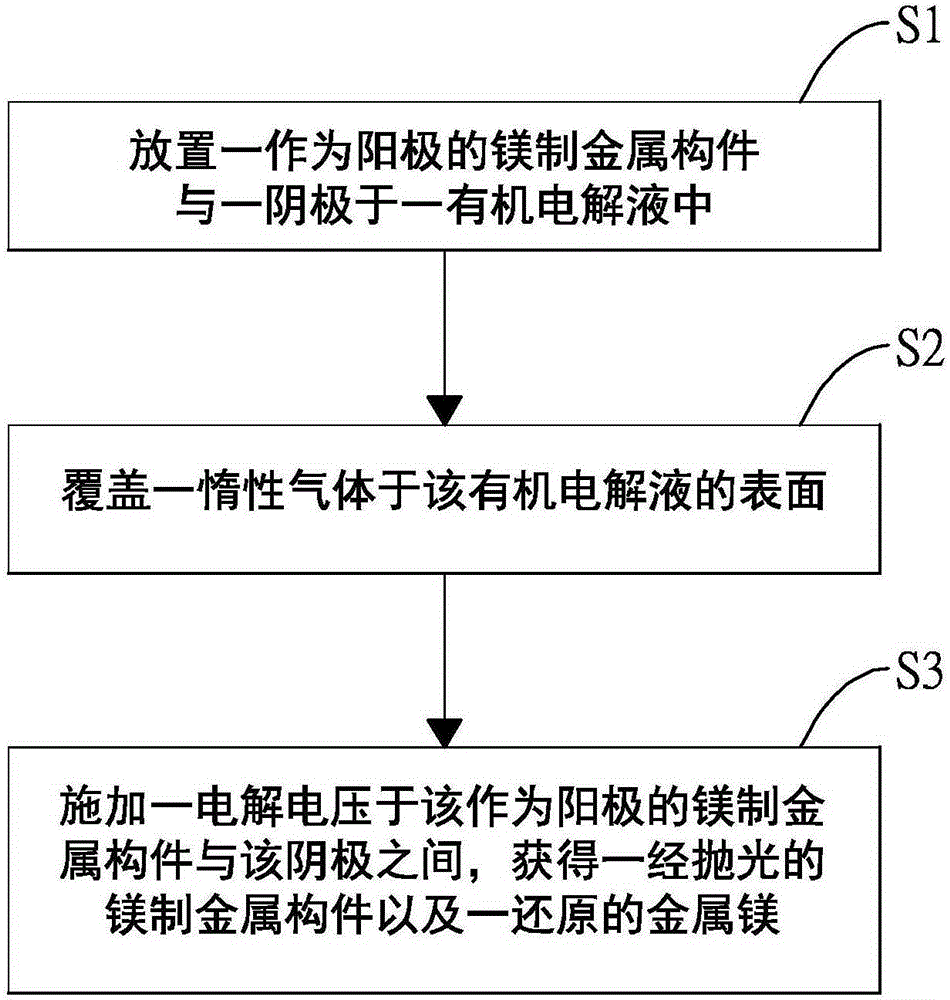
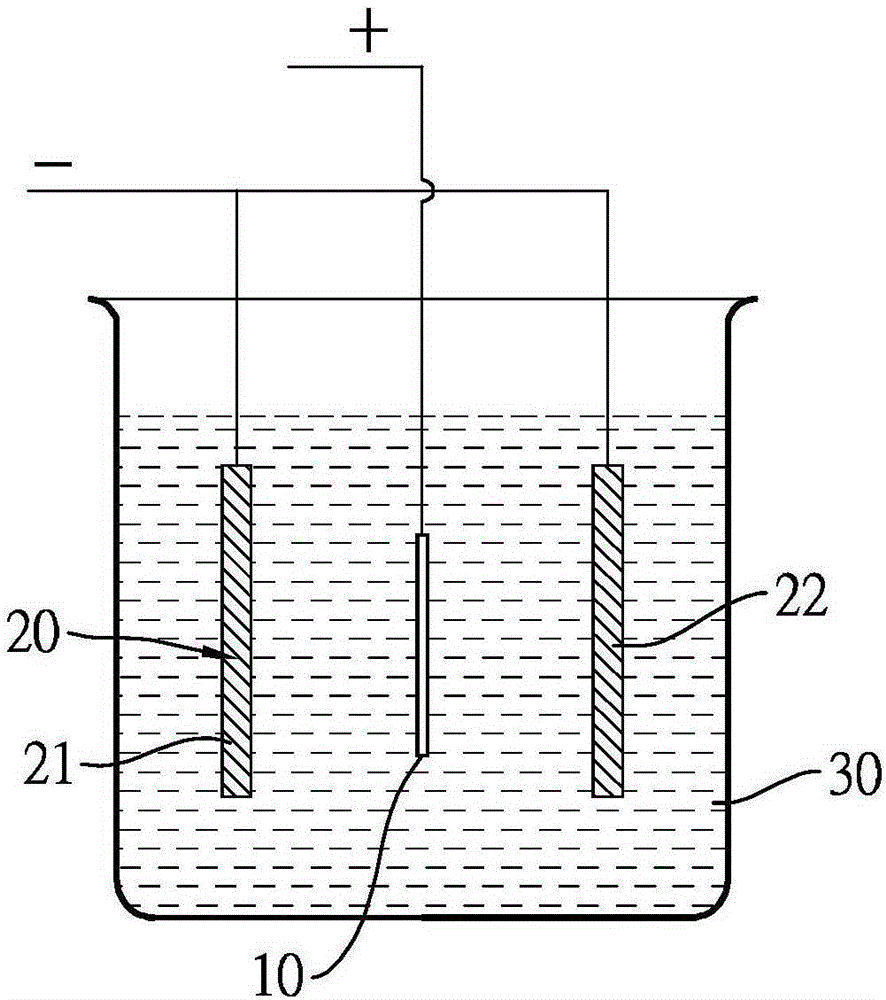
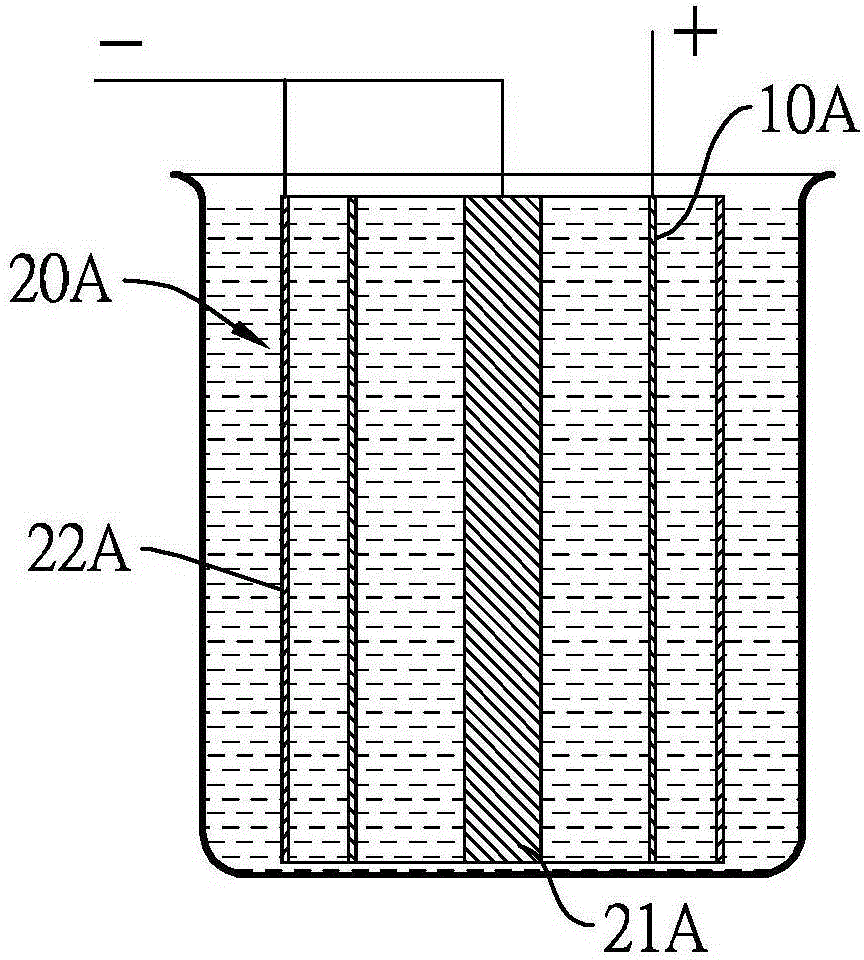
Electrochemical polishing method for magnesium metal component
ActiveCN106435706AEasy to polishElectrolysis componentsElectrochemical machining apparatusElectrolysisMethylmagnesium chloride
The invention discloses an electrochemical polishing method for a magnesium metal component. The electrochemical polishing method comprises the following steps of firstly, putting an anode and a cathode into an organic magnesium electrolyte solution, wherein the anode is the magnesium metal component; and then, applying an electrolysis voltage between the anode and the cathode to obtain a polished magnesium metal component. The organic electrolyte solution comprises a solvent selected from a group consisting of ethyl ether, tetrahydrofuran, 2-methyl tetrahydrofuran and cyclopentyl methyl ether, and an organic magnesium electrolyte selected from a group consisting of methyl magnesium chloride, ethyl magnesium chloride, phenyl magnesium chloride, alkoxy magnesium bromide and alkoxy magnesium chloride. The electrochemical polishing method for the magnesium metal component disclosed by the invention has the advantages of saving time, saving labor, and being suitable for fine magnesium metal components.
