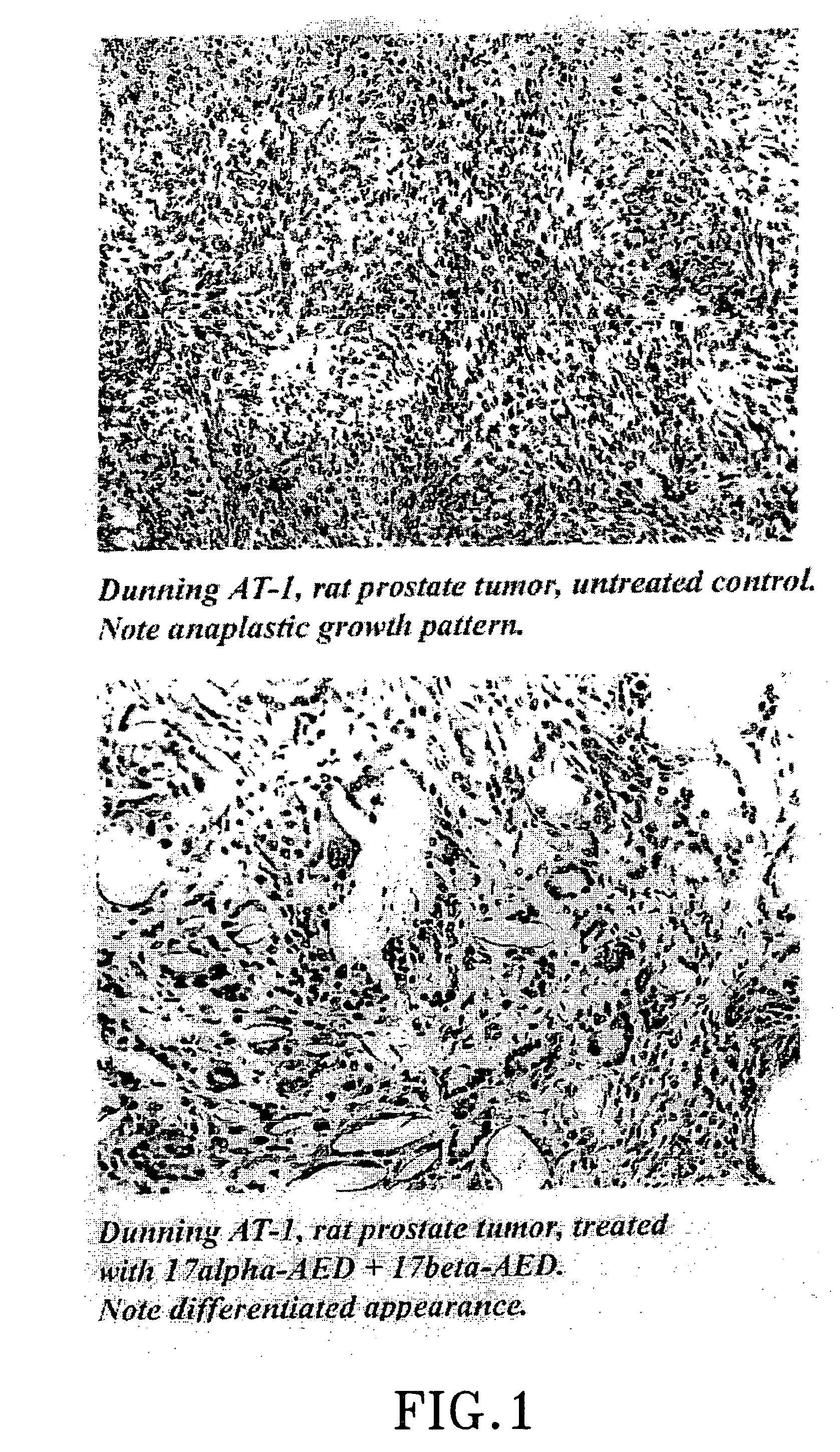
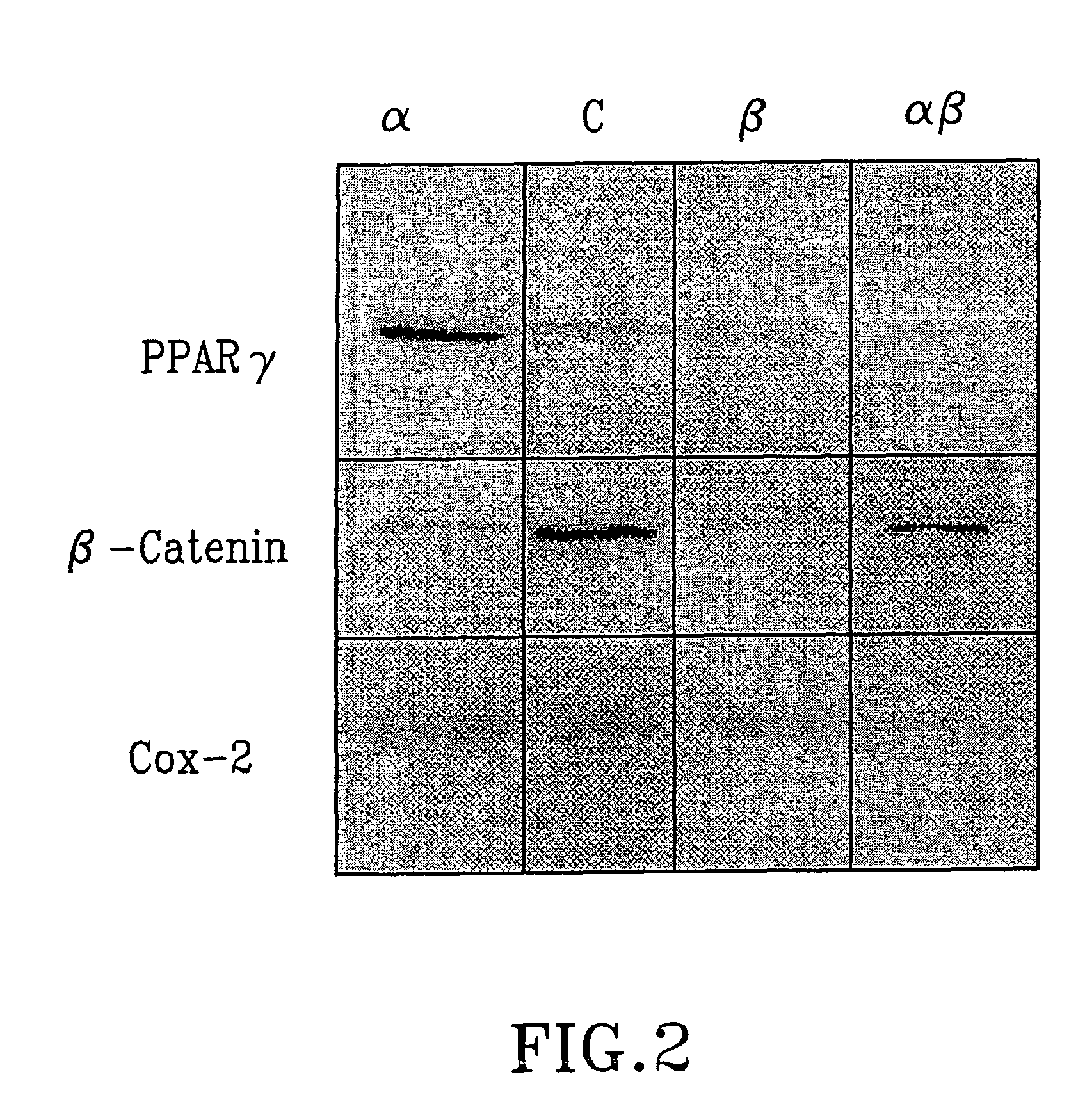
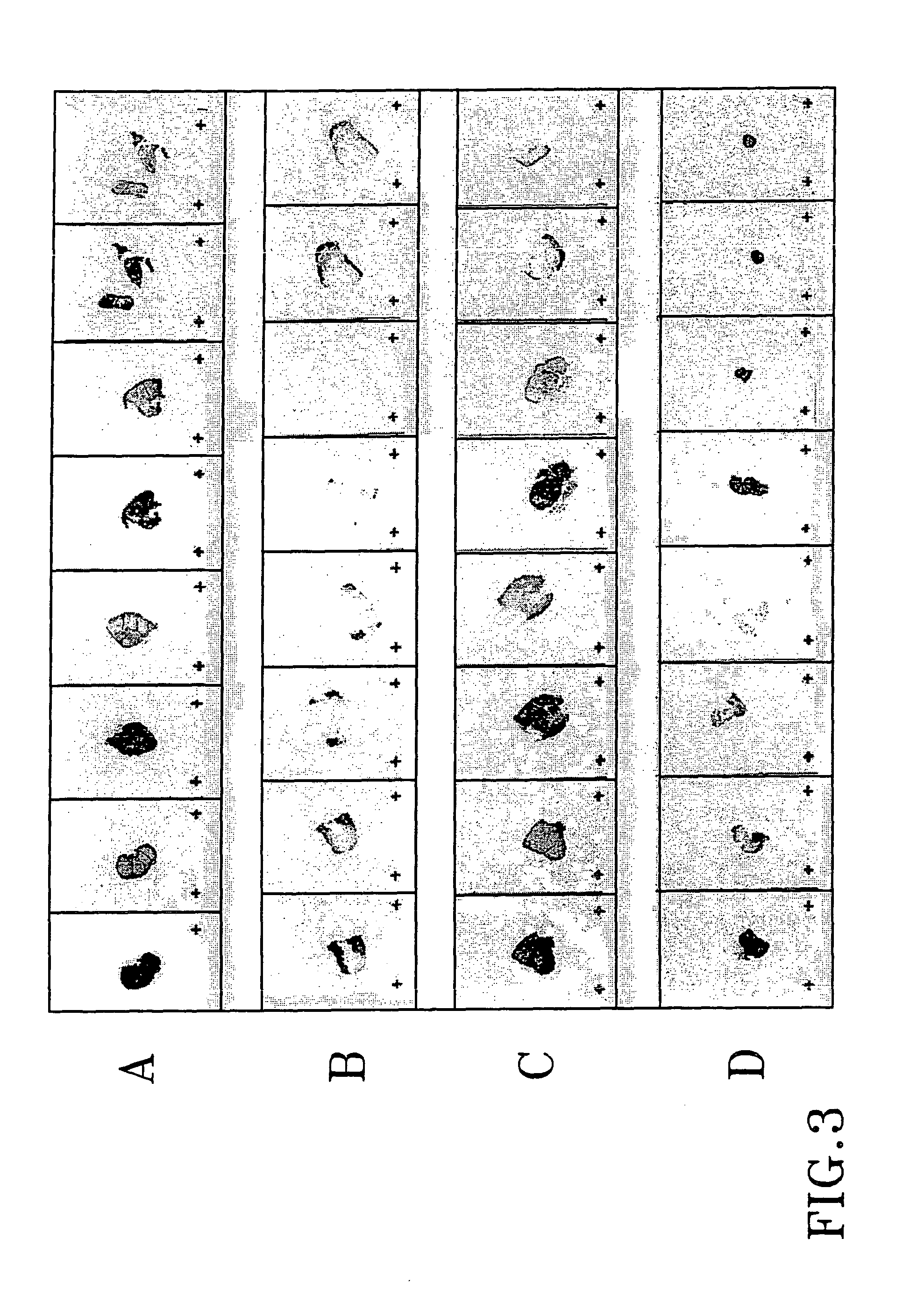
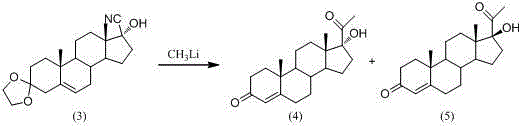
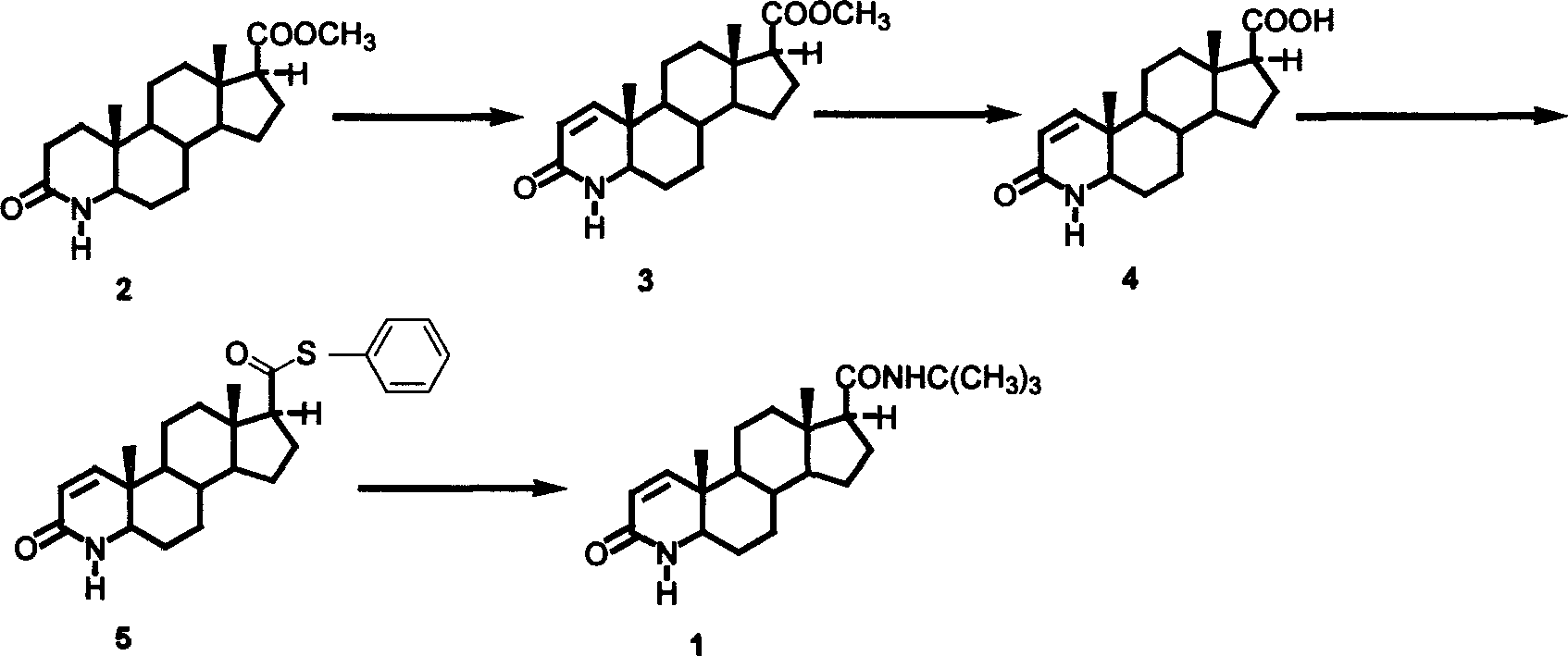
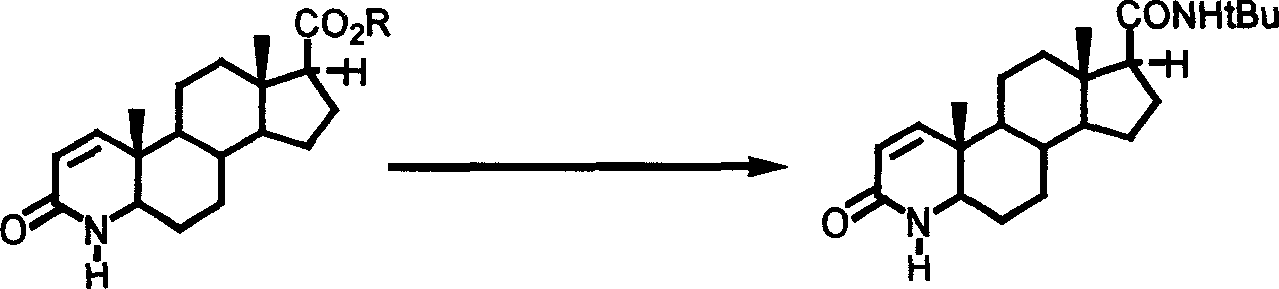
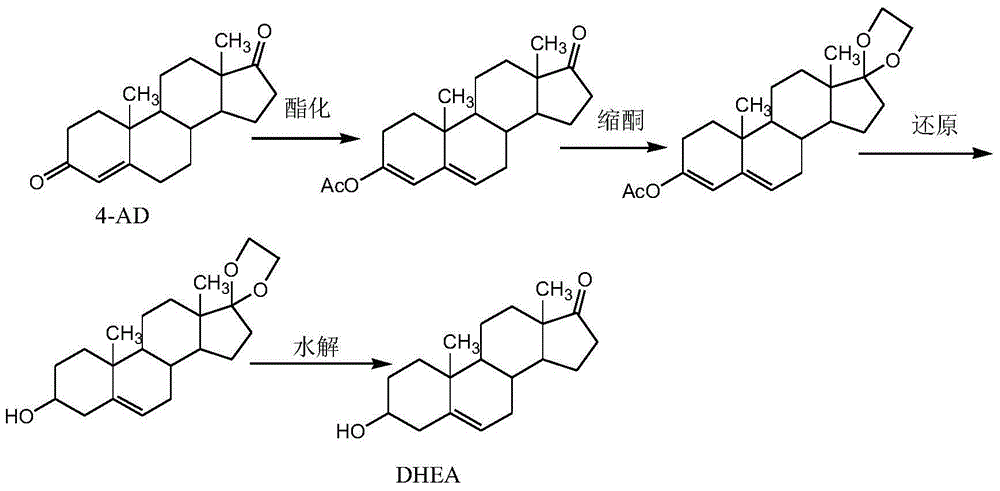
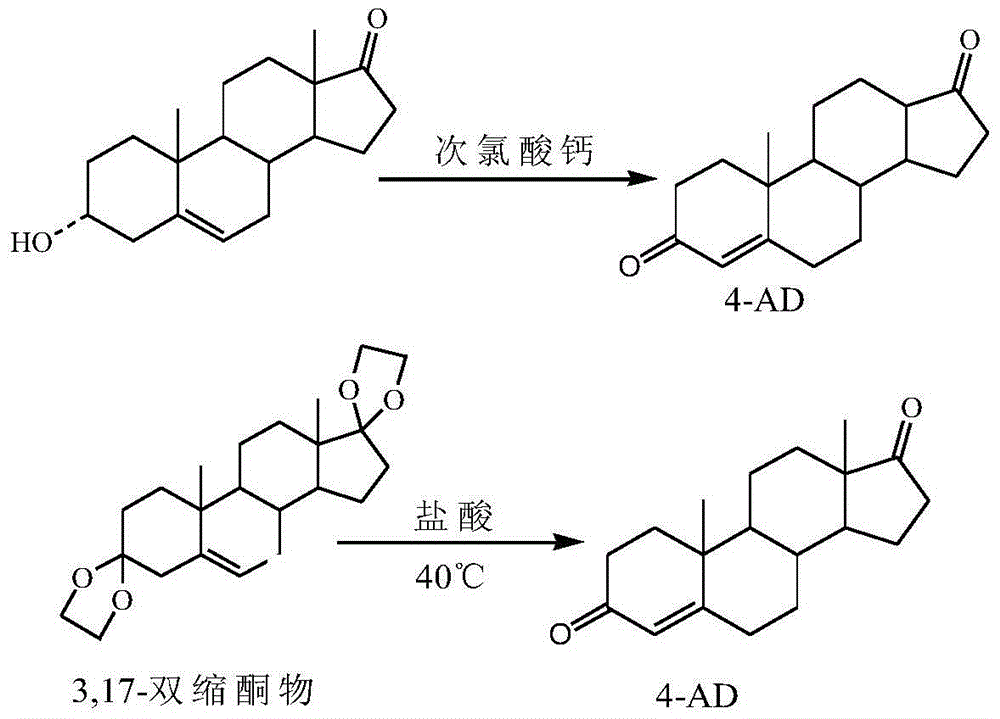
