A New Method for Synthesizing 17α-Hydroxyprogesterone
A technology of hydroxyprogesterone and hydroxyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low 3-position ketal protection yield, low overall yield, incomplete reaction, etc., and achieves easy industrial implementation and simple post-processing , the effect of less raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 117
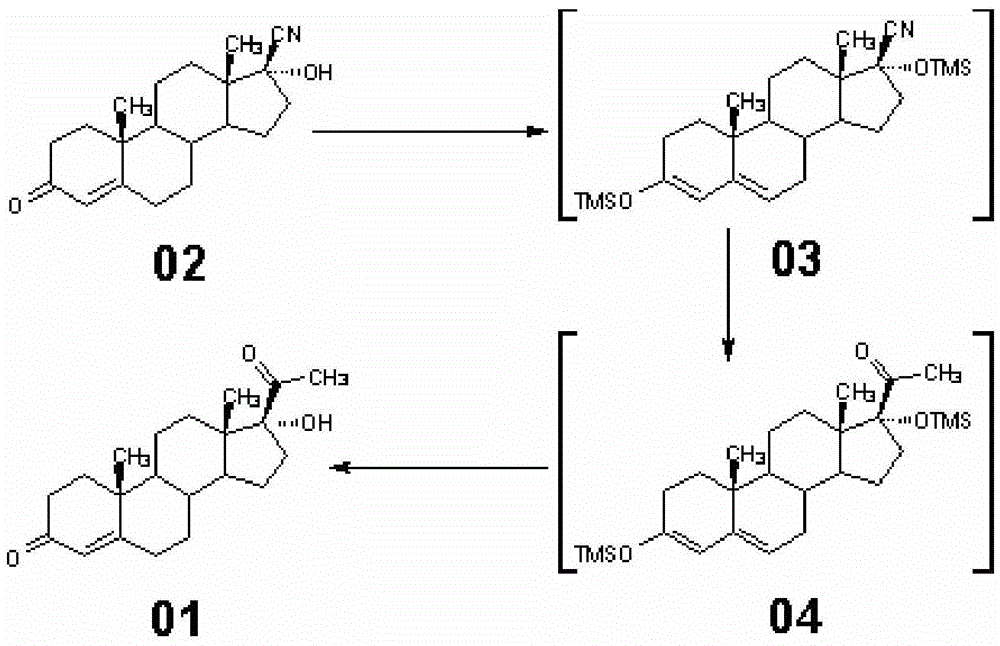
[0038] Synthesis of embodiment 117α-hydroxyprogesterone (01)
[0039] At room temperature, in a 5000L enamel reaction kettle equipped with a thermometer and a stirrer, add 1000L of toluene, stir, add 200kg of 17β-cyano-17α-hydroxy-4-androsten-3-one (02) and 87kg of imidazole , lower the temperature to -5~0°C, add 153kg of trimethylchlorosilane, stir evenly, slowly add 2400kg of 2M methylmagnesium chloride solution dropwise, keep the temperature at -5~0°C for 2 hours, then slowly raise the temperature to 55°C~60 ℃, heat preservation reaction for 4 hours, after the plate reaction is complete, cool the ice brine to below 10 ℃, slowly transfer to the ammonium chloride aqueous solution cooled by circulating water, stir for 30 minutes, stand still for 30 minutes, separate the brine layer, and use 200kg Wash once with tap water, separate the tap water, add 6kg of activated carbon, decolorize at 55-60°C for 2 hours, heat-retain and filter, wash with an appropriate amount of toluene, t...
Embodiment 217
[0040] The synthesis of embodiment 217α-hydroxyprogesterone (01)
[0041] The synthesis method is the same as in Example 1, except that in this example, the 87kg of imidazole in Example 1 is replaced with 105kg of 2-methylimidazole. Finally, 195.92kg of 17α-hydroxyprogesterone (01) was obtained, with a weight yield of 97.96%, a molar yield of 92.91%, and a melting point of 216.0-220.0°C. The synthesis of embodiment 317α-hydroxyprogesterone
Embodiment 317
[0042] Synthetic method is the same as embodiment 1, and difference is only in the present embodiment, the methylmagnesium chloride solution 2400kg of 2M among the example 1 is reduced to 2000kg. Finally, 194.87kg of 17α-hydroxyprogesterone (01) was obtained, with a weight yield of 97.44%, a molar yield of 92.42%, and a melting point of 215.0-219.0°C. The synthesis of embodiment 417α-hydroxyprogesterone
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com