Synthesis method of 4-isopropylresorcinol
A technology of propyl resorcinol and its synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, production of bulk chemicals, etc., can solve problems such as complex product composition and difficult purification, shorten hydrogenation time, The effect of reducing temperature and pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
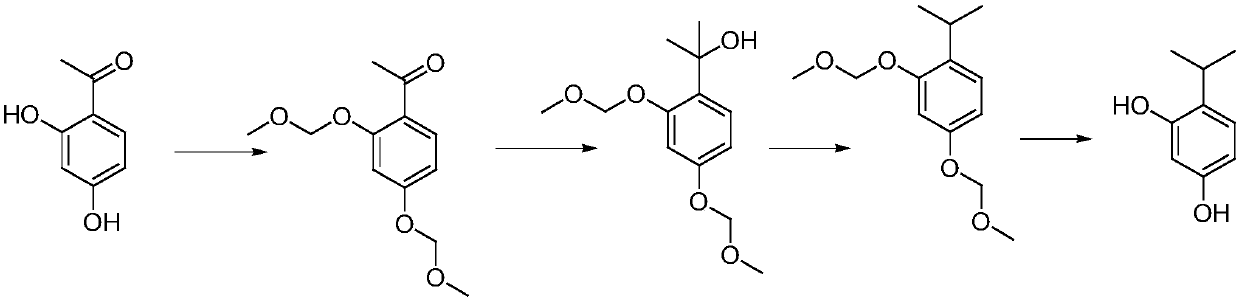
[0020] Such as figure 1 Shown, a kind of synthetic method of 4-isopropyl resorcinol comprises the steps:
[0021] S1, group protection: in the presence of solvent and potassium carbonate, 2,4-dihydroxyacetophenone and halomethyl ether react to generate 2,4-dioxymethoxyacetophenone;
[0022] S2, Grignard reaction: 2,4-dioxymethoxyacetophenone reacts with Grignard reagent to prepare 2,4-dioxymethoxyphenylpropanol;
[0023] S3. Hydrodeoxygenation: 2,4-dioxymethoxyphenylpropanol is subjected to a hydrogenation reaction in the presence of a hydrogenation catalyst to obtain 2,4-dioxymethoxycumene;
[0024] S4. Deprotection: 2,4-dioxymethoxycumene is deprotected in the presence of a solvent and concentrated hydrochloric acid to obtain 4-isopropylresorcinol.
[0025] In S1, the solvent is N,N-dimethylformamide, and the halomethyl ether is chloromethyl ether.
[0026] In S1, add 2,4-dihydroxyacetophenone to N,N-dimethylformamide, then add anhydrous potassium carbonate and chlorometh...
specific Embodiment 1
[0031] S1: Add 100 grams of 2,4-dihydroxyacetophenone to 1 liter of N,N-dimethylformamide, add 200 grams of anhydrous potassium carbonate, then add 115 grams of chloromethyl methyl ether, and react at 60 degrees After 8 hours, cool and filter, add 3 L of water to separate the liquid, then extract the water phase with ethyl acetate three times and combine it with the organic phase, dry and evaporate the solvent to obtain 150 g of the product;
[0032] S2: Add 150 grams of the product obtained in S1 into 2 liters of anhydrous tetrahydrofuran, cool to 0°C, add 250 ml of 3M methylmagnesium chloride dropwise, then react at 0°C for 2 hours, add 1 liter of saturated ammonium chloride aqueous solution to quench The water layer obtained after the liquid separation was extracted 3 times with ethyl acetate, then combined in the organic phase, dried, and evaporated to remove the solvent to obtain 144 grams of the product;
[0033] S3: Add 144 grams of the product obtained in S2 to 1 liter...
specific Embodiment 2
[0035] S1: Add 100 grams of 2,4-dihydroxyacetophenone to 1 liter of N,N-dimethylformamide, add 200 grams of anhydrous potassium carbonate, then add 115 grams of chloromethyl methyl ether, and react at 50 degrees After 10 hours, cool and filter, add 3L of water to separate the liquid, then extract the water phase with ethyl acetate 4 times and combine it with the organic phase, dry and evaporate the solvent to obtain 148 g of the product;
[0036] S2: Add 148 grams of the product obtained in S1 to 2 liters of anhydrous tetrahydrofuran, cool to 0°C, add 250 ml of 3M methylmagnesium bromide dropwise, then react at 0°C for 2 hours, add 1 liter of saturated ammonium chloride The aqueous solution was quenched, and the aqueous layer obtained after the separation was extracted 3 times with ethyl acetate, then combined in the organic phase, dried, and evaporated to remove the solvent to obtain 140 grams of the product;
[0037] S3: Add 140 grams of the product obtained in S2 to 1 liter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com