Preparation method of 4-(1-hydroxy-1-methylethyl)-2-propylimidazolium iodide-5-carboxylate
A technology of propylimidazole and methyl ethyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of large environmental pollution, complicated process, and low yield, and achieve the effects of environmental friendliness, short process route, and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
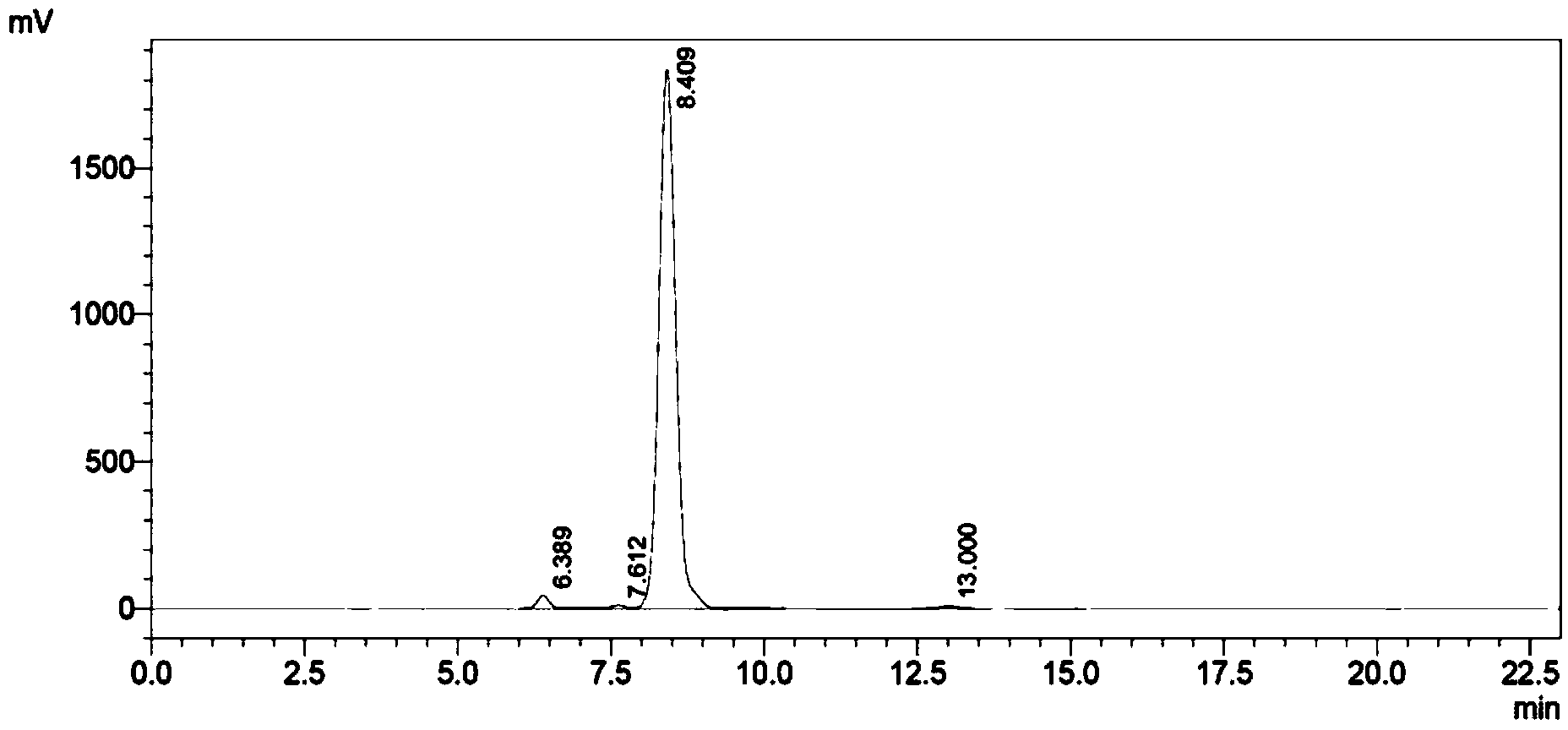
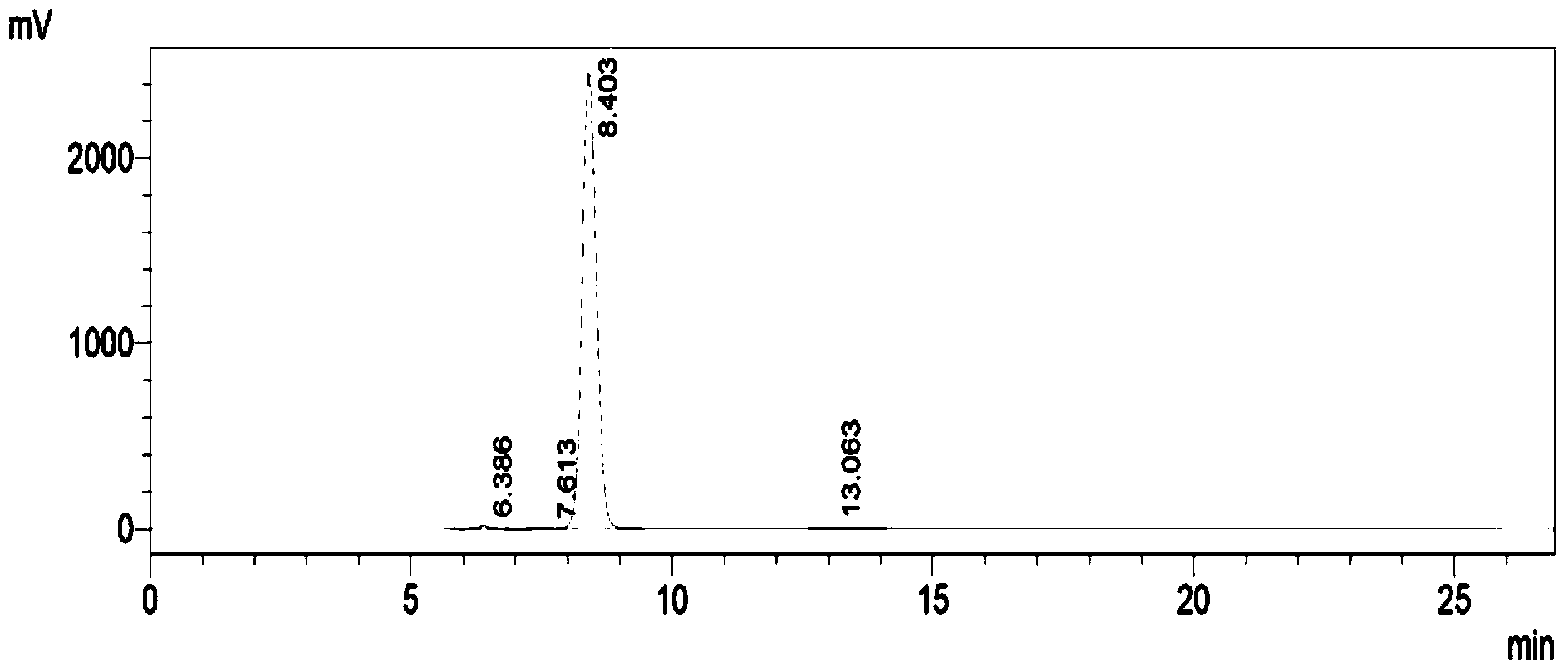
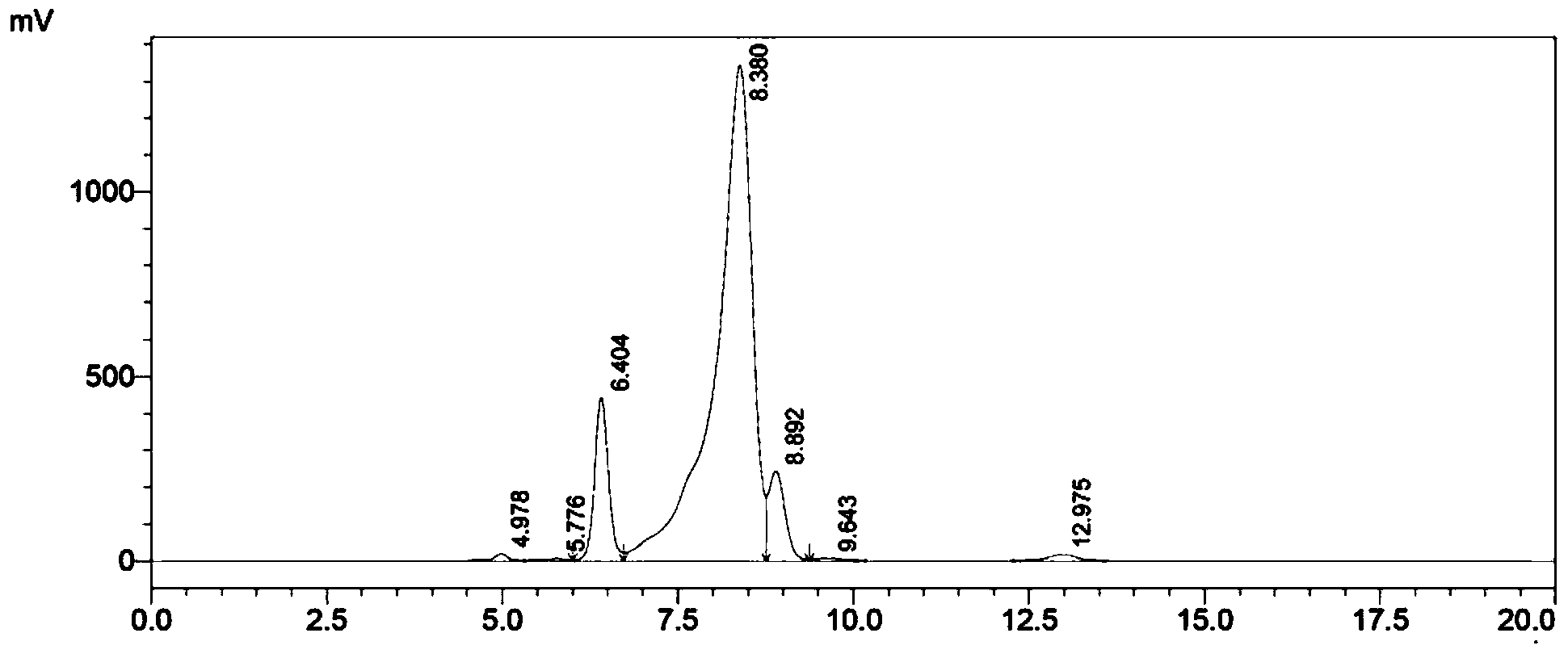
Image
Examples
Embodiment 1
[0039] Add 120mL (2mol / L, 0.24mol) of tetrahydrofuran solution of methylmagnesium bromide into a 250mL three-necked flask, preferably blown with nitrogen for protection, cool down to -10°C, and start adding 2-propylimidazole-4 dropwise, 10g (0.04mol) of ethyl 5-dicarboxylate in 30mL of dichloromethane solution, keep the temperature below 10°C during the dropwise addition, after the dropwise addition, control the temperature at 15°C to react for 1 hour, after the reaction , lower the temperature to 0°C, add 100mL ethyl acetate and 60mL saturated aqueous ammonium chloride solution, stir for 15 minutes, let stand, separate, and collect the organic