Owner:张无量
Preparation method for 2,3,3,3-tetrafluoropropene
ActiveCN106316777ARaw materials are cheap and easy to getShort processPreparation by hydrogen halide split-offOrganic solventMethylmagnesium chloride
The invention discloses a preparation method for 2,3,3,3-tetrafluoropropene. The preparation method comprises the following steps: (a) reacting chloropenta fluoroethane and methylmagnesium chloride in an organic solvent, and after the reaction is ended, performing cooling, filteration and rectification to obtain 1,1,1,2,2-perfluoropropane, wherein the methylmagnesium chloride and the chloropenta fluoroethane are in the molar ratio of 1:(1-5), the reacting temperature is 20 to 60 DEG C, and the reacting time is 1 to 5 hours; (b) introducing the 1,1,1,2,2-perfluoropropane which is obtained in the step (a) into an alkaline liquor at 50 to 90 DEG C, collecting a gas product, and drying and condensing the gas product to obtain the product, namely, the 2,3,3,3-tetrafluoropropene; or performing gas phase catalysis on the 1,1,1,2,2-perfluoropropane which is obtained in the step (a) under the action of a catalyst to remove HF and obtain the 2,3,3,3-tetrafluoropropene. The preparation method for the 2,3,3,3-tetrafluoropropene has the advantages that the process is simple, raw materials are easily obtained, and the cost is low.
Owner:JUHUA GROUP TECH CENT
Preparation method of methyl-magnesium chloride
ActiveCN101555254AReduce manufacturing costLow priceMagnesium organic compoundsGrignard reagentMethylmagnesium chloride
The invention relates to a preparation method of methyl-magnesium chloride, belonging to the technical field of preparation method of Grignard reagent. The method is characterized in that: reaction is implemented by taking methyl chloride and magnesium metal as raw materials and methylal as a solvent, wherein the molar ratio between the magnesium metal and the methyl chloride is 1:0.5 to 3, and the weight ratio between the magnesium metal and the methylal is 1:2 to 100. The method carries out the reaction by taking the methyl chloride and the magnesium metal as raw materials and the methylal as the solvent, thus lowering the production cost; furthermore, the recycling of the methylal is easy, thus further lowering the production cost. The raw material can also be prepared from byproducts of glyphosate production, therefore, the source of raw materials are rich, the cost is low, furthermore, the purification and separation cost of the byproducts is reduced, and the production technology is simple and easy, thus having obvious economic benefit.
Owner:HANGZHOU JINFADA CHEM IND
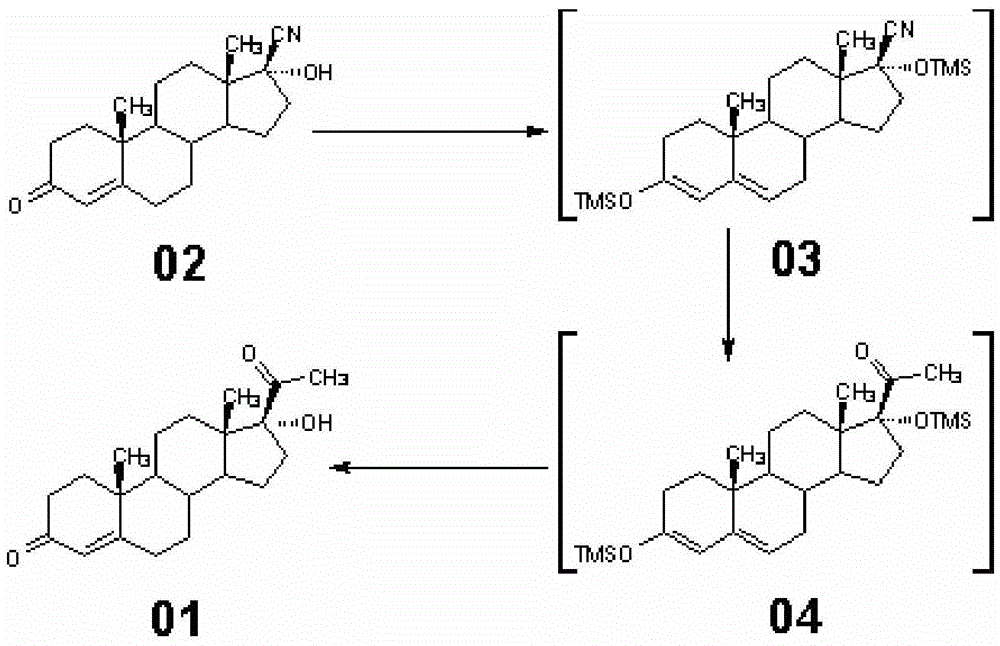
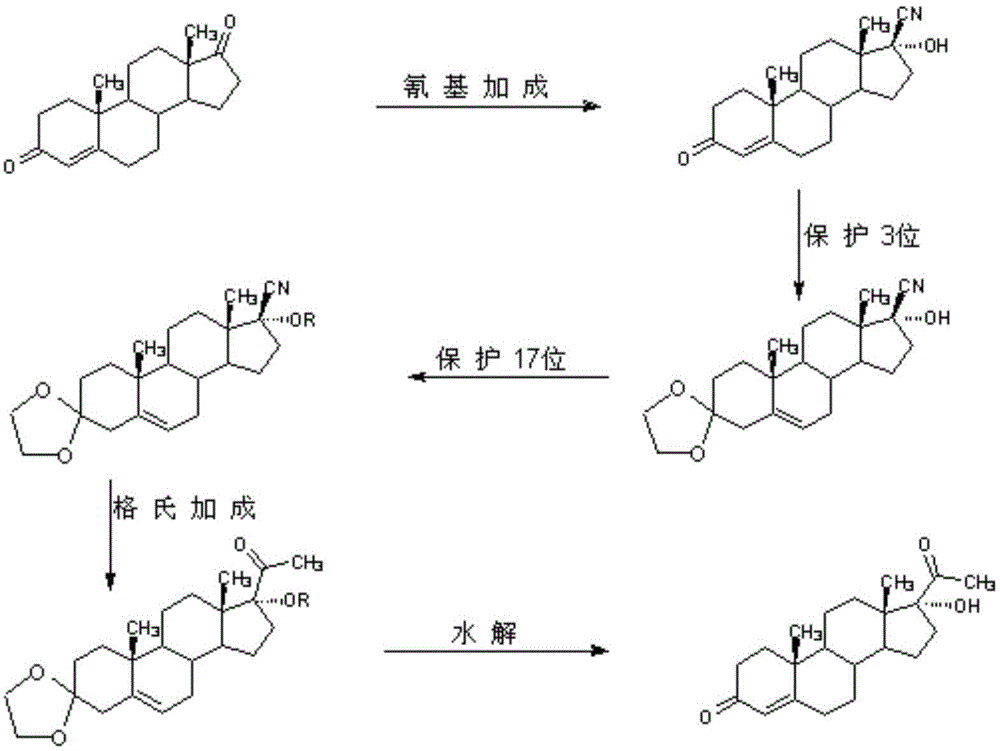
A New Method for Synthesizing 17α-Hydroxyprogesterone
The invention provides a novel method of synthesizing 17 alpha-hydroxyl progesterone (01). The novel method comprises the following steps: 1) in the presence of trimethylsilyl chloride and a methyl magnesium chloride solution, performing double-protection reaction on 17 bata-cyano-17 alpha-hydroxyl-4-androstene-3-one (02) to produce a compound (03); 2) heating up to perform grignard addition reaction, and transferring into an ammonium chloride aqueous solution to produce a compound (04); 3) preserving the heat and discoloring by active carbon, adding diluted acid water to reflux and perform hydrolysis reaction of double protection groups, and separating and purifying to obtain 17 alpha-hydroxyl progesterone (01). According to the novel method disclosed by the invention, the conventional 3-position ketal protection, 17 alpha-hydroxyl vinyl ether protection, grignard addition, re-hydration of protection groups on position 3 and position 17 and discoloring-refining are simplified into one-step operation.