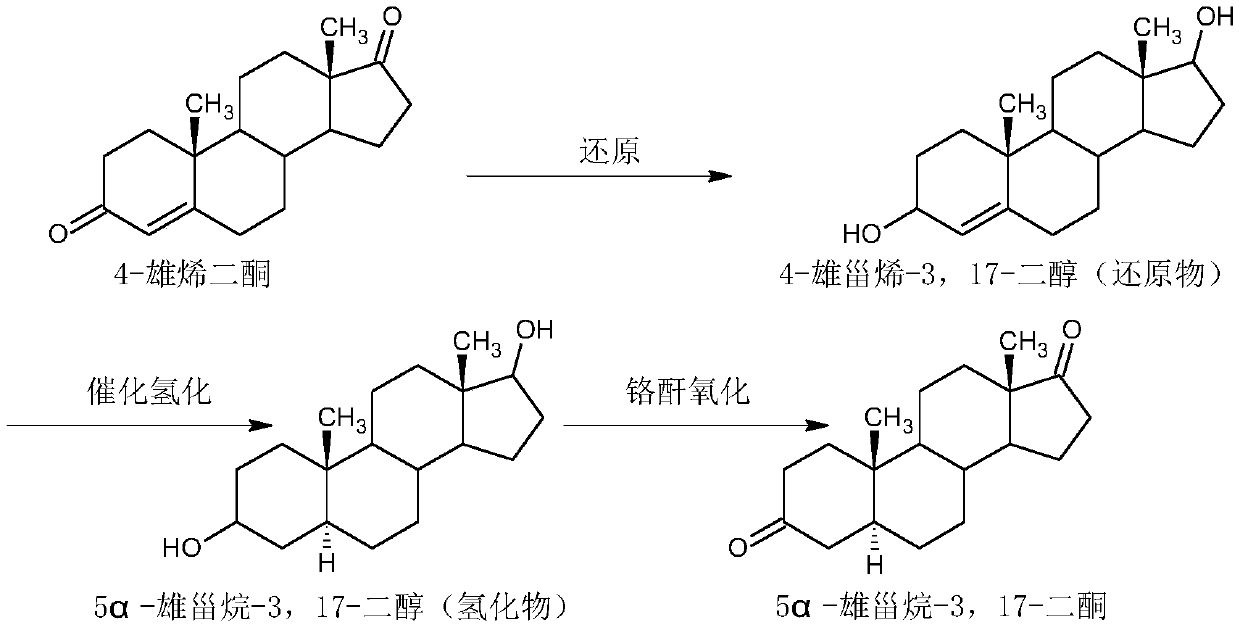
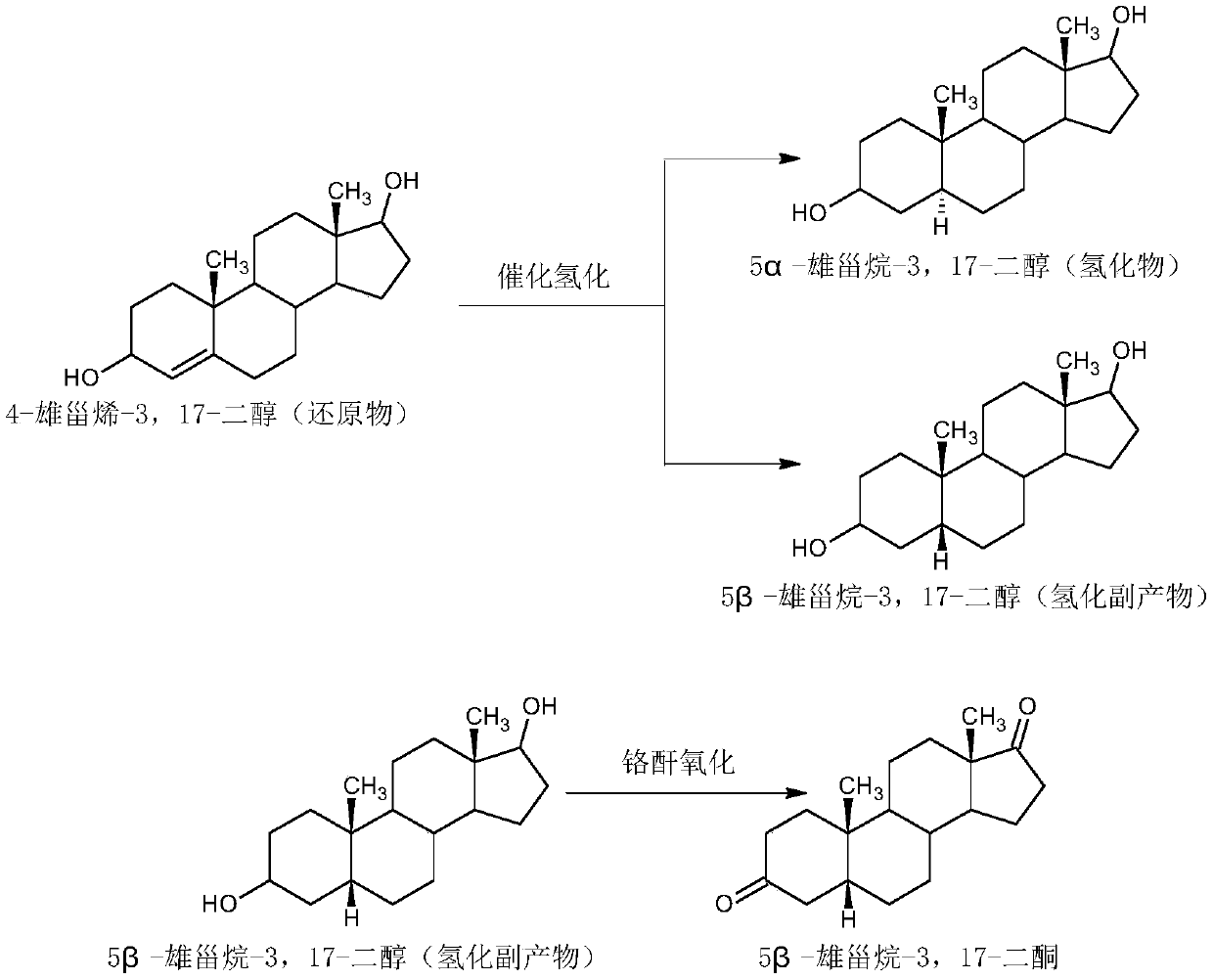
Patents
Literature
86 results about "Androstenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Unsaturated derivatives of the steroid androstane containing at least one double bond at any site in any of the rings.
