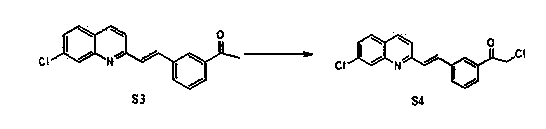
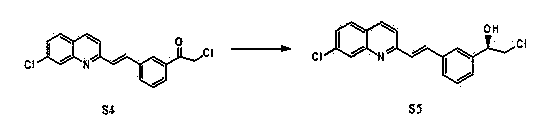
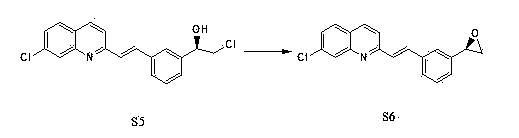
Preparation method of montelukast chiral intermediate
A technology of chiral intermediates and compounds, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as the great influence of quality on the reaction, harsh reaction conditions, and expensive raw materials, so as to improve optical purity and chemical purity, mild reaction conditions, and cheap raw materials. Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Compound S2 from Compound S1
[0038]
[0039] Suspend 146.5g S1 (0.5mol) in 1.5L anhydrous tetrahydrofuran, cool down to -5~0℃, add 300ml tetrahydrofuran solution with a concentration of 1.75mol / L methylmagnesium chloride dropwise within 1~2h, and keep warm for 0.5h after dropping When the reaction is complete, add 500ml of 25% ammonium chloride solution dropwise to terminate, return to 20-30°C, extract twice with 500ml×2 ethyl acetate, combine the organic phases, wash with 500ml saturated brine, separate the organic phase and concentrate to If there is no fraction, add 400ml of methyl tert-butyl ether and stir for crystallization at 10-20°C for 2h, filter with suction, and dry to obtain 136g of beige compound S2 as a solid, with a yield of 88%.
Embodiment 2
[0040] Example 2 Preparation of Compound S2 from Compound S1
[0041] 314ml of tetrahydrofuran solution with a concentration of 1.75mol / L methylmagnesium chloride was added dropwise, and the solution was incubated for 1h until the reaction was complete. Others were the same as in Example 1, and the yield of compound S2 was 89%.
Embodiment 3
[0042] Example 3 Preparation of Compound S2 from Compound S1
[0043] Add dropwise 329ml of tetrahydrofuran solution with a concentration of 1.75mol / L methylmagnesium chloride, and keep it warm for 0.8h until the reaction is complete. Others are the same as in Example 1, and the yield of compound S2 is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com