Owner:HUNAN KEREY BIOTECH
Synthetic method of ultra-high molecular weight polyethylene
The invention discloses a synthetic method of ultra-high molecular weight polyethylene. The method comprises the following steps: in water-free and oxygen-free conditions, adding a methyl magnesium chloride Grignard reagent into a carrier, carrying out primary filtration after stirring for 2 to 3 h, after washing a filter cake with a solvent, mixing with TiCl4, carrying out secondary filtration after stirring for 2 to 3 h, and drying the filter cake to obtain a supported catalyst; adding the obtained support catalyst, normal hexane and triisobutyl aluminum into a reaction still, and at the temperature of 50 to 60 DEG C, carrying out an ethylene reaction for 30 to 120 min at 0.5 to 1 MPa to obtain the ultra-high molecular weight polyethylene. The most coomon polyolefin coordination polymerization catalyst Z-N catalyst is loaded by simply using the carrier with a porous structure, and the ultra-high molecular weight polyethylene can be obtained by using the catalyst to catalyze vinyl polymerization.
Owner:HANGZHOU NORMAL UNIVERSITY
Magnesium chloride/mesoporous molecular sieve bisupported Ziegler-Natta polyethylene catalyst, preparation and use
The invention relates to a Ziegler-Natta polyethylene catalyst loaded by double carriers of magnesium chloride / mesoporous molecular sieve, a preparation method and an application thereof. The catalyst is made up by taking titanium tetrachloride as a main catalyst of active components and organic aluminum as a cocatalyst. The double carriers of the magnesium chloride / mesoporous molecular sieve aretaken as a carrier; an organic reagent of magnesium is used, by the breaking and creation of chemical bonds, Si-O-Mg bond is generated so as to obtain a complex carrier with clear structure. The dried mesoporous molecular sieve is suspended in toluene and added with tetrahydrofuran solvent of methyl magnesium chloride; the methyl magnesium chloride on a surface is reacted with silanol group to form the Si-O-Mg bond; and an obtained complex carrier has the frame of the mesoporous molecular sieve and the surface of the magnesium chloride, and can possess the advantages of the double carriers. The active titanium is loaded on the double carriers of chloride / mesoporous molecular sieve to catalyze the polymerization of ethane, thereby obtaining polyethylene with a super molecular weight of 600thousand to 7 million.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
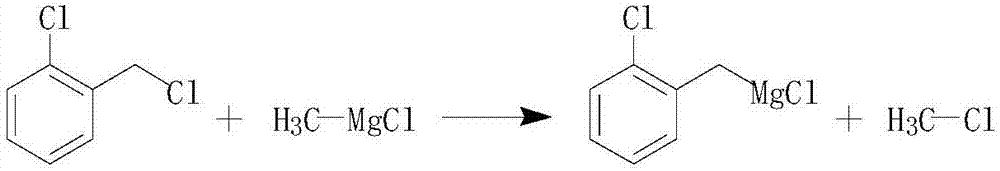
Preparation method of 2-chlorobenzyl chloride Grignard reagent
InactiveCN108003179AHigh purityReduce conversion reaction timeMagnesium organic compoundsMethylmagnesium chlorideGrignard reagent
The invention discloses a preparation method of a fungicide prothioconazole key intermediate of a 2-chlorobenzyl chloride Grignard reagent. Compared with the prior art: in the presence of a catalyst,a methyl magnesium chloride Grignard reagent is added dropwise into 2-chlorobenzyl chloride to conduct Grignard reaction, the method changes a process route and choice of raw materials; a product witha total yield reaching 98 % (based on the 2-chlorobenzyl chloride) is obtained; a preparation process is simple and easy to implement, reaction speed is fast, side reaction is small, a by-product methyl chloride can be recycled to achieve clean and environment-friendly production, and economic benefits are remarkable.
Owner:LIMIN CHEM CO LTD
Preparation process of montelukast sodium and its intermediate product
ActiveCN104293850BEfficient reuseReduce typesOrganic chemistryChemical recyclingMethylmagnesium chlorideCoupling
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
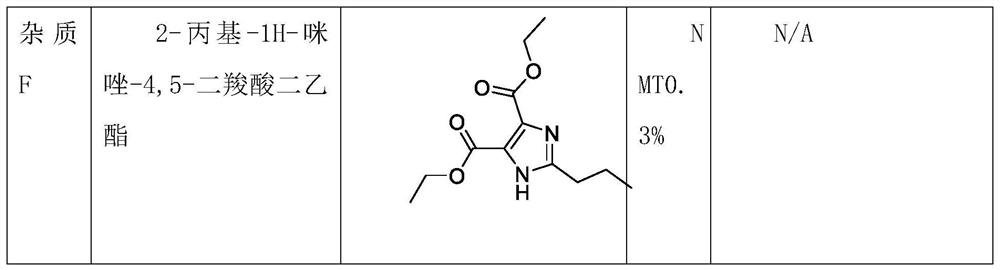
Preparation method of 4-(1-hydroxy-1-methylethyl)-2-propylimidazolium iodide-5-carboxylate
The invention relates to a preparation method of 4-(1-hydroxy-1-methylethyl)-2-propylimidazolium iodide-5-carboxylate, which belongs to the technical field of pharmaceutical intermediate synthesis. For solving the problems that the content of products is low, and a large amount of organic solvents are required to be adopted for recrystallization, a preparation method of 4-(1-hydroxy-1-methylethyl)-2-propylimidazolium iodide-5-carboxylate is provided. The method comprises the following steps: reacting 2-propylimidazolium iodide-4,5-dicarboxylate with methylmagnesium chloride so as to obtain an oily matter; adding an inorganic acid and activated carbon into the oily matter, and heating the obtained mixture so as to carry out a salt forming reaction, so that the obtained mixture is transferred into a corresponding acidic salt; filtering the acidic salt so as to obtain filter liquor of the corresponding acidic salt; and controlling the temperature of the filter liquor, adding an alkaline reagent into the filter liquor so as to adjust the pH value of the obtained mixture to 7, and then carrying out cooling crystallization on the obtained mixture so as to obtain 4-(1-hydroxy-1-methylethyl)-2-propylimidazolium iodide-5-carboxylate. The method disclosed by the invention is simple and short in process, an organic solvent is not required to be adopted for recrystallization, and the purity and yield of obtained products are high.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Halogen-free rubber foam insulation material and preparation method thereof
InactiveCN107522956AImprove insulation effectImprove flame retardant performancePolymer sciencePhosphate
The invention discloses a preparation method of a halogen-free rubber foam insulation material. The preparation method comprises the following steps: mixing an ethylene-propylene-diene monomer with ethylene propylene, adjusting the temperature to 110-120 DEG C, and performing a stirring reaction for 20-30 minutes; further adding polyvinyl chloride, polydipentanone and methyl magnesium chloride, adjusting the temperature to 120-125 DEG C, and performing a reaction for 15-25 minutes; further adding polycaprolactone and trioctyl phosphate, adjusting the temperature to 128-132 DEG C, and performing a reaction for 20-30 minutes continuously; adding 2-methyl-6-isopropyl phenylamine, aluminum silicate and azodicarbonamide, and leaving to stand at 135-140 DEG C to react for 30-40 minutes; further performing a stirring reaction for 20-30 minutes at a velocity of 800-900r / minute; cooling after the reaction, drying, and pelletizing, thereby obtaining the halogen-free rubber foam insulation material. The halogen-free rubber foam insulation material prepared by using the method is good in both flame retardancy and heat preservation.