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
Use of Δ5-androstene-3β-ol-7,17-dione in the treatment of lupus erythematosus
Lupus erythematosus can be treated by administering therapeutic amounts of Δ5-androstene-3β-ol-7,17-dione and metabolizable precursors thereof, such as Δ5-androstene-3b-acetoxy-7,17-dione, which are readily metabolized in vivo to Δ5-androstene-3β-ol-7,17-dione but are not appreciably metabolizable in vivo to androgens, estrogens or dehydroepiandrosterone. Such treatment can be prophylactic, ameliorative or curative in nature.
Owner:INTERHEALTH NUTRACEUTICALS
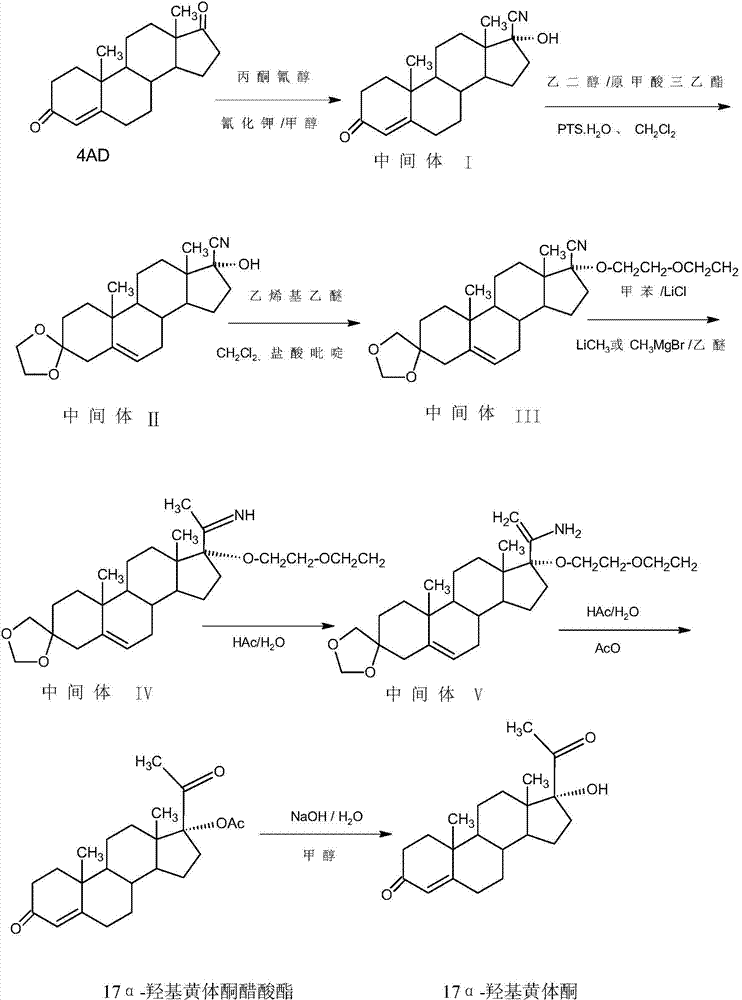
Synthesis method of 17alpha-hydroxyl progesterone
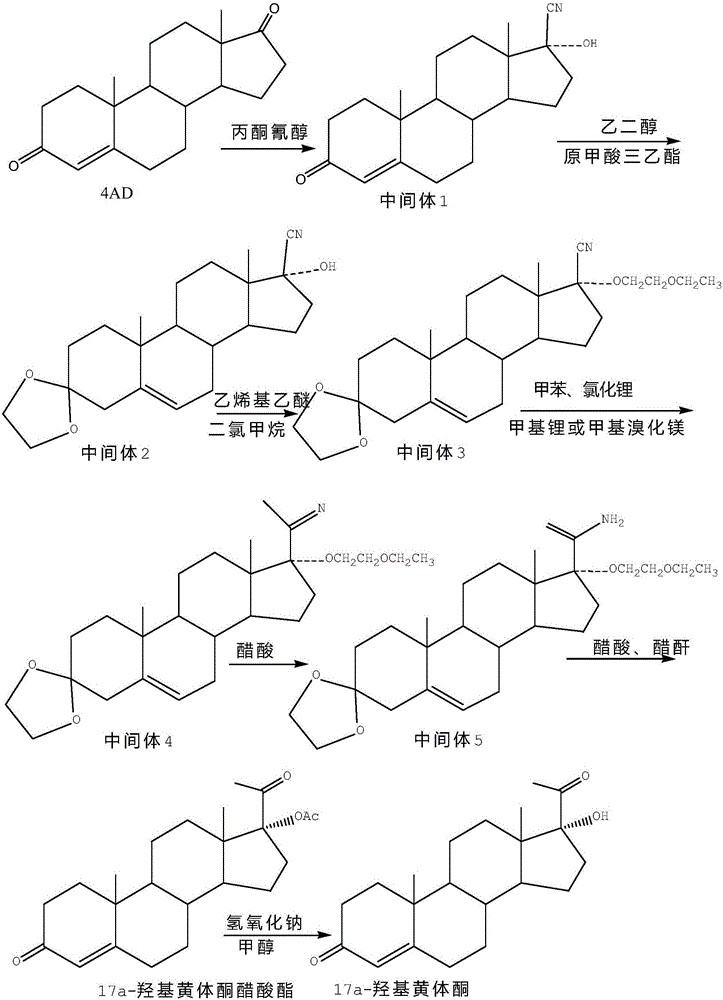
The invention discloses a synthesis method of 17alpha-hydroxyl progesterone, which comprises the steps of by taking 4-androstene-diketone as a starting raw material, carrying out vyanation via acetone cyanohydrin, protecting 3carbonyl by using triethyl orthoformate and ethyl alcohol, protecting 17hydroxy by using butyl vinyl ether, and carrying out hydrolysis after Grignard reaction to generate the 17alpha-hydroxyl progesterone. According to the synthesis method, the cost is reduced, the environment pollution is decreased, the reaction time is shortened, the aftertreatment process of the industrial production is simplified, the production time and cost are greatly saved, the productivity is improved and convenience is brought to the industrial implementation. Compared with the traditional process, the synthesis method has the characteristics of low raw material cost, simple and convenient method, high yield, good selectivity, mild reaction condition, small pollution and applicability to industrial production; and the method is stable and easy to realize.
Owner:ZHEJIANG PURUI PHARMA
High-sensitivity nanometer cobalt oxide-doped talampicillin molecular imprinting electrochemical sensor and preparation method thereof
