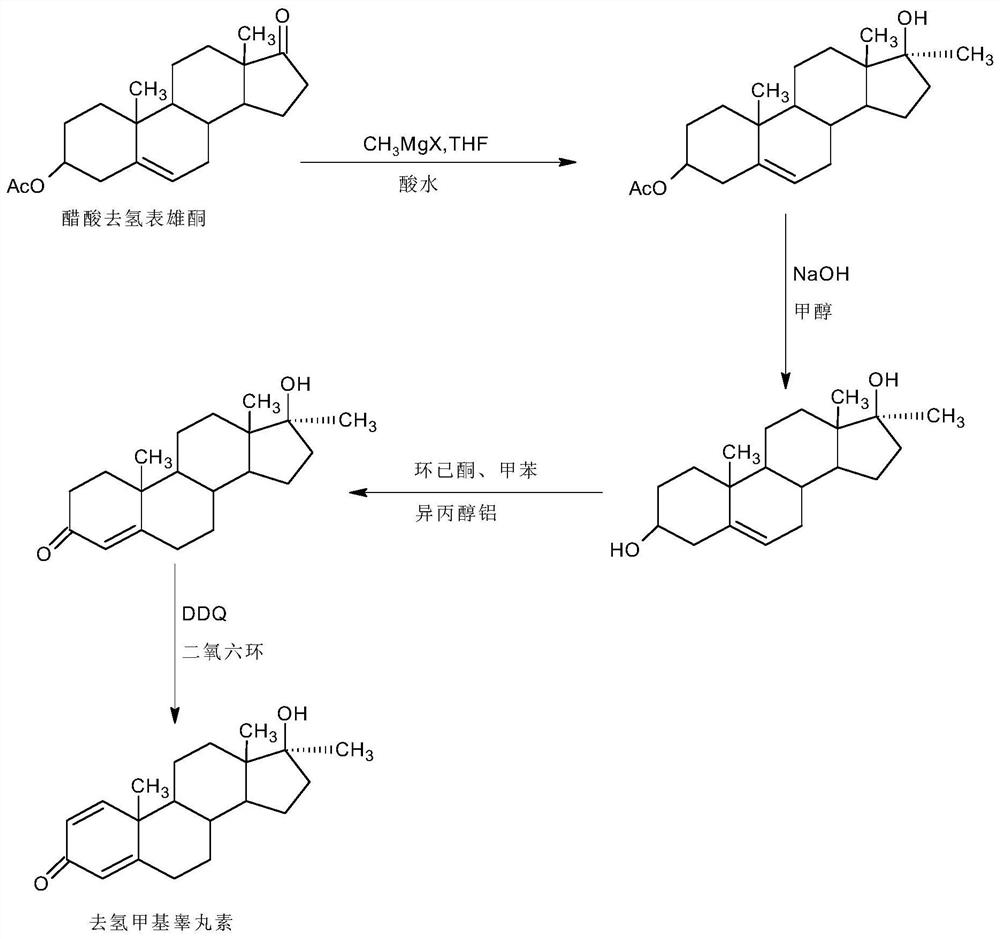
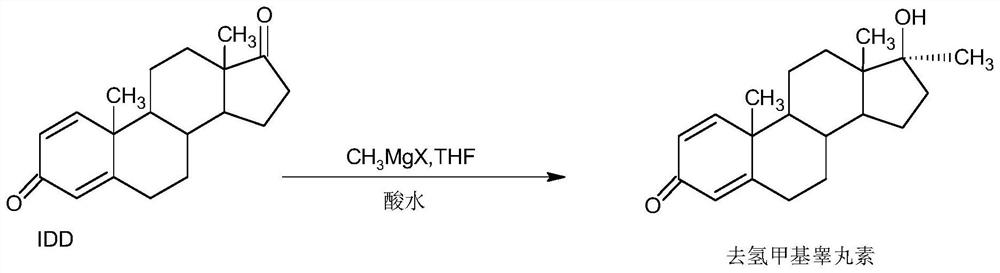
A kind of preparation method of dehydromethyl testosterone product
A technology of hydromethyl testosterone and products, applied in the field of preparation of anabolic hormone drug dehydromethyl testosterone products, can solve the production cost of dehydromethyl testosterone and the increase in market price, saponin, diene production cost problems such as growth, rising planting costs, etc., to achieve the effect of reducing the cost of production raw materials, high product yield, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A. Preparation of Grignard reagents
[0038]In a 1000ml three-neck flask, add 35g of magnesium powder and 800ml of tetrahydrofuran, stir, keep warm at 30-35°C, and feed 98g of methyl chloride. After the passage, continue to stir and react for 4 to 6 hours until the magnesium powder basically disappears, and the Grignard Reagent spare;
[0039] B, the preparation of dehydromethyltestosterone
[0040] In a 1000ml three-neck flask, add 100g IDD and 500ml THF, stir and heat up to 50-55°C, slowly add about 800ml of the Grignard reagent solution prepared above, dropwise for about 1-1.5 hours, then continue to keep warm and stir for 2~ After 3 hours, TLC detects the end point of the reaction. After the reaction, slowly add 2N hydrochloric acid to pH 2-3. After the drop, continue the hydrolysis reaction at 50-55°C for 2-3 hours. TLC detects that the hydrolysis is complete. After the reaction, reduce the pressure About 90-95% THF was concentrated, and the recovered THF was use...
Embodiment 2
[0044] A. Preparation of Grignard reagents
[0045] In a 1000ml three-necked flask, add 35g of magnesium powder and 800ml of tetrahydrofuran, stir, keep warm at 40-45°C, and feed 120g of methyl bromide. After the passage, continue to stir and react for 2 to 3 hours until the magnesium powder basically disappears to obtain the Grignard reagent spare;
[0046] B, the preparation of dehydromethyltestosterone
[0047] In a 1000ml three-neck flask, add 100g IDD and 500ml toluene, stir and heat up to 50-55°C, slowly add about 800ml of the Grignard reagent solution prepared above, dropwise for about 1-1.5 hours, then continue to keep warm and stir for reaction 2 ~3 hours, TLC detects the end point of the reaction. After the reaction, slowly add 2N hydrochloric acid to PH2-3. After the drop, continue the hydrolysis reaction at 50-55°C for 2-3 hours. The mixture of THF and toluene about 90-95% was concentrated under reduced pressure, and the recovered mixture of THF and toluene was u...
Embodiment 3
[0051] A. Preparation of Grignard reagents
[0052] In a 1000ml three-necked flask, add 35g of magnesium powder and 800ml of ether, stir, keep warm at 30-35°C and add 150g of methyl iodide dropwise. After passing through, continue to stir and react for 4 to 6 hours until the magnesium powder basically disappears to obtain Grignard Reagent spare;
[0053] B, the preparation of dehydromethyltestosterone
[0054] In a 1000ml three-neck flask, add 100g IDD and 500ml benzene, stir and heat up to 50-55°C, slowly add about 800ml of the Grignard reagent solution prepared above, dropwise for about 1-1.5 hours, then continue to keep warm and stir for reaction 2 ~3 hours, TLC detects the end point of the reaction. After the reaction, slowly add 2N hydrochloric acid to pH2-3. After the drop, continue the hydrolysis reaction at 50-55°C for 2-3 hours. Concentrate under high pressure to get about 90-95% of the mixture of ether and benzene, and the recovered mixture of ether and benzene can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com