Owner:FOSHAN FEISHIDA NEW MATERIAL TECH CO LTD
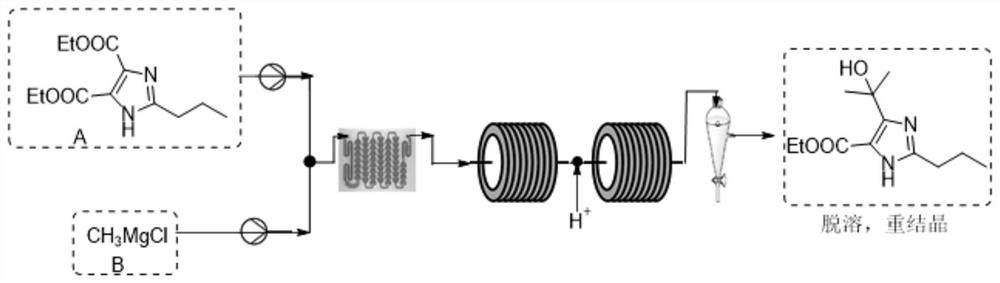
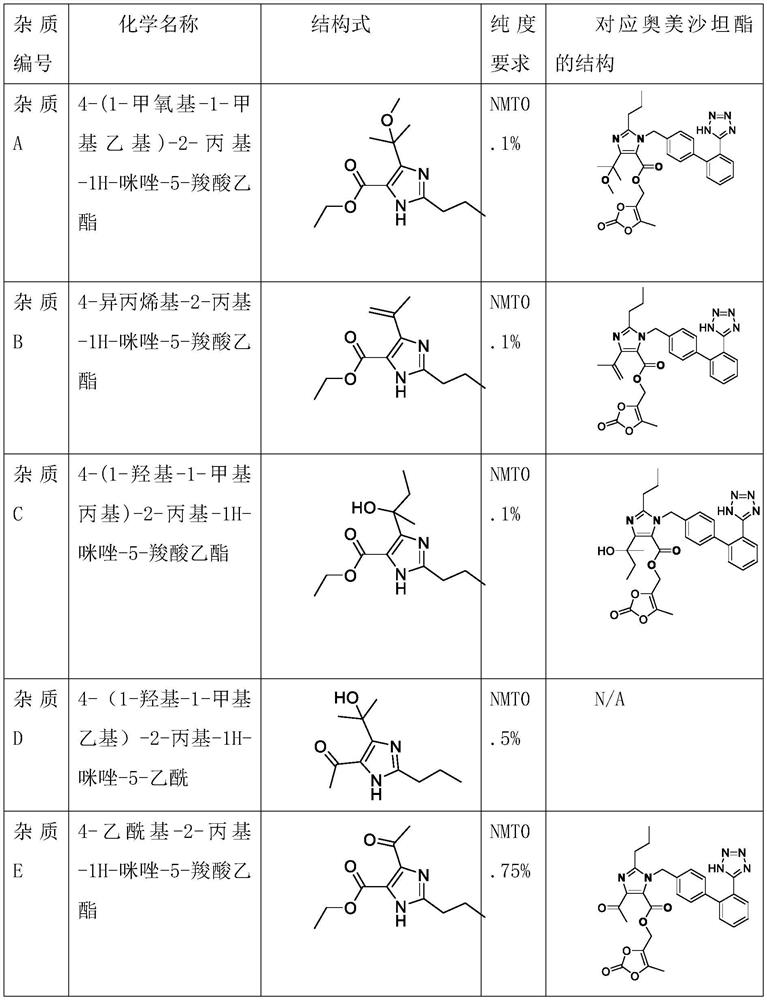
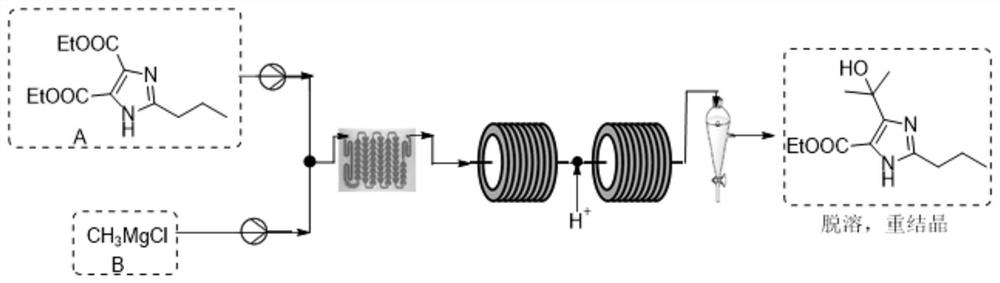
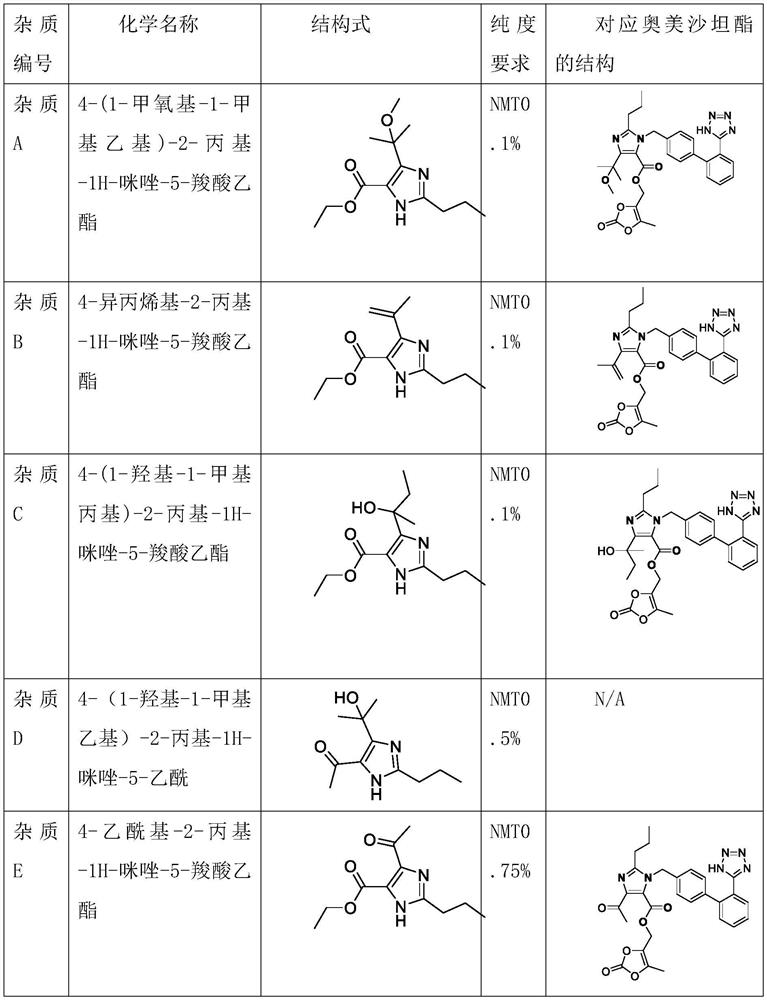
Synthetic method for preparing olmesartan medoxomil intermediate through continuous flow
The invention discloses a synthetic method for preparing an olmesartan medoxomil intermediate through continuous flow, which belongs to the technical field of drug synthesis. The method comprises the following steps: step (1), dissolving a compound 2-propyl-4,5-imidazole dicarboxylic acid ethyl ester I in an organic solvent to form a reaction phase A; step (2), taking a commercially purchased methyl magnesium chloride solution as a reaction phase B; step (3), pumping the reaction phase A and the reaction phase B into a mixer by using a plunger pump at a certain flow rate, then feeding the mixture into a reactor, retaining for a certain time, quenching by using an acid water phase C, feeding the mixture into an oil-water continuous liquid separator, separating liquid to obtain an organic solution of a compound as shown in a formula II, desolventizing, and recrystallizing to obtain the compound as shown in the formula II. The technical problems of low purity and high cost in the existing preparation process are solved, the product is high in purity and few in impurities, the content of the key impurity A, the key impurity B and the key impurity C can be controlled to be 0.1% or below, the raw materials are cheap and easy to obtain, the reaction condition is mild, operation is easy and convenient, the synthesis efficiency is high, and the method is suitable for industrial production. A novel continuous flow path is provided for preparing the olmesartan medoxomil and the intermediate.