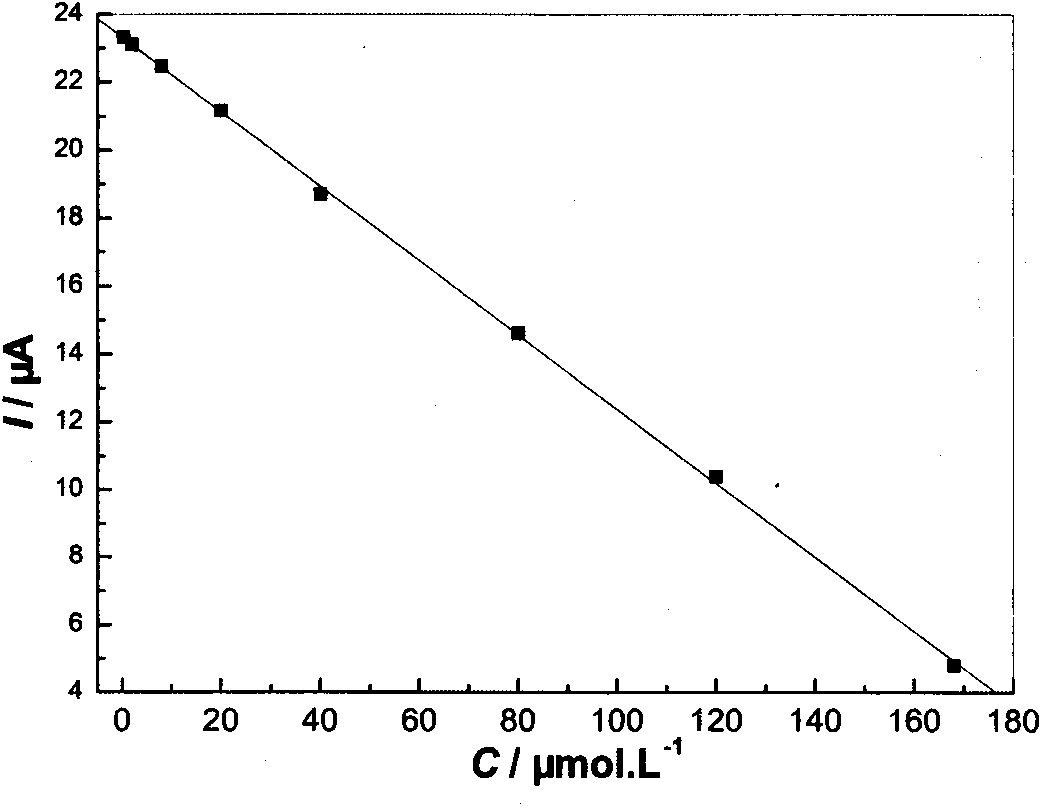
InactiveCN103926286AHigh sensitivityEasy to manufactureMaterial electrochemical variablesFunctional monomerKetone
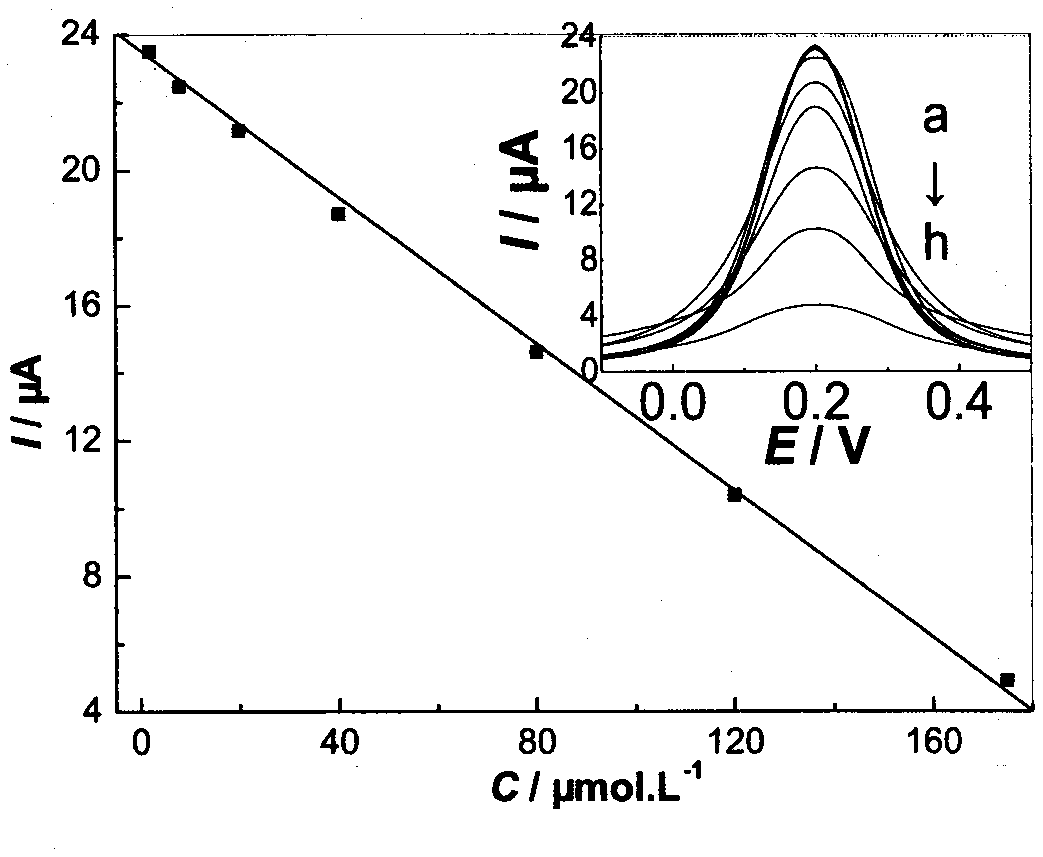
The invention discloses a high-sensitivity nanometer cobalt oxide-doped talampicillin molecular imprinting electrochemical sensor and a preparation method thereof. Talampicillin serves as a template molecule, N-tert-butyl-2alpha-hydroxyl-5alpha-androstene-3-ketone-17beta-formamide serves as a functional monomer, azodiisobutyronitrile serves as an initiator, nanometer cobalt oxide serves as a doping agent, and maleated rosin ethylene glycol acrylate synthesized by taking rosin as a raw material serves as a crosslinking agent, so as to prepare a high-sensitivity nanometer cobalt oxide-doped talampicillin molecular imprinting electrochemical sensor. The analytical method is simple and practical, and the defects that the previous analytical method is complex, expensive in equipment and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
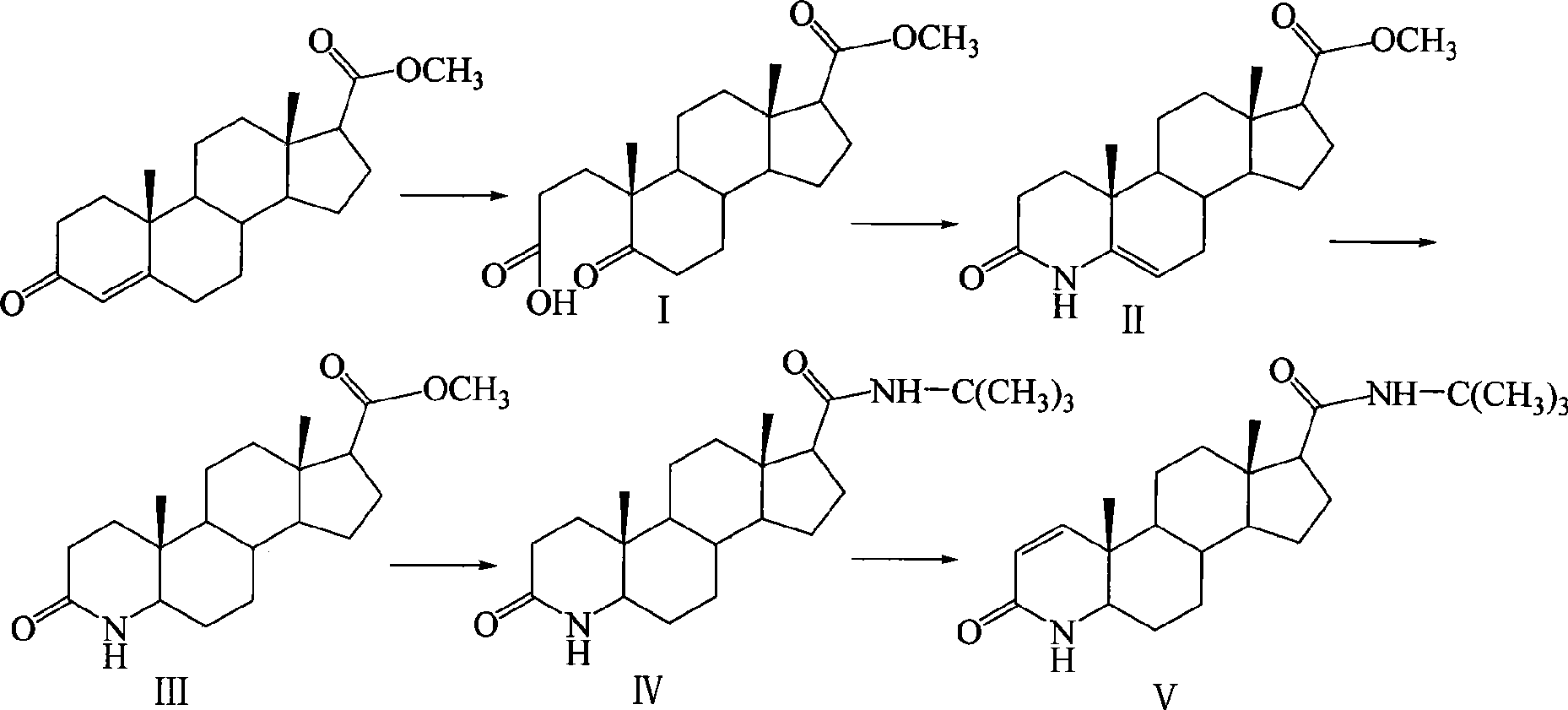
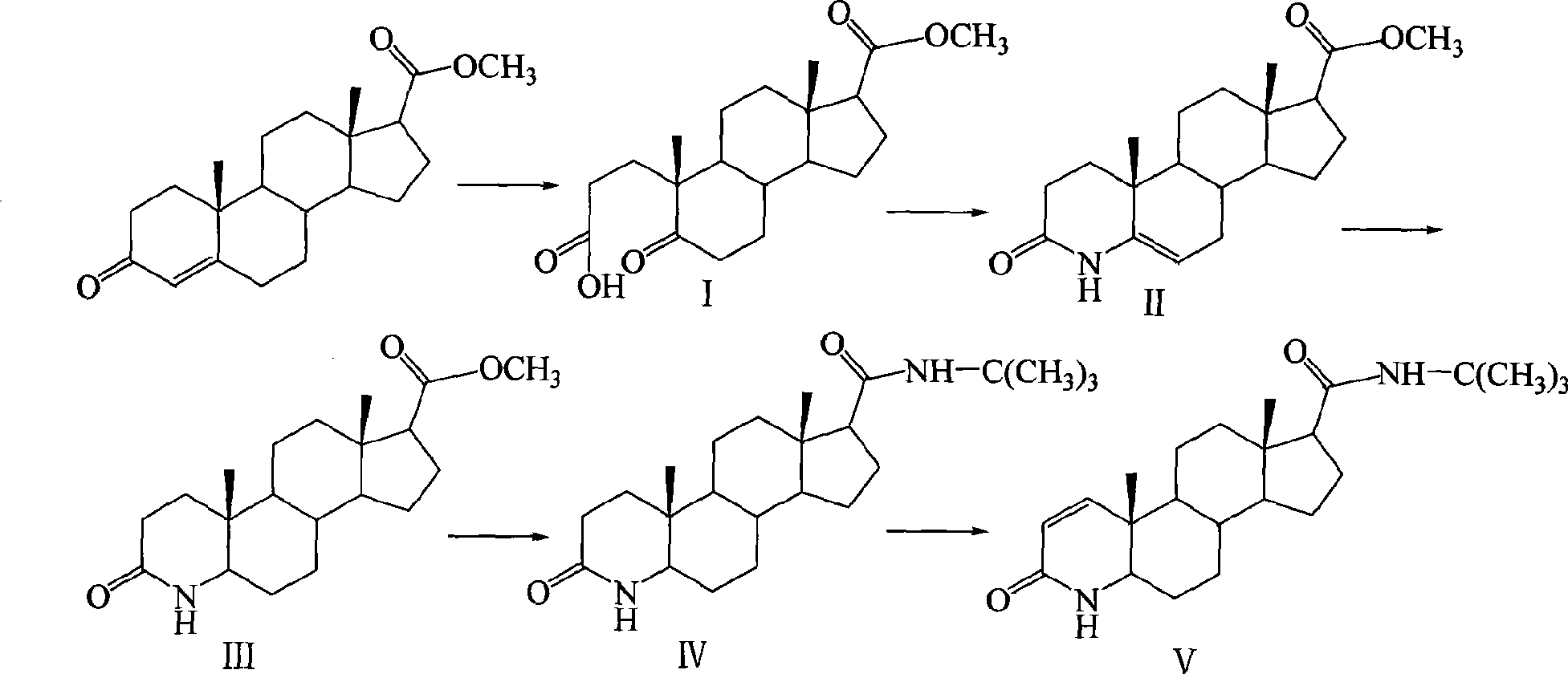
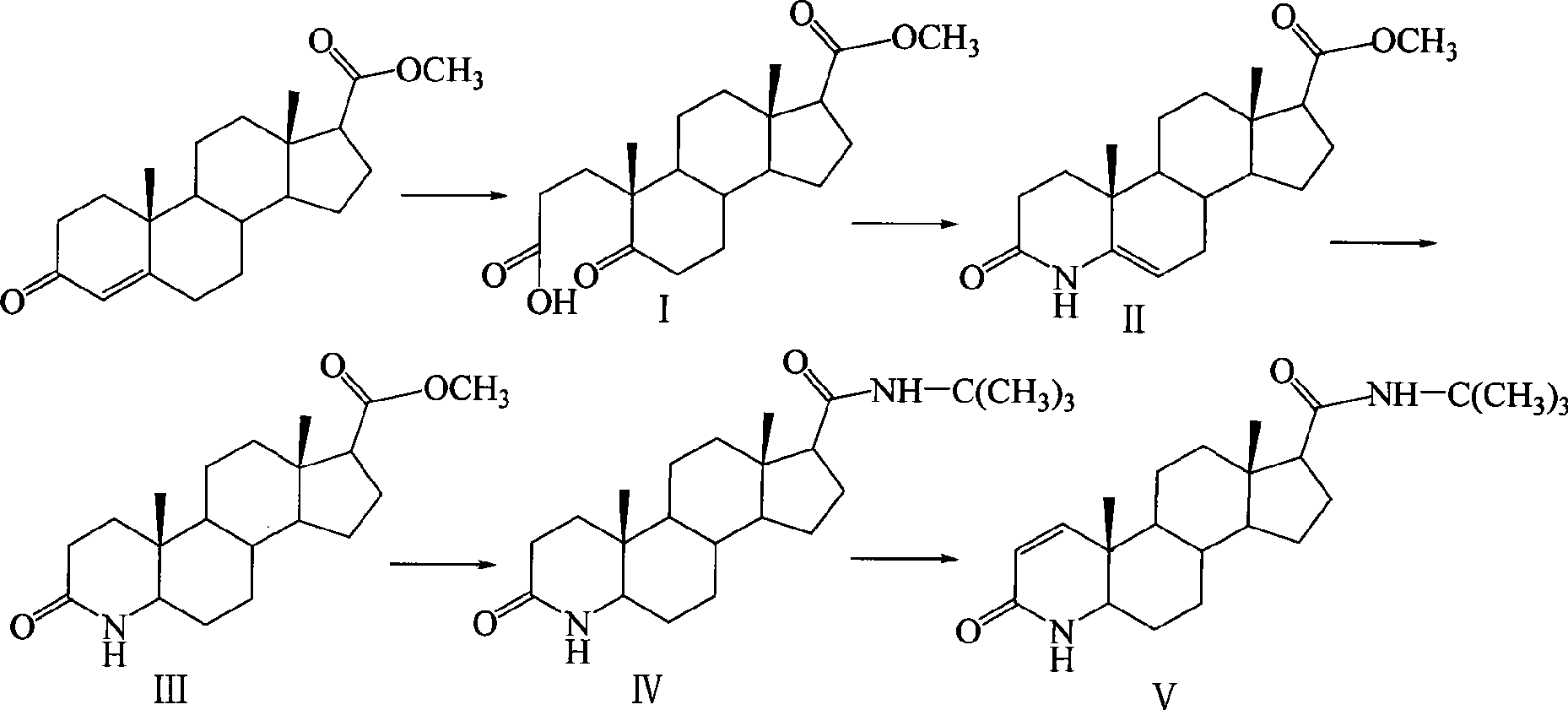
Method for synthesizing finasteride
InactiveCN101863956AStandards compliantThe synthesis process is simpleSteroidsSynthesis methodsAndrostanes
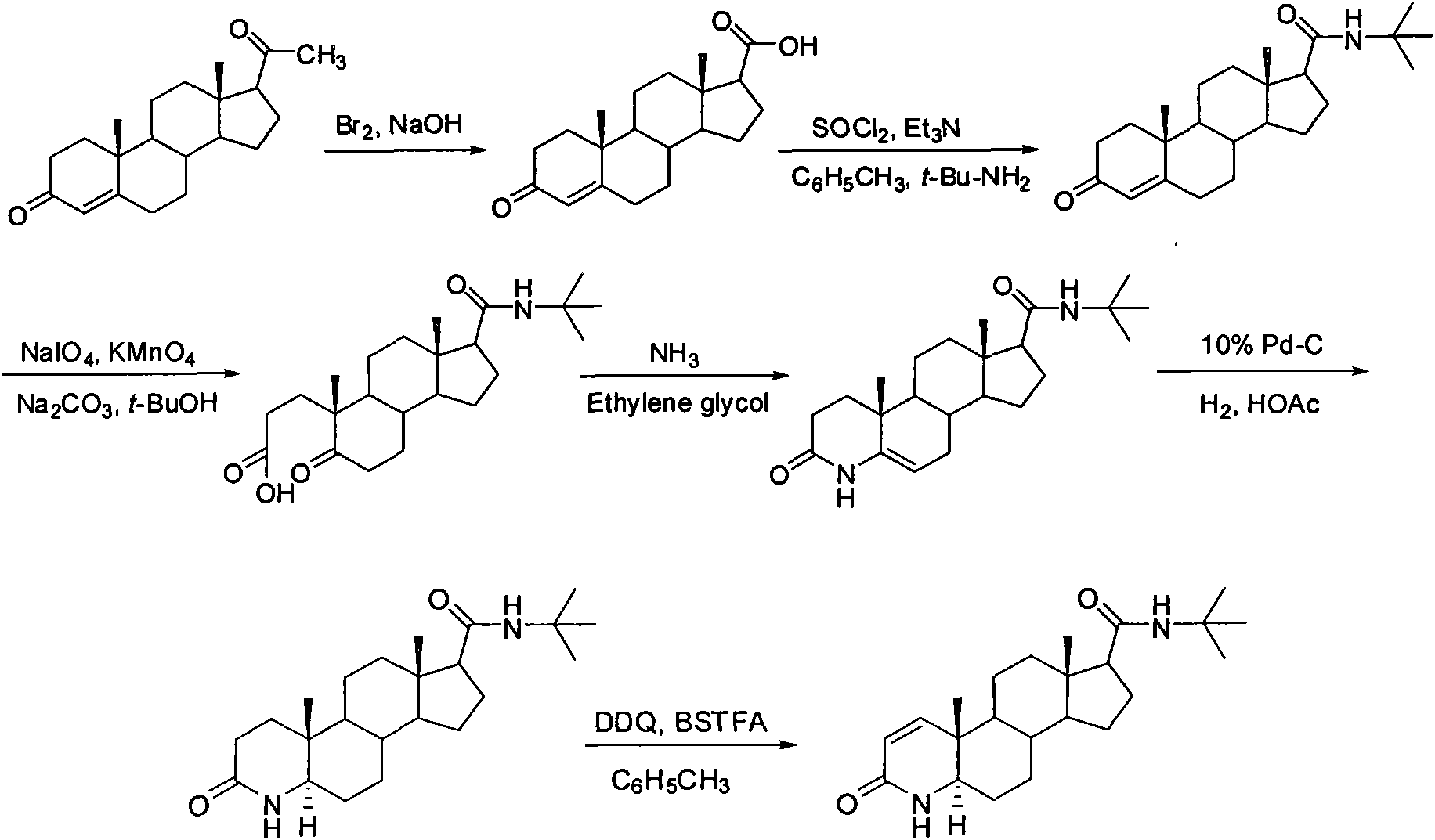
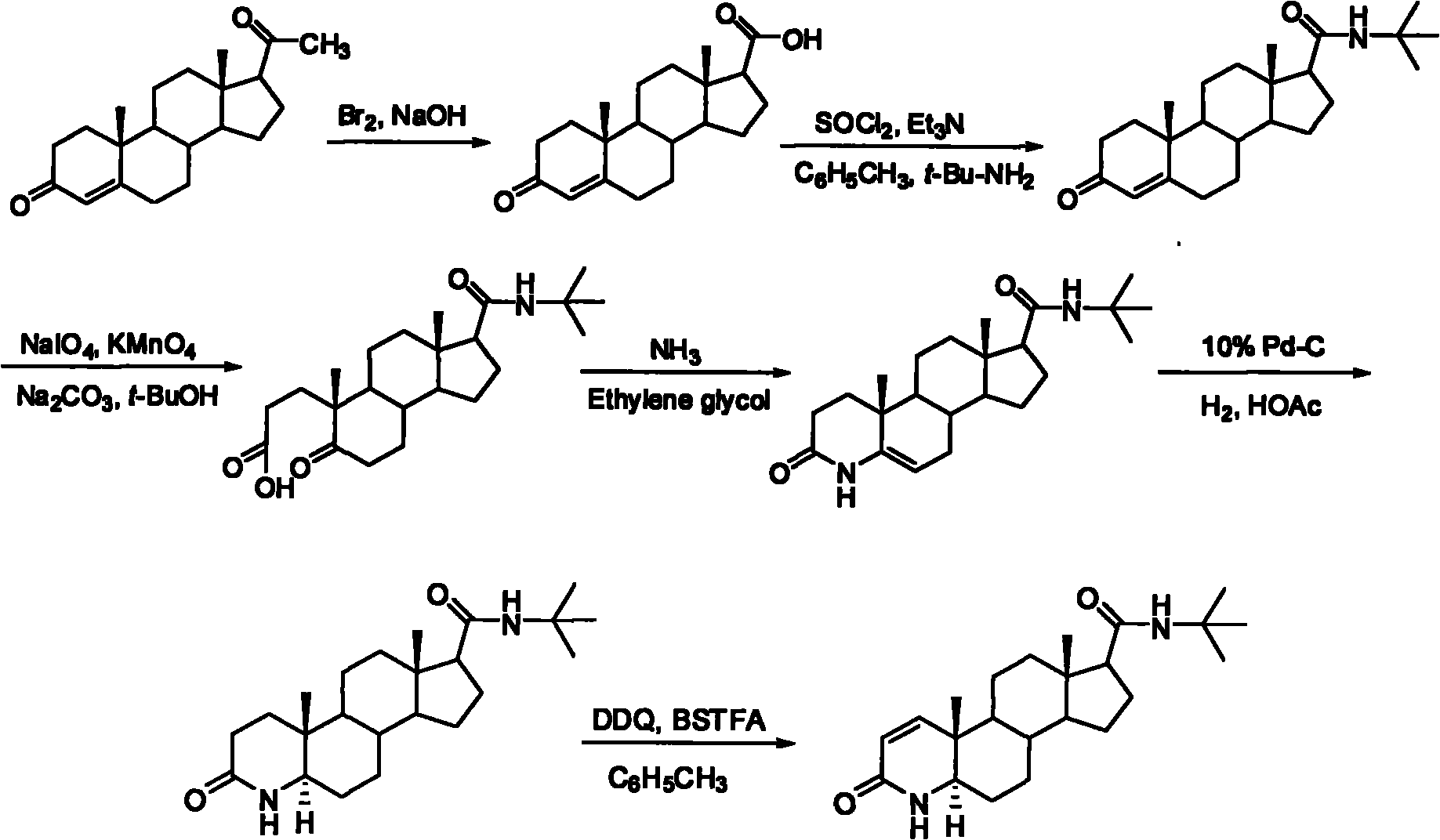
The invention discloses a synthesis method of finasteride, which comprises the following steps that: taking inexpensive luteosterone and the like as the raw materials to finally prepare finasteride through 6 steps of synthetic reaction, i.e. the synthesis of 3- carbonyl-4- androstene-17beta-carboxylic acid, the synthesis of N-tertiary-butyl-3- carbonyl-4- androstene-17beta-formamide, the synthesis of N-tertiary-butyl-5- carbonyl-17beta- carbamoyl-A-lost