A kind of synthetic method of continuous flow preparation olmesartan medoxomil intermediate
A technology for olmesartan medoxomil and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of long reaction time, limited production capacity, and no controlled impurities, and achieves the effects of short reaction time, high purity and good quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
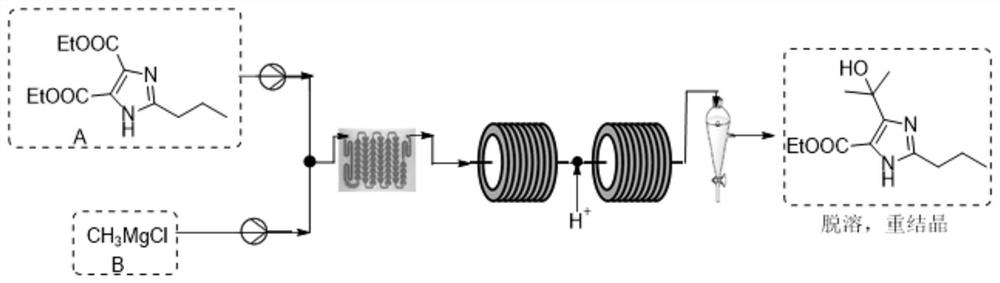
[0037] Step 1, the preparation of formula II compound 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-carboxylic acid ethyl ester, 190g2-propyl-imidazole-4,5-dicarboxylate Acid diethyl ester (compound of formula I) was dissolved in a mixed solution of 338.2 g of tetrahydrofuran and 1316.3 g of toluene, stirred and dissolved into phase A, and the relative concentration was 0.1 g / mL. The commercially available methylmagnesium chloride tetrahydrofuran solution (3M) was used as phase B with a density of 1.04g / mL; the temperature of the reactor was set at 15°C, the flow rate of phase A was set at 10mL / min, and the flow rate of phase B was set at 1.13mL / min. Pump phase A and phase B into the mixer and then enter the reactor, retention time T Res =1.3min; set 10% hydrochloric acid aqueous solution as item C, and pump it into the quenching module at a feed rate of 5.52mL / min. The reaction liquid directly enters the continuous oil-water separator for continuous liquid separation, and...
Embodiment 2
[0042] Step 1, the preparation of formula II compound 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-carboxylic acid ethyl ester, 950g2-propyl-imidazole-4,5-dicarboxylate Acid diethyl ester (compound of formula I) was dissolved in 1.69kg of 2-methyltetrahydrofuran and 6.58kg of toluene mixed solution, stirred and dissolved into phase A, and the relative concentration was 0.1g / mL. The commercially available methylmagnesium chloride tetrahydrofuran solution (3M) was used as phase B with a density of 1.04g / mL; the temperature of the reactor was set at 15°C, the flow rate of phase A was set at 50mL / min, and the flow rate of phase B was set at 5.65mL / min. Pump phase A and phase B into the mixer and then enter the reactor, retention time T Res =1.3min; set 10% hydrochloric acid aqueous solution as item C, and pump it into the quenching module at a feed rate of 27.6mL / min. The reaction liquid directly entered the continuous oil-water separator for continuous liquid separation, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com