Owner:拓信达(启东)医药生物科技有限公司 +2
Synthesis method of 4-isopropylresorcinol
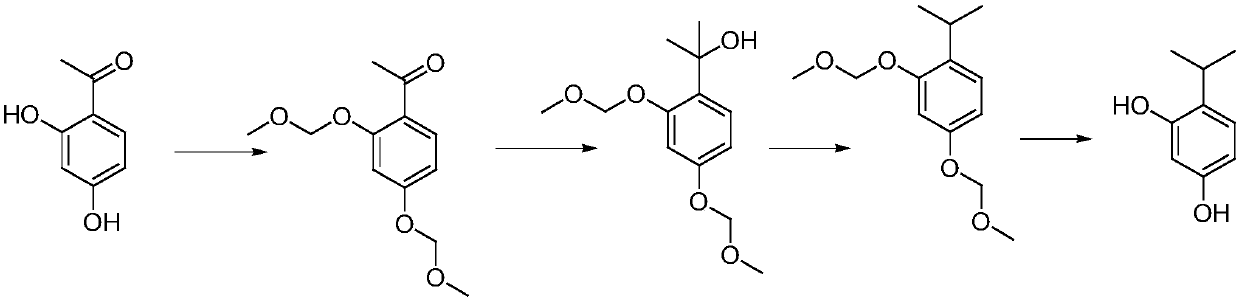
ActiveCN107556164AReduce temperature and pressureEasy to deal withOrganic chemistryOrganic compound preparationSynthesis methodsHydrogenation reaction
The invention discloses a synthesis method of 4-isopropylresorcinol. The method comprises the following steps: group protection, Grignard reaction, hydrodeoxygenation and deprotection. According to the method, a novel protecting group halomethyl methyl ether is adopted for protecting phenolic hydroxy, then the Grignard reaction is performed on 3 milliliters of methylmagnesium chloride or methylmagnesium bromide, hydrogenation is performed under low pressure and normal temperature in the presence of acid, and the protecting group is removed with hydrochloric acid to obtain the 4-isopropylresorcinol. The problem of difficulty in treatment after Witting reaction is solved, the temperature and the pressure of hydrogenation reaction are reduced, the hydrogenation time is shortened, deprotectionis finally implemented under a mild condition, and the whole process is a safe production process capable of implementing industrial production.
Owner:济南美高生物医药科技有限公司
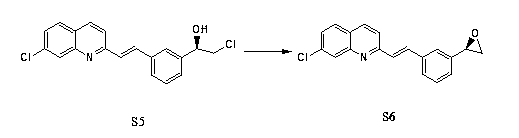
Preparation method of montelukast chiral intermediate
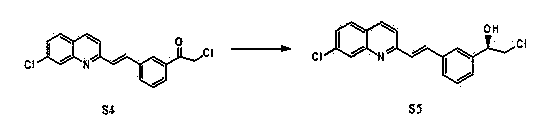
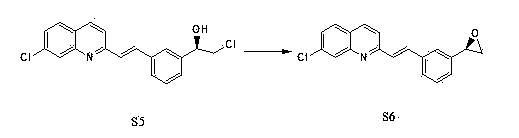
ActiveCN103554017BMild reaction conditionsReaction conditions are easy to controlOrganic chemistryChemical synthesisMethylmagnesium chloride
The invention discloses a preparation method of a montelukast chiral intermediate, which belongs to the field of medical chemical synthesis. The preparation method comprises the following steps of: reacting compound S1 serving as the raw material with methylmagnesium chloride, oxidizing by using manganese dioxide, chlorinating by using NCS (Succinchlorimide), carrying out chiral reduction by using (-)-B-chlorodiisopinocampherylborane, carrying out cyclization under the action of anhydrous potassium carbonate, and carrying out addition reaction with 2-methyl formate benzylmagnesium bromide to obtain the medical intermediate S7. By adopting the preparation method disclosed by the invention, the application of precious metal for catalysis is avoided, the reaction conditions are mild and easily controlled, the reaction product is single, the yield is high, the optical purity and the chemical purity of the product are greatly improved, the process is simple, the raw materials are low in price and easily available, and therefore the preparation method is more suitable for industrial large-scale production.
Owner:CANGZHOU SENARY CHEM SCI TEC
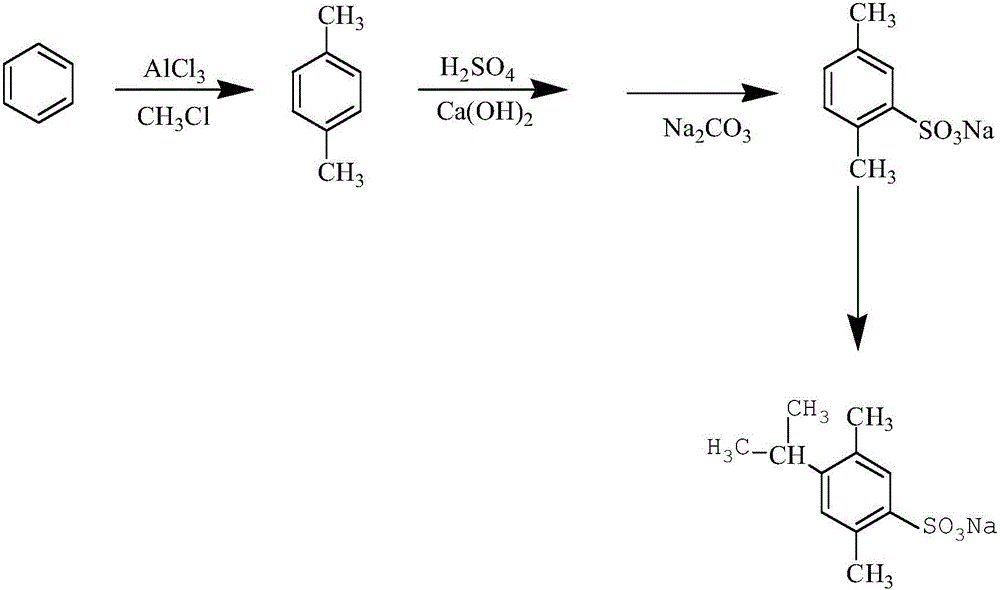
Synthesis method of 4-isopropyl-2,5-sodium dimethylbenzenesulfonate
InactiveCN105777589ASulfonic acids salts preparationSulfonic acid preparationChemical synthesisMethylmagnesium chloride
The invention discloses a synthesis method of 4-isopropyl-2,5-sodium dimethylbenzenesulfonate, belonging to the field of organic chemical synthesis. The synthesis method comprises the following steps: firstly putting benzene in a closed container, introducing nitrogen and adding aluminum chloride and methane chloride; sealing and heating; stirring for reacting; naturally cooling; introducing chlorine and adding methyl magnesium chloride; heating, standing and cooling; mixing the mixture with sulfuric acid; heating at constant temperature; putting in deionized water and adjusting the pH value; filtering; adding sodium carbonate into the filtrate; filtering to obtain 2,5-sodium dimethylbenzenesulfonate; putting the 2,5-sodium dimethylbenzenesulfonate into a reaction kettle, adding copper, introducing hydrogen and adding isopropylbenzyl alcohol; and heating, preserving heat, cooling, filtering and drying to obtain the 4-isopropyl-2,5-sodium dimethylbenzenesulfonate. The synthesis method disclosed by the invention has the beneficial effects of simple synthesis conditions, no secondary pollution, few byproducts and relatively high yield.