carbon-3,5-cracking- androstane-3-acid, the synthesis of N-tertiary-butyl-3-carbonyl-4- aza-5- androstene-17beta-formamide, the synthesis of N-tertiary-butyl-3- carbonyl-4- aza-5alpha- androstane-17beta- formamide, and the synthesis of finasteride. The synthesis process has the advantages of inexpensive and easily available raw materials and stable yield, is applicable to industrial production, and the product quality meets the pharmacopeia standards.
Owner:SHANGHAI INST OF TECH
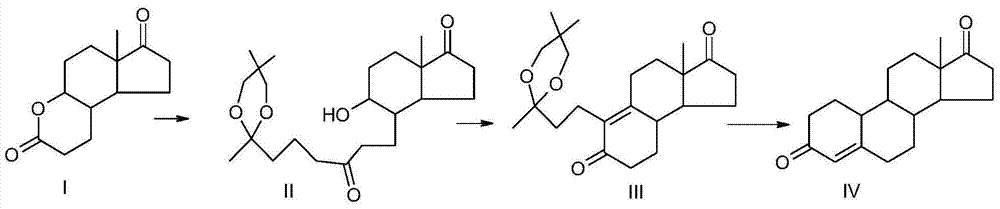
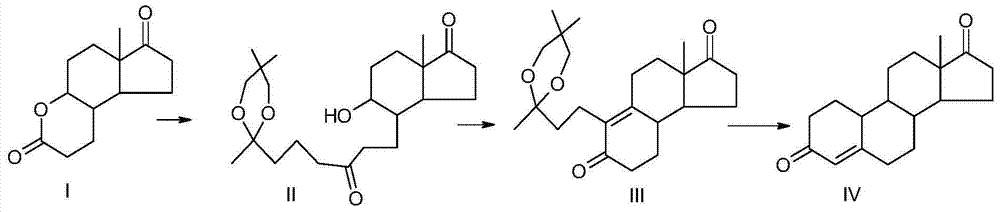
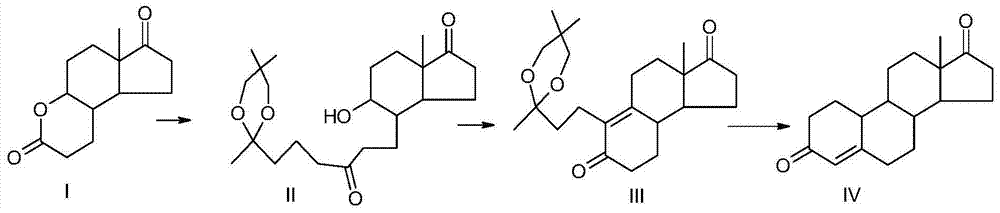
Preparation method of compound 19-desmethyl-4-androstene-3,17 diketone
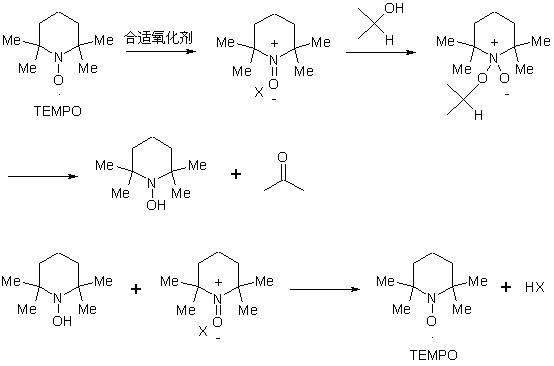
The invention discloses a preparation method of a compound 19-desmethyl-4-androstene-3,17 diketone, comprises the following step of by taking compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as raw materials, and carrying out oxidation reaction on the compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as well as an intermediate compound 19-hydroxy-4-androstene-3,17-diketone with N-halogenated amide oxidant in a mixed solution of an organic solvent and an alkaline buffer solution in the presence of a catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide (TENPO) in the mild condition. In the two steps of oxidation reaction, the catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide and the N-halogenated amide oxidant are both adopted to replace a mixed solution of chromium trioxide, sulfuric acid and water. In the oxidation method, without using the chromium trioxide, the use of carcinogenic substances are avoided being used, limitation by environmental protection is avoided and a great amount of wastes containing heavy metals in preparation are avoided being generated and accumulated, thereby no money and labor is consumed for removing the wastes.
