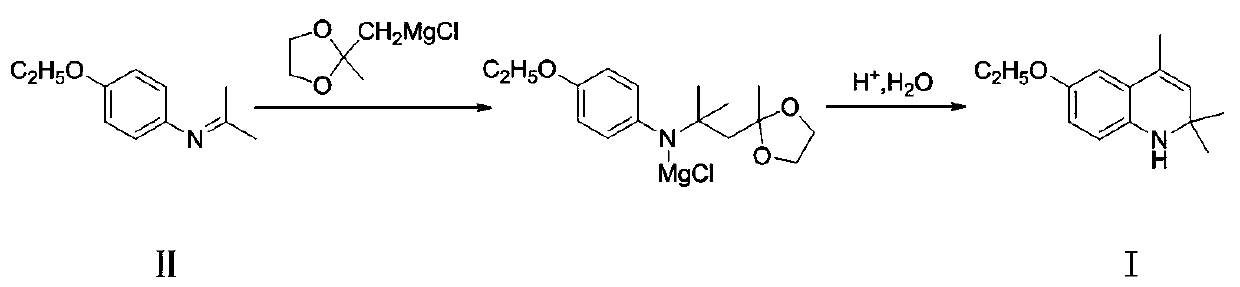
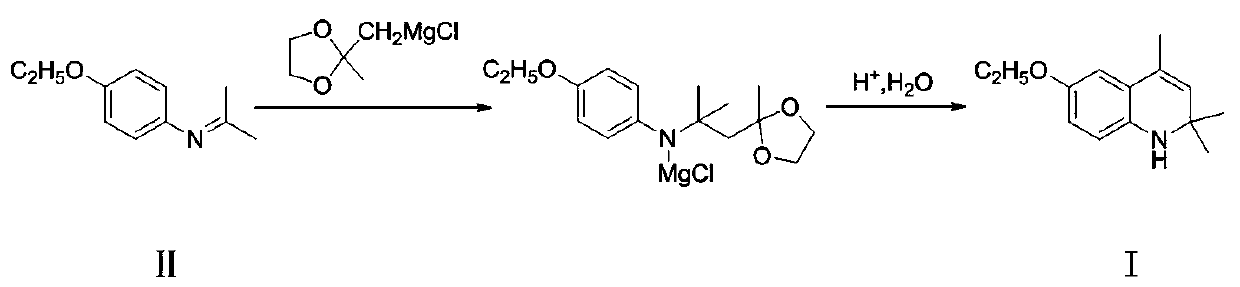
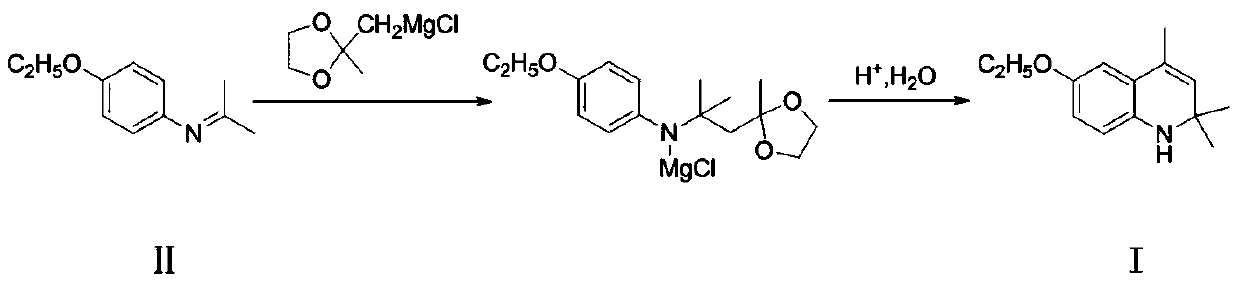
A kind of preparation method of ethoxyquinoline
A technology of ethoxyquinoline and ethoxyquinoline, which is applied in the field of preparation of ethoxyquinoline, can solve the problems of being unable to supply the high-end feed market, low production efficiency, and low product content, so as to achieve good industrial prospects and control costs , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 4-ethoxy-N-(propane-2-enyl)aniline (177g, 1.0mol) and toluene (880g) into the reactor, after stirring evenly, add the newly prepared ((2- Methyl-1,3-dioxolan-2-yl)methyl)magnesium chloride in THF, where ((2-methyl-1,3-dioxolan-2-yl)methyl)magnesium chloride is Compound (II) (176.5g, 1.1mol) and tetrahydrofuran (880g) were kept at 55-60°C for 12 hours after the dropwise addition, and the reaction was completed.
[0029] Then most of the tetrahydrofuran was removed by distillation under normal pressure, then dilute hydrochloric acid (885g) with a concentration of 5% was added, and stirring at room temperature was continued for 5 hours. Aqueous sodium oxide solution was used to adjust the pH to 10, and toluene (1770g) was added to the neutralized mixture, separated after extraction, and the organic phase was loaded into a new reactor, then p-toluenesulfonic acid (8.85g) was added, and the temperature was raised to Stir and divide water under reflux for 6 hours, then d...
Embodiment 2
[0032] Add 4-ethoxy-N-(propane-2-enyl)aniline (II) (177g, 1.0mol) and toluene (550g) into the reactor, after stirring evenly, add the newly prepared ( (2-Methyl-1,3-dioxolan-2-yl)methyl)magnesium chloride in tetrahydrofuran, where ((2-methyl-1,3-dioxolan-2-yl)methyl ) Magnesium chloride is compound (II) (160.5g, 1.0mol), tetrahydrofuran is (550g), after the dropwise addition is completed, keep warm at 55-60°C and continue to react for 4 hours, and the reaction ends.
[0033] Then most of the tetrahydrofuran was removed by distillation under normal pressure, then dilute hydrochloric acid (885g) with a concentration of 5% was added, and stirring at room temperature was continued for 5 hours. Aqueous sodium oxide solution was used to adjust the pH to 10, and toluene (1770g) was added to the neutralized mixture, separated after extraction, and the organic phase was loaded into a new reactor, then p-toluenesulfonic acid (8.85g) was added, and the temperature was raised to Stir and...
Embodiment 3
[0036] Add 4-ethoxy-N-(propane-2-enyl)aniline (II) (177g, 1.0mol) and toluene (720g) into the reactor, after stirring evenly, add the newly prepared ( (2-Methyl-1,3-dioxolan-2-yl)methyl)magnesium chloride in tetrahydrofuran, where ((2-methyl-1,3-dioxolan-2-yl)methyl ) Magnesium chloride is (168.5g, 1.05mol) of compound (II), tetrahydrofuran is (720g), the temperature of the mixture is kept below 60°C during the dropwise addition, and the reaction is continued at 55-60°C for 8 hours after the dropwise addition, and the reaction ends.
[0037] Then most of the tetrahydrofuran was removed by distillation under normal pressure, then dilute hydrochloric acid (885g) with a concentration of 5% was added, and stirring at room temperature was continued for 5 hours. Aqueous sodium oxide solution was used to adjust the pH to 10, and toluene (1770g) was added to the neutralized mixture, separated after extraction, and the organic phase was loaded into a new reactor, then p-toluenesulfonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com