Owner:CHANGZHOU UNIV
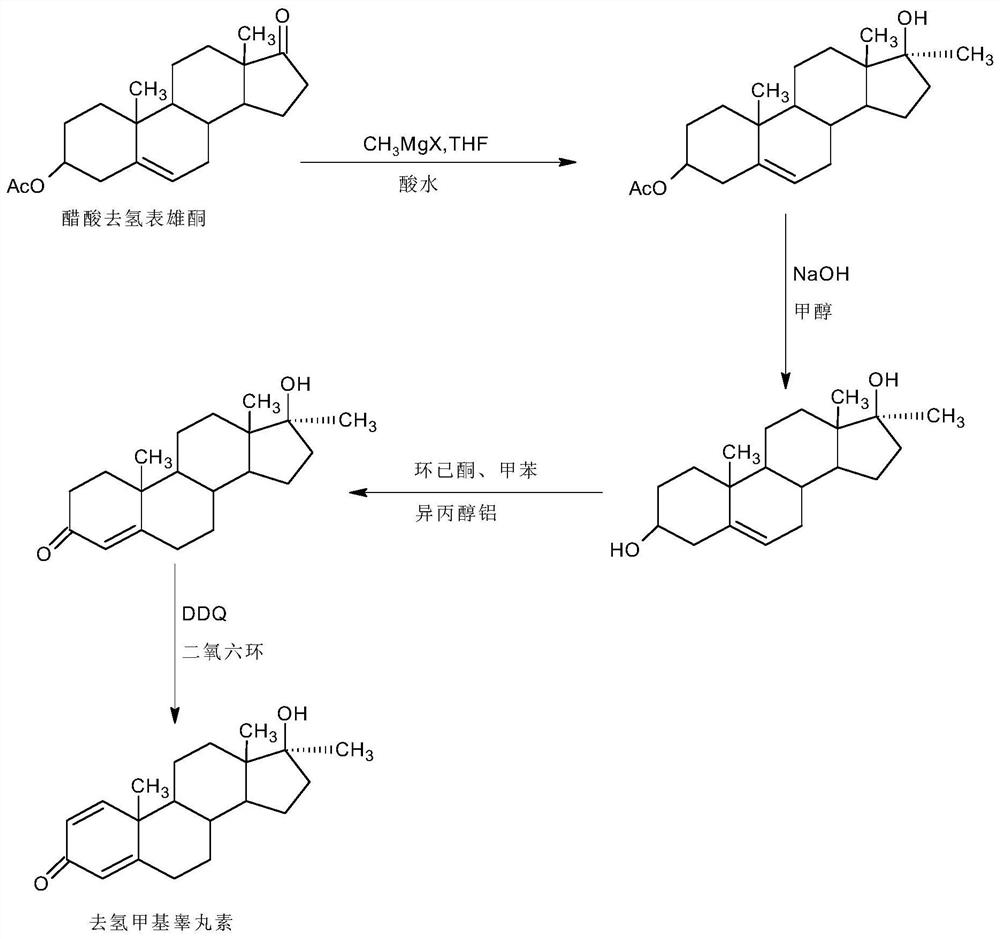
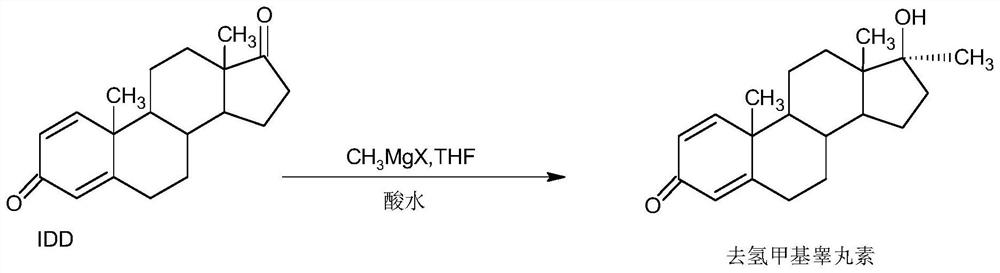
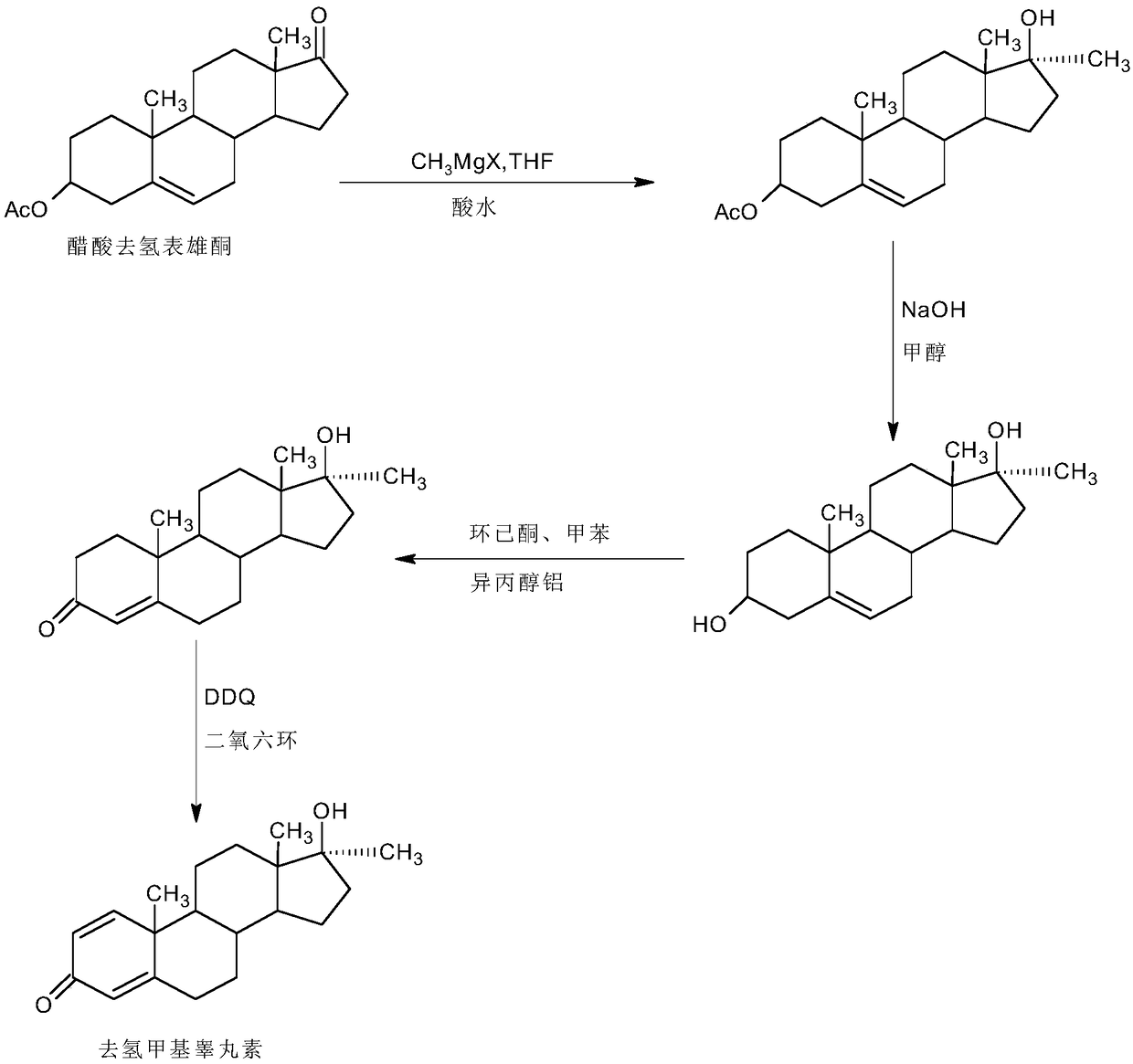
A kind of preparation method of dehydromethyl testosterone product
ActiveCN109456379BWide variety of sourcesProcess economy and environmental protectionSteroidsMethylmagnesium chlorideOrganosolv
The invention provides a preparation method of a metandienone product. The preparation method comprises the following steps that 1,4-androstenedione, namely IDD, is adopted as a raw material, in the presence of methylmagnesium chloride, an organic solvent and acid, alpha-CH3 and beta-OH are introduced at the 17 position, and metandienone is prepared; and then the obtained metandienone is heated byactivated carbon in acetone or lower alcohol below C4 for reflow discoloration and is recrystallized, and the metandienone product is obtained. According to the preparation method, the IDD serves asthe raw material to prepare the metandienone, compared with a traditional method adopting diosgenin as a raw material, the raw material source is wide, a process is economic and environmentally friendly, and the production cost is significantly lowered. Compared with a traditional production method, according to the preparation method, a synthesis route is short, the process is easy, convenient and environmentally friendly, the product yield is high, the quality is good, and the production raw material cost is lowered by 40-45% by calculating through the current raw material price.
Owner:HUNAN KEREY BIOTECH
Synthesis method of cis-jasmonone
PendingCN114409519AShort process routeHigh selectivityOrganic compound preparationOrganic chemistry methodsCyclopenteneMethylmagnesium chloride
The invention discloses a synthesis method of cis-jasmone, which specifically comprises the following steps: carrying out Grignard reaction on 1, 4-dichlorobutene and methylmagnesium chloride in a THF (tetrahydrofuran) medium to obtain 1-chloro-2-pentene; the method comprises the following steps: carrying out cyclization reaction on 2, 5-hexanedione in a dibromomethane medium under a strong alkaline condition to obtain 3-methyl-2-cyclopentene-1-ketone. According to the method, 1-chloro-2-pentene and 3-methyl-2-cyclopentene-1-ketone are subjected to an addition / elimination reaction in an ethanol medium under an alkaline condition, and cis-jasmonone is obtained. Compared with other synthesis methods, the method has the advantages of short process route, high reaction selectivity and conversion rate, high product yield, low cost and the like.