Owner:ZHEJIANG XIANJU PHARMA
Nanometer cobaltous oxide doped micronomicin molecular imprinting electrochemical sensor with high sensitivity and preparation method of sensor
InactiveCN103926289AEasy to manufactureMaterial electrochemical variablesCross-linkFunctional monomer
The invention discloses a nanometer cobaltous oxide doped micronomicin molecular imprinting electrochemical sensor with the high sensitivity and a preparation method of the sensor. The preparation method is characterized in that micronomicin is utilized as a template molecule, 20(s)-O-3beta-hydroxyl-5-androstene-17beta-acyl camptothecin is utilized as a functional monomer, azodiisobutyronitrile is utilized as an initiator, nanometer cobaltous oxide is utilized as a doping agent, and maleated rosin crylic acid gylcol ester which is compounding by taking rosin as a raw material is utilized as a cross-linking agent, so that the nanometer cobaltous oxide doped micronomicin molecular imprinting electrochemical sensor with the high sensitivity is prepared, an analytical method is simple and practical, and the disadvantages that an existing analytical method is complex, equipment is expensive, and the sensitivity is low are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Method of treatment of prostate cancer
InactiveUS7241753B2Heavy metal active ingredientsPhosphorous compound active ingredientsCancer preventionHormone dependence
The present invention relates to the field of cancer, and in particular hormone dependent cancers including, but not limited to prostate, breast, endometrial, ovarian, thyroid, bone, and testis. The present invention also relates to the use of steroid analogues, and in particular analogues of Δ5-androstene-3-β, 17α-diol, and its epimer Δ5-androstene-3-β, 17β-diol for the treatment and prevention of cancer.
Owner:HARBOR DIVERSIFIED
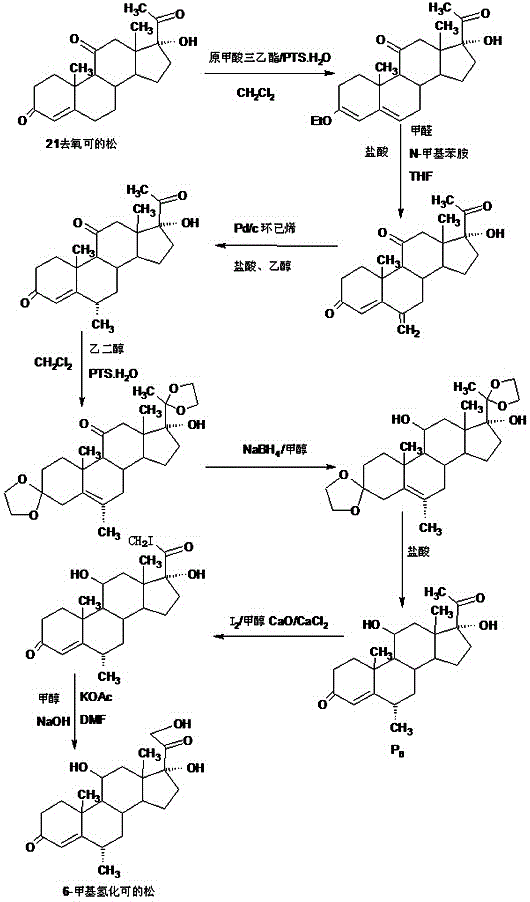
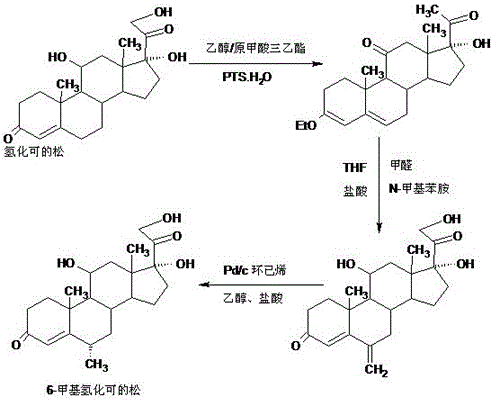
Preparation method of 6a-methyl hydrocortisone
InactiveCN106518945AWide variety of sourcesProcess economy and environmental protectionSteroidsSolventMethyl group
The invention provides a preparation method of 6a-methyl hydrocortisone. The preparation method comprises the steps that hydrocortisone prepared from 4-androstene-3,17-dione (called as 4AD for short) is adopted as a raw material to generate an acid catalytic reaction with triethyl orthoformate in an organic solvent, and etherate 3-ether enol hydrocortisone is obtained; the etherate generates a Manlixi reaction with N-methylaniline and formaldehyde in an organic solvent, and a methylene product 6-methylene hydrocortisone is obtained; the methylene product generates a catalytic hydrogenation reaction in an organic solvent, and 6a-methyl hydrocortisone is obtained. Compared with a production method achieved by taking a mold removal product obtained by processing diosgenin as a raw material, the method for producing 6a-methyl hydrocortisone has the advantages that raw material sources are wide, the processes are economical and environmentally friendly, production operation is easy and convenient, the synthetic route is short, and the product yield is high; by producing 6a-methyl hydrocortisone through the method, the production cost is reduced by 40%-50% compared with a traditional method; the solvents used in production can be recycled and cyclically reused, and implementation of industrialized production is promoted.
Owner:HUNAN KEREY BIOTECH
Nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity and preparation method thereof
InactiveCN103926287AEasy to manufactureMaterial electrochemical variablesCross-linkFunctional monomer