phase; the water phase can be extracted twice with ethyl acetate, 30mL each time, combined and collected The organic phase was washed three times with saturated brine, 30 mL each time, and finally dried with anhydrous sodium sulfate, filtered, and the filtrate was collected, and then the solvent was removed by distillation under reduced ...
Embodiment 2
[0041] Adopt the method of Example 1 to obtain the oily substance, then add 100mL of dilute hydrochloric acid solution and 1g of activated carbon to the oily substance with a mass concentration of 10%, the addition of the activated carbon is 2-propylimidazole-4,5-dicarboxylate 10% of the mass of ethyl acetate, and then control the temperature at 50°C to conduct a salt-forming reaction for 30 minutes, filter while it is hot, wash the filter cake with cold water three times, 20ml each time, combine the filtrate, cool the filtrate to 0°C, and slowly add mass The concentration is 10% sodium hydroxide aqueous solution to adjust the pH to 7 to remove hydrochloric acid. After adjusting the pH value, stop stirring, and continue to cool the temperature of the reaction liquid (filtrate) to -5°C to make the filtrate completely condense into a solid, and then place it in Slowly melt the ice at room temperature, and a large amount of white solid precipitates out. Stir again for 5 minutes, f...
Embodiment 3
[0043] Add 120mL (2mol / L, 0.24mol) of tetrahydrofuran solution of methylmagnesium bromide into a 250mL three-necked flask, preferably blown with nitrogen for protection, cool down to -10°C, and start adding 2-propylimidazole-4 dropwise, 10g (0.04mol) of ethyl 5-dicarboxylate in 30mL of dichloromethane solution, keep the temperature at -10°C to 10°C during the dropwise addition, after the dropwise addition, control the temperature at 13°C for 1.5 hours , after the reaction, cool down to 0°C, add 100mL ethyl acetate and 60mL saturated aqueous ammonium chloride solution, stir for 15 minutes, let stand, separate, and collect the organic phase; the water phase can be extracted twice with ethyl acetate, each time 30 mL, combined the collected organic phases, washed three times with saturated brine, 30 mL each time, finally dried with anhydrous sodium sulfate, filtered, collected the filtrate, and then distilled under reduced pressure to remove the solvent to obtain 9.4 g of crude pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com