Owner:江苏宏邦化工科技有限公司
Method for preparing 2,3,3,3-tetrafluoropropene using methyl magnesium chloride
ActiveUS10183903B2Short processing flowHigh product yieldPreparation by hydrogen halide split-offPreparation by halogen additionTetrafluoroethyleneMethylmagnesium chloride
The present invention discloses a method for preparing 2,3,3,3-tetrafluoropropene using methylmagnesium chloride, comprising the following steps: 1) preparing 1,1,2-trifluoropropene (CH3CF═CF2); 2) preparing 1,1,1,2,2-pentafluoropane (CF3CF2CH3); 3) preparing 2,3,3,3-tetrafluoropropene (CF3CF═CH2). In the present invention, using a Grignard reagent, namely methylmagnesium chloride, and tetrafluoroethylene as starting raw materials, 2,3,3,3-tetrafluoropropene is prepared by three steps of nucleophilic addition-elimination, fluorine addition, and dehydrofluorination in sequence. The process flow is relatively short, and the product yield is high.
Owner:JUHUA GROUP TECH CENT
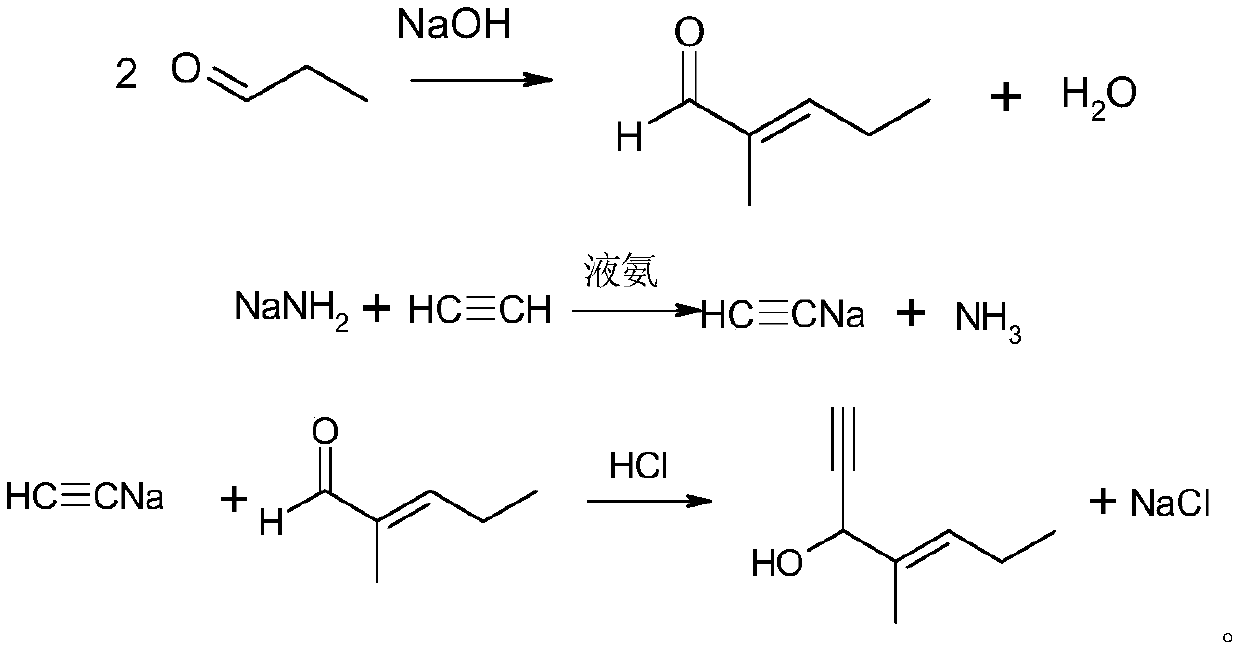
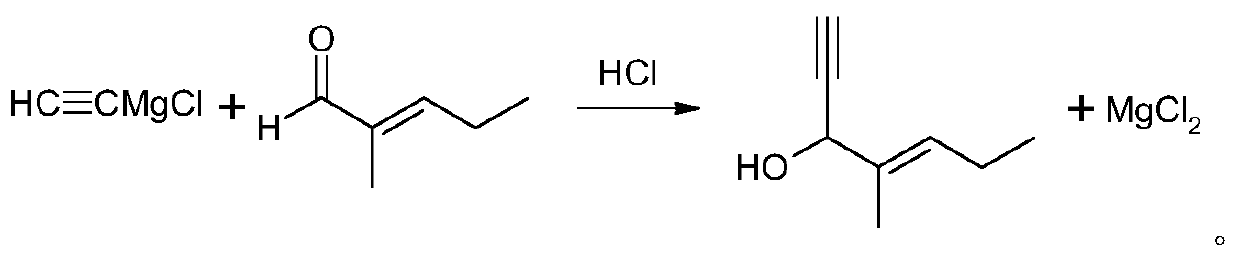
The preparation method of 2-methyl-1-ethynyl-2-penten-1-ol
ActiveCN108059585BEasy dischargeAvoid safety hazardsOrganic compound preparationHydroxy compound preparationPtru catalystMethylmagnesium chloride
The invention discloses a preparation method of 2-methyl-1-ethynyl-2-penten-1-ol, wherein the preparation method comprises the steps: firstly, introducing chloromethane into tetrahydrofuran and magnesium chips, carrying out a Grignard reaction, and generating methyl magnesium chloride; cooling, adding a catalyst, and introducing acetylene to generate acetylene magnesium chloride; and after completion of the reaction, dropwise adding 2-methyl-2-pentenal, hydrolyzing with dilute hydrochloric acid, washing an organic layer by water, carrying out reduced pressure distillation, and rectifying a residual material to obtain 2-methyl-1-ethynyl-2-penten-1-ol. The method is easy to operate, cheap and easily obtained chloromethane is used as a raw material, dangerous sodium amide or liquid ammonia isnot used, and tetrahydrofuran, toluene and other solvents can be reused, the product yield is high, the purity is high, and the cost is low.
Owner:JIANGSU YANGNONG CHEM +1
Method for synthesizing trimethylphosphine oxide
ActiveCN105566388AReduced purification stepsHigh yieldGroup 5/15 element organic compoundsMethylmagnesium chlorideDistillation
The invention discloses a method for synthesizing trimethylphosphine oxide and belongs to the field of compound preparation. The method includes: under nitrogen protection, allowing chloromethane and magnesium chips to react in the mixed solution of toluene and tetrahydrofuran by using bromoethane as initiator so as to obtain methylmagnesium chloride; directly adding trichlorine into the methylmagnesium chloride, which needs not to be purified, after cooling, stirring under low temperature to allow for reaction, extracting after the reaction is completed, and performing vacuum refinery distillation and cooling crystallization to obtain the trimethylphosphine oxide. The method has the advantages that the purity of the obtained trimethylphosphine oxide is above 99.5%, the yield of the trimethylphosphine oxide reaches 95%, and the solvent used by the method is easy to recycle.
Owner:SHANDONG WEITIAN FINE CHEM TECH CO LTD
A kind of synthetic method of continuous flow preparation olmesartan medoxomil intermediate
The invention discloses a continuous flow method for preparing an intermediate of olmesartan medoxomil, which belongs to the technical field of pharmaceutical synthesis and comprises the following steps: step (1), compounding formula I compound 2-propyl-4,5-imidazole dicarboxylic acid Ethyl ester I is dissolved in organic solvent to form reaction phase A, step (2), the methylmagnesium chloride solution of commercialization purchase is used as reaction phase B, step (3), reaction phase A and reaction phase B are used plunger pump to Pump it into the mixer at a certain flow rate, then enter the reactor, keep it for a certain period of time, quench it with the acidic water phase C, and enter the oil-water continuous separator for liquid separation, that is, the organic solution of the compound formula II is obtained. The compound of formula II can be obtained by recrystallization, which solves the technical problems of low purity and high cost in the existing production process. The product of the present invention has high purity and few impurities, and the content of key impurity A, key impurity B and key impurity C can be controlled at 0.1 % or less, and the raw materials are cheap and easy to obtain, the reaction conditions are mild, the operation is simple, the synthesis efficiency is high, and it is suitable for industrial production. It provides a new continuous flow route for the preparation of olmesartan medoxomil and intermediates.