The invention discloses a nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity and a preparation method of the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor. The minocycline hydrochloride is taken as a template molecule, 20(s)-O-3beta-acetoxyl-5-androstene-17beta-acyl camptothecin is taken as a functional monomer, azodiisobutyronitrile is taken as an initiator, nano cobaltous oxide is taken as a dopant, and maleic rosin ethylene glycol acrylate synthesized from rosin as a raw material is taken as a cross-linking agent, so as to prepare the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity. The analysis method is simple and practical, and the defects that the traditional analysis method is complicated, expensive in equipment, and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Synthesis technology of finasteride
InactiveCN101531698AReduce usageReduce manufacturing costOrganic-compounds/hydrides/coordination-complexes catalystsUrinary disorderCarboxylic acidDouble bond
This invention discloses a synthesis technology of finasteride, it takes 3-oxo-4-androstene-17 beta-carboxylic acid methyle as raw material, and synthesizes finasteride through the following five steps, (a). hydrogen peroxide; (b). cyclization; (c). catalyzed hydrogenation reduction; (d). ester-amide condensation into amide; and (e). oxidizing to form double bond. Compare with the existing technology, this technology has short route, high efficiency, simple operation and low cost.
Owner:重庆浩康医药化工集团有限公司 +1
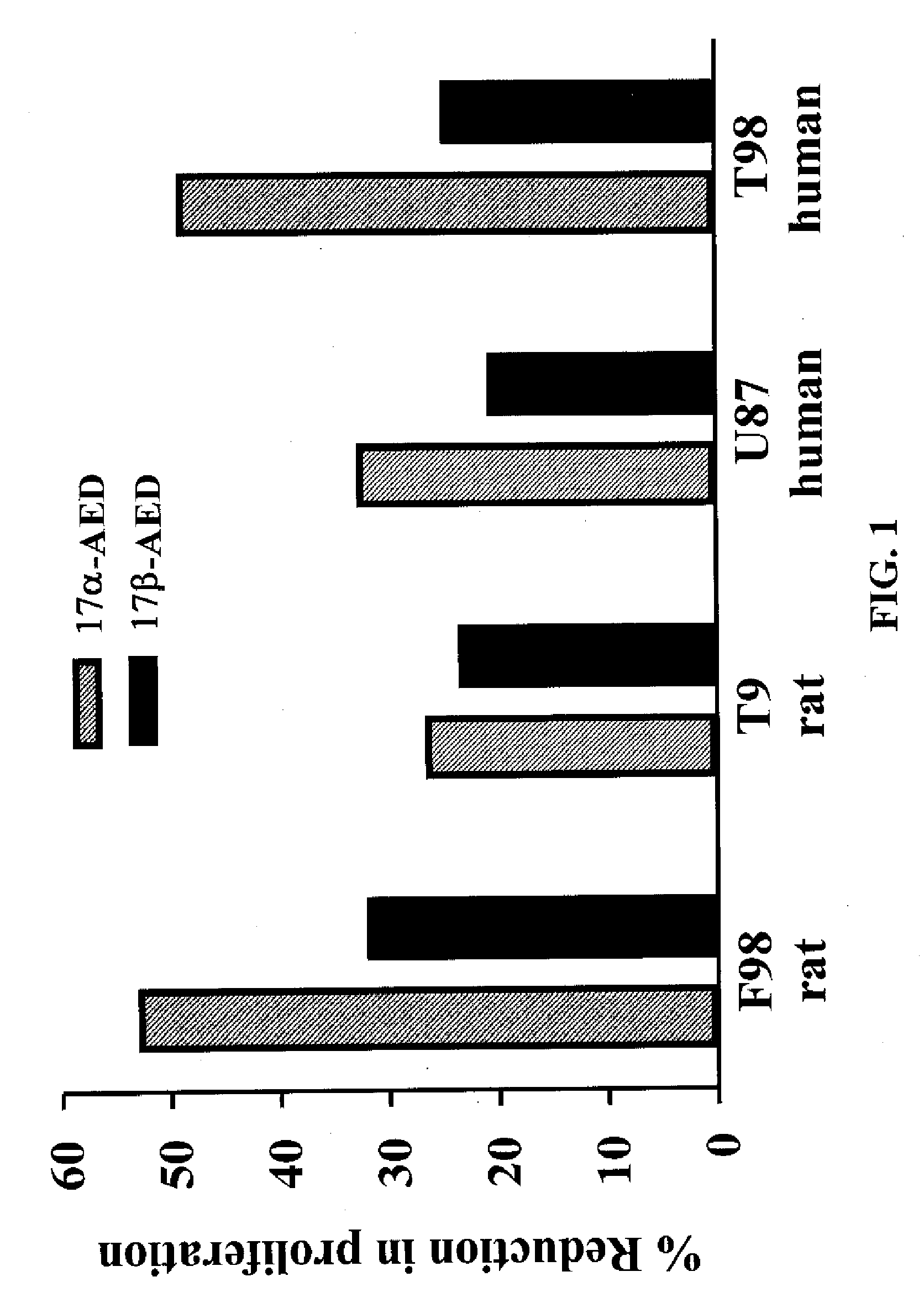
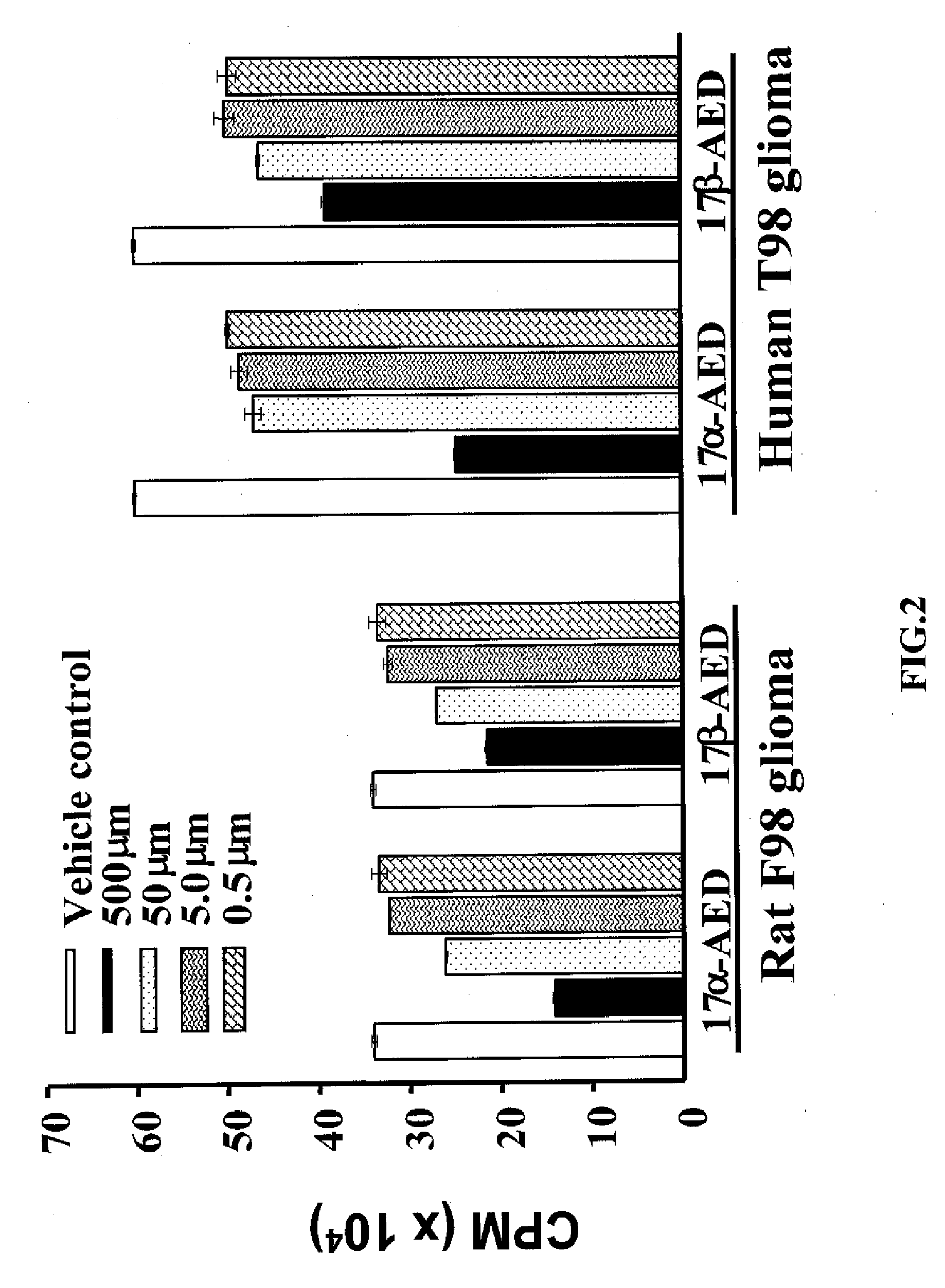
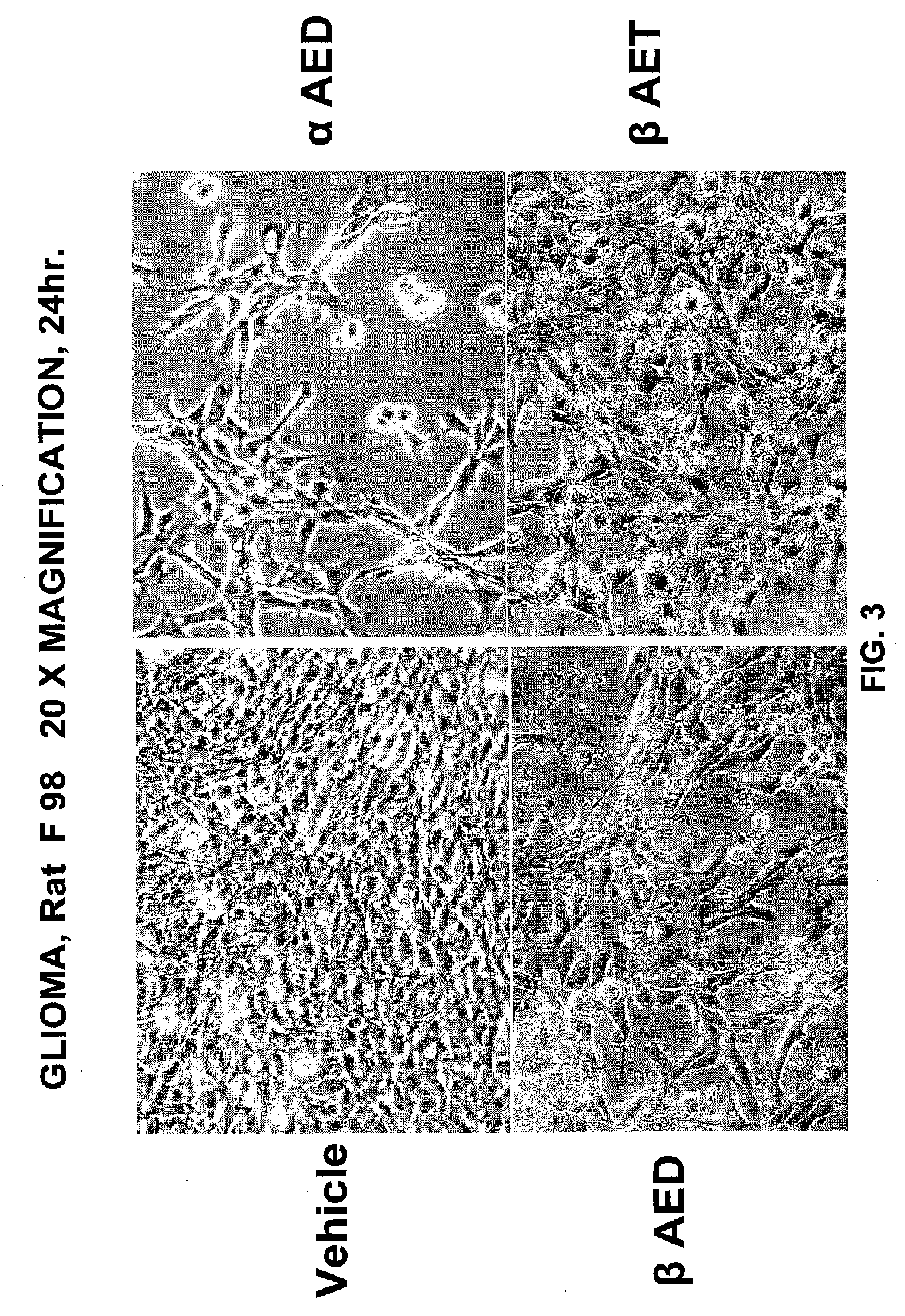
5-Androstenediol As An Inhibitor of Gliomas
The invention relates to the field of pharmaceuticals for tumor-inhibitory effects. The 5-androstene 3β,17α diol (αAED) and 5-androstene 3β,17β diol (βAED), their esters and ethers, are taught herein to achieve tumor-inhibiting effect. The invention also relates to the field of pharmaceuticals for tumor-inhibitory effects and the use of 5-androstene 3β,7β,17β triol (βAET), 5-androstene 3β,7α,17β triol (αAET or 17α-AET) and their esters and ethers, are taught herein to achieve tumor-inhibiting effect.
Owner:LORIA ROGER M
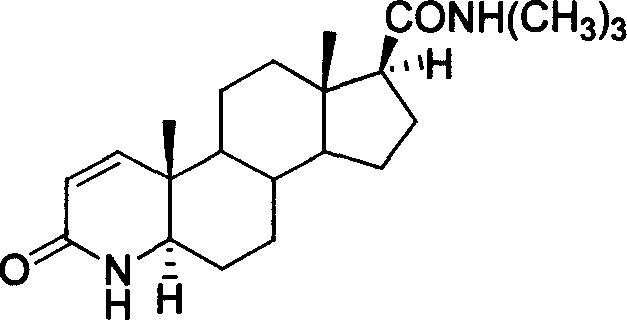
Synthesis method of N-tertiary butyl-3-carbonyl-4-aza-5 alpha-androl-1-end-17 beta-formamide
A process for synthesizing N-tert-butyl-3-carbonyl-4-a2Q-5 alpha-androst-1-ene-17 beta-formamide, that is phenandrostamine, includes such steps as providing 4-androstene-3, 17-dione as raw material, oxidizing for opening loop to obtain 5, 17-dicarbonyl-3, 5-openloop-androst-3-acid, reacting on ammonium acetate for closing loop to obtain 4-azasterol compound, cyanoalcoholizing, dewatering, Ritter reaction on tert-butanol, hydrogenating and dehydrogenating.
Owner:WUHAN UNIV
Method for processing dehydroepiandrosterone mother liquor objects
The invention discloses a method for processing dehydroepiandrosterone mother liquor objects. DHEA, 3alpha-hydroxide radical-5-androstene-17-ketone and 3, 17-diketal objects can be obtained through column chromatography isolation; then the obtained 3alpha-hydroxide radical-5-androstene-17-ketone and the obtained 3, 17-diketal objects synthesize a starting material 4-AD. By means of the method, the purity of the dehydroepiandrosterone (DHEA) obtained through the column chromatography isolation is larger than or equal to 99.5%, and the DHEA total yield is larger than or equal to 75%; materials obtained through the column chromatography isolation can synthesize the 4-AD, reusing can be carried out, and the using rate is high; pollution of hormone waste to the environment is reduced.
Owner:湖北竹溪人福药业有限责任公司
Method for preparing 19- nor-4-androstene-3, 17-diketone
The invention provides a method for preparing 19- nor-4-androstene-3, 17-diketone. The preparation method comprises the following step: with a compound I as a raw material, carrying out a Grignard reaction, an oxidation and ring-closure reaction and a reduction and ring-closure reaction, and the reaction route is shown as follows. The starting raw material of the preparation method is relatively cheap, the product yield is high and the production cost can be saved. The formula is described in the description.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Method for preparing 17alpha-hydroxyprogesteron
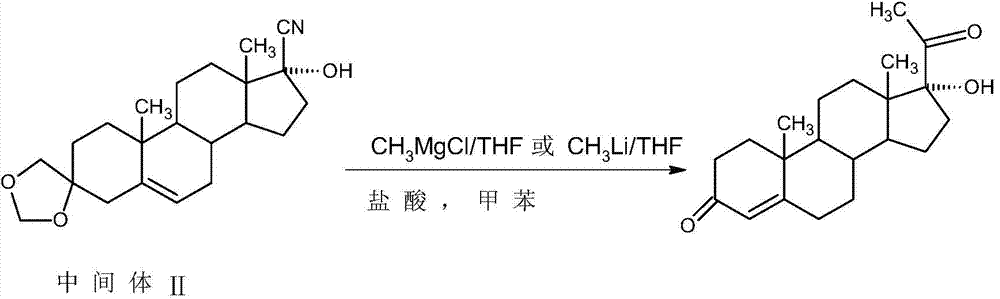
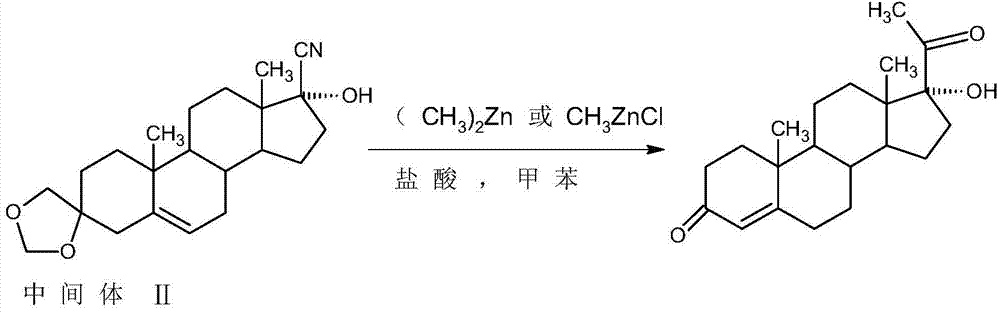
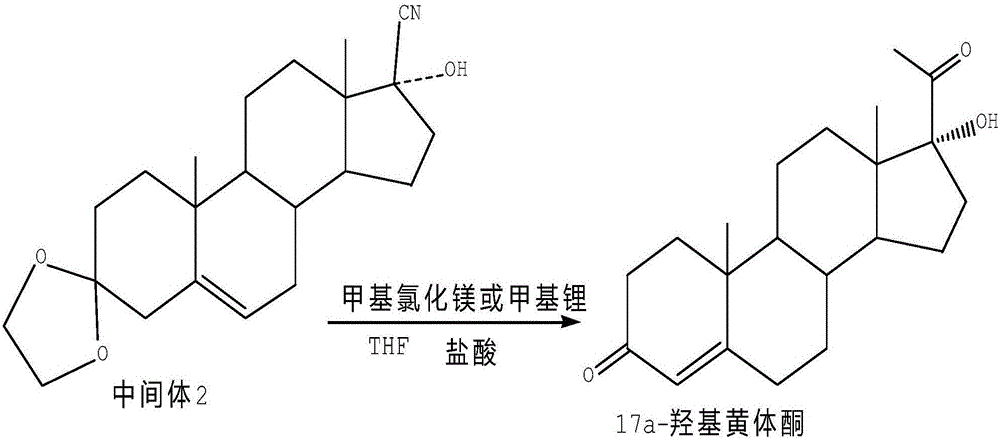
The invention relates to a method for preparing 17alpha-hydroxyprogesterone. The17alpha-hydroxyprogesterone is prepared by taking 17beta- cyano-5-androstene-17-ol-3,3-diethylene ketal (referred as an intermediate II) as a raw material and dimethylzinc or methylzinc chloride as a reagent; the content of the 17alpha-hydroxyprogesterone by HPLC is above 99.5% and the weight yield is 83-87%. The method comprises the following steps of dissolving the intermediate II in an organic solvent, adding lithium chloride as a catalyst, stirring, raising the temperature to 40-80 DEG C, dropwise adding a toluene solution of dimethylzinc or methylzinc chloride of which the concentration is 2M, and continuing to complete the reaction; and then adding an ammonium chloride solution of which the concentration is 25% to destruct an organic zinc reagent, separating the aqueous layer out and extracting, merging the organic layer and the extract and concentrating the solvent to near dryness, and then adding lower alcohol, stirring, raising the temperature to 40-60 DEG C, adding the acid of which the concentration is 2M, hydrolyzing, adjusting the pH value with a weak base after the reaction is completed, evaporating 90% of the solvent out, adding tap water, cooling and crystallizing to obtain a crude 17alpha-hydroxyprogesterone product; and then carrying out reflux decolorizing on the crude product with activated carbon by virtue of alcohol, and refining to obtain the commercial grade 17alpha-hydroxyprogesterone. The 17alpha-hydroxyprogesterone produced by the method disclosed by the invention has the advantages of good purity and high yield and is economic and environment-friendly, and the solvent can be recycled.
Owner:HUNAN KEREY BIOTECH
Synthesis and application of four new conjugates of camptothecin-steroid
The invention discloses the synthesis and application of four new conjugates of camptothecin-steroid. The four new conjugates of camptothecin-steroid are: 1) the name of the first new conjugate is 20 (S)-O-3 beta-hydroxy-5-androstene-17 beta-acyl camptothecin, CPT-1 for short; 2) the name of the second new conjugate is 20 (S)-O-3 beta-acetoxy-5-androstene-17 beta-acyl camptothecin, CPT-2 for short; 3) the name of the third new conjugate is 20 (S)-O-androst-4-alkene-17 beta-acyl camptothecin, CPT-3 for short; 4) the name of the forth new conjugate is 20 (S)-O-4-aza-5 alpha-androst-3-ketone-17 beta-acyl camptothecin, CPT-4 for short; By employing EDCI-DMAP coupling method, all the four new conjugates of camptothecin-steroid are synthesized based on raw materials of camptothecin and four steroid acids. The compounds of the invention have good antineoplastic activity, and great prospect in pharmaceutical development.
Owner:GUANGXI UNIV FOR NATITIES
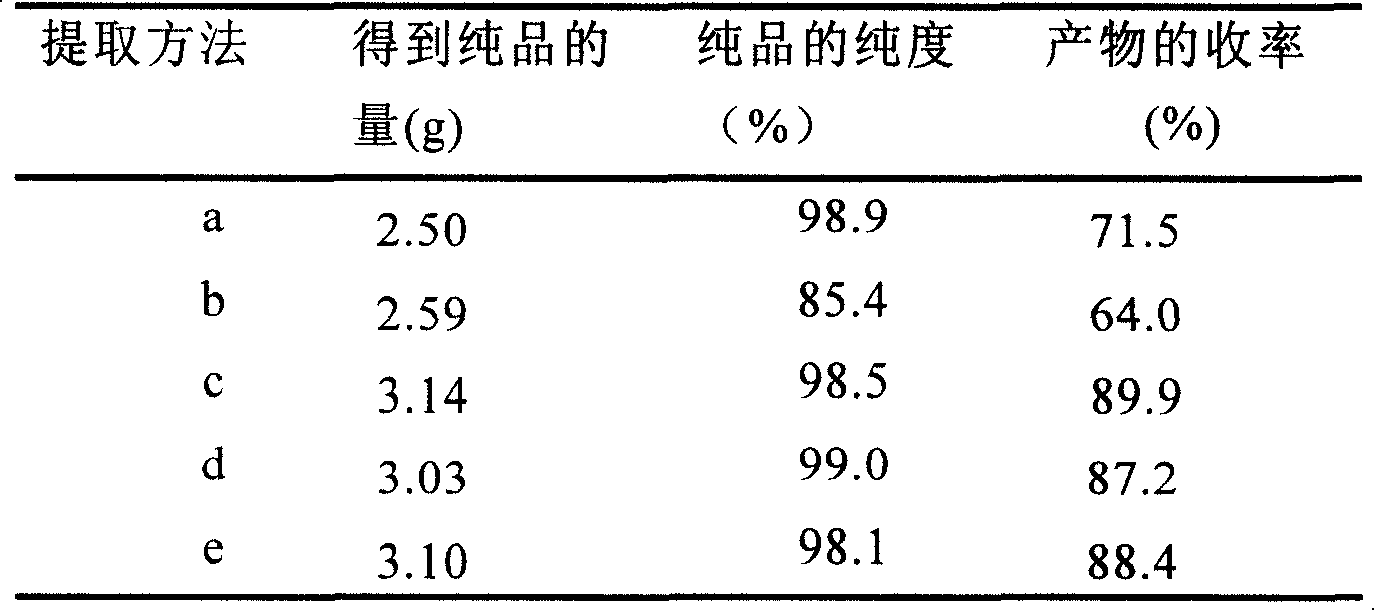
Extraction and purification method of 7 Alpha, 15Alpha-dihydroxy androstene alcohol ketone
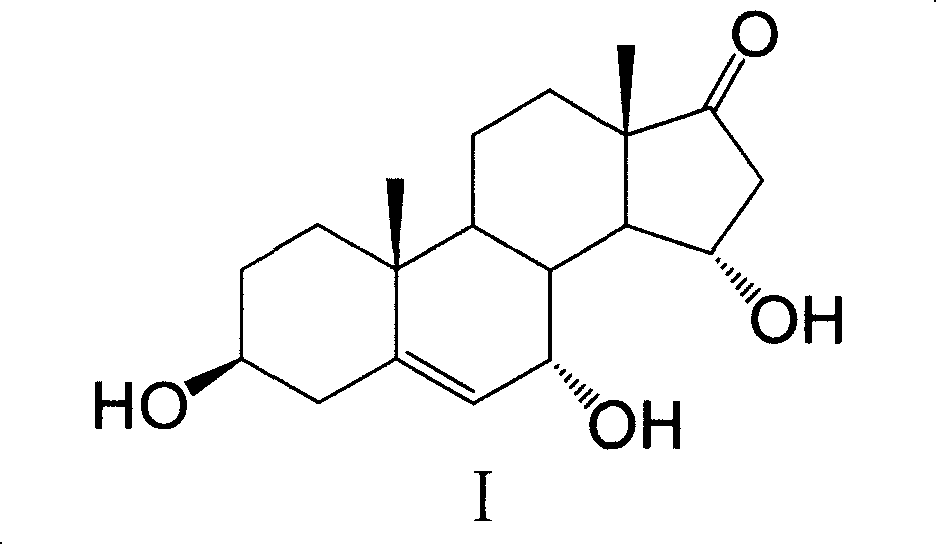
InactiveCN101182565AImprove extraction efficiencyReduce generation costMicroorganism based processesSteroidsColletotrichum liniPurification methods
The invention provides a method for extracting and purifying 7α, 15α-dihydroxyandrostenolone. The 7α, 15α-dihydroxyandrostenolone is prepared from dehydroepiandrosterone through the biological process of Colletotrichum lini AS 3.4486. obtained by transformation, after the transformation is completed, the fermentation liquid is centrifuged, and the supernatant is extracted with a non-polar organic solvent. The steps of organic solvent extraction. Using this method for extraction and purification, the yield of the product can reach nearly 90%, and the total yield of I prepared by conversion of dehydroepiandrosterone can reach 75%, thereby effectively improving the total yield of I by conversion of dehydroepiandrosterone. Yield, reducing the production cost of I.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Method for synthesizing drospirenone
The invention relates to a method for synthesizing drospirenone and belongs to the field of pharmaceutical chemicals, which comprises: reacting a 3beta,5beta-dyhydroxy-6beta,7beta,15beta,16beta-imethylene-17alpha-(3'-hydroxypropyl)-androstene-17ol compound serving as a raw material in dichloromethane in the presence of dichlorodimethylhydantoin, potassium bicarbonate and crown ether, which serve as catalysts, to obtain an 3-oxo-5beta-hydroxy-6beta, 7beta,15beta,16beta-dimethylene-17alpha(spiro)butyrolactone intermediate; removing excessive oxidant by using a small amount of sodium sulfite, filtering the solution, adding a certain amount of phosphorus pentoxide into solution of dichloromethane for dehydration, adding water for washing the reaction product for one time at the end ( detected by thin-layer chromatography) of the reaction and washing the reaction product for one time with saturated solution of sodium bicarbonate; drying the reaction product with anhydrous sodium sulfate, filtering the reaction product, distilling and recovering solvent and crystallizing the solid with water solution (in a volume ratio of 3:1) of methanol; and finally, recrystallizing the obtained solid with isopropylacetate to obtain a qualified drospirenone product. The synthesis yield of the method is about 67 percent. The reaction is mild and easy to operate and consumes a small amount of organic solvent.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
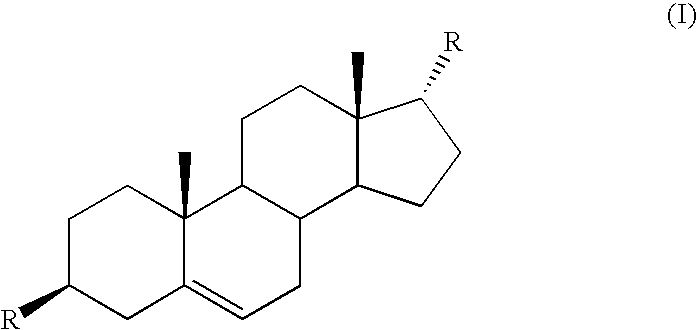