Owner:拓信达(启东)医药生物科技有限公司 +2
Preparation method of metandienone product
ActiveCN109456379AWide variety of sourcesProcess economy and environmental protectionSteroidsActivated carbonOrganic solvent
The invention provides a preparation method of a metandienone product. The preparation method comprises the following steps that 1,4-androstenedione, namely IDD, is adopted as a raw material, in the presence of methylmagnesium chloride, an organic solvent and acid, alpha-CH3 and beta-OH are introduced at the 17 position, and metandienone is prepared; and then the obtained metandienone is heated byactivated carbon in acetone or lower alcohol below C4 for reflow discoloration and is recrystallized, and the metandienone product is obtained. According to the preparation method, the IDD serves asthe raw material to prepare the metandienone, compared with a traditional method adopting diosgenin as a raw material, the raw material source is wide, a process is economic and environmentally friendly, and the production cost is significantly lowered. Compared with a traditional production method, according to the preparation method, a synthesis route is short, the process is easy, convenient and environmentally friendly, the product yield is high, the quality is good, and the production raw material cost is lowered by 40-45% by calculating through the current raw material price.
Owner:HUNAN KEREY BIOTECH
A kind of synthetic method of trimethylphosphine oxide
ActiveCN105566388BReduced purification stepsHigh yieldGroup 5/15 element organic compoundsDistillationMethylmagnesium chloride
The invention discloses a method for synthesizing trimethylphosphine oxide and belongs to the field of compound preparation. The method includes: under nitrogen protection, allowing chloromethane and magnesium chips to react in the mixed solution of toluene and tetrahydrofuran by using bromoethane as initiator so as to obtain methylmagnesium chloride; directly adding trichlorine into the methylmagnesium chloride, which needs not to be purified, after cooling, stirring under low temperature to allow for reaction, extracting after the reaction is completed, and performing vacuum refinery distillation and cooling crystallization to obtain the trimethylphosphine oxide. The method has the advantages that the purity of the obtained trimethylphosphine oxide is above 99.5%, the yield of the trimethylphosphine oxide reaches 95%, and the solvent used by the method is easy to recycle.
Owner:SHANDONG WEITIAN FINE CHEM TECH CO LTD
Preparation method of montelukast chiral intermediate
ActiveCN103554017AMild reaction conditionsReaction conditions are easy to controlOrganic chemistryChemical synthesisMethylmagnesium chloride
The invention discloses a preparation method of a montelukast chiral intermediate, which belongs to the field of medical chemical synthesis. The preparation method comprises the following steps of: reacting compound S1 serving as the raw material with methylmagnesium chloride, oxidizing by using manganese dioxide, chlorinating by using NCS (Succinchlorimide), carrying out chiral reduction by using (-)-B-chlorodiisopinocampherylborane, carrying out cyclization under the action of anhydrous potassium carbonate, and carrying out addition reaction with 2-methyl formate benzylmagnesium bromide to obtain the medical intermediate S7. By adopting the preparation method disclosed by the invention, the application of precious metal for catalysis is avoided, the reaction conditions are mild and easily controlled, the reaction product is single, the yield is high, the optical purity and the chemical purity of the product are greatly improved, the process is simple, the raw materials are low in price and easily available, and therefore the preparation method is more suitable for industrial large-scale production.
Owner:CANGZHOU SENARY CHEM SCI TEC
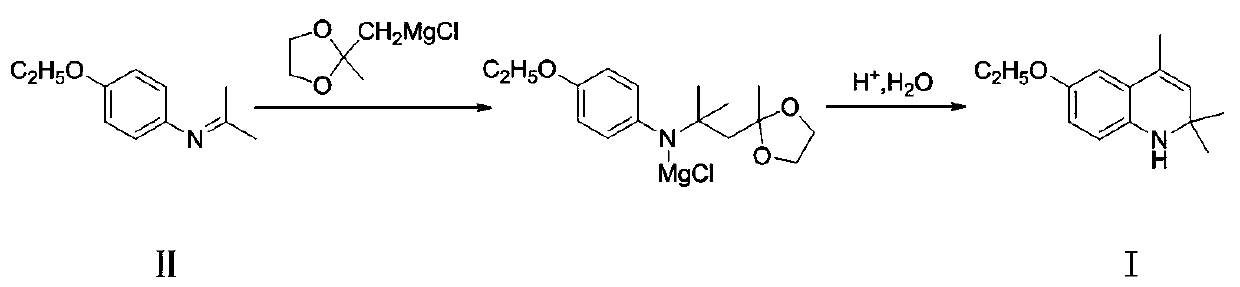
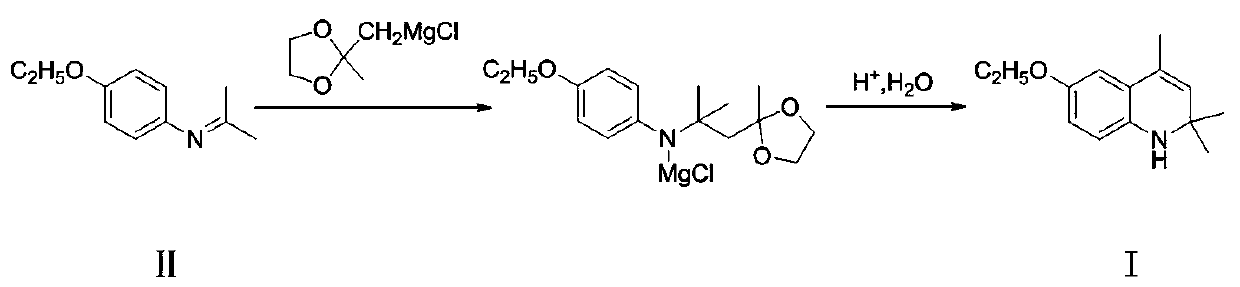
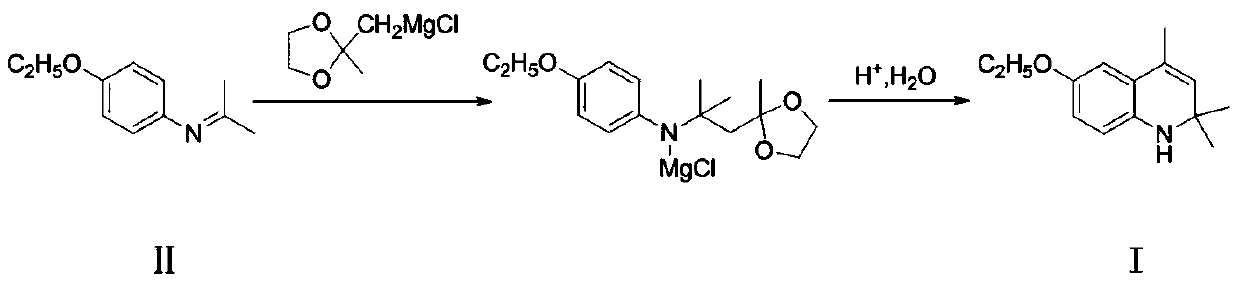
A kind of preparation method of ethoxyquinoline
ActiveCN106749003BImprove conversion rateRelieve pressureOrganic chemistryMethylmagnesium chlorideQuinoline
The invention discloses a preparation method of ethoxyquin. The method comprises the following concrete steps: 1, enabling 4-ethoxy-N-(propane-2-alkenyl)aniline, which is taken as a raw material, to react with ((2-methyl-1,3-dioxolane-2-yl)methyl)magnesium chloride to obtain an intermediate product; 2, hydrolyzing the obtained intermediate product for deprotection; 3, enabling the hydrolyzed product to make a cyclization reaction under an acidic condition to prepare the ethoxyquin. The ethoxyquin is prepared by a one-step method, the intermediate produced in the preparation process does not need to be separated, and a great deal of acetone and methylbenzene are not needed by the reaction, so that the conversion rate of the raw material is better improved, a reaction solvent is saved, and the pressure of environmental protection is reduced; the preparation method has the advantages of mild reaction conditions, simple technology, convenient operation and environment friendliness; furthermore, the obtained product is also higher in purity and can meet the demands of feed markets at all levels, thus having better industrial prospect.
Owner:宜兴市天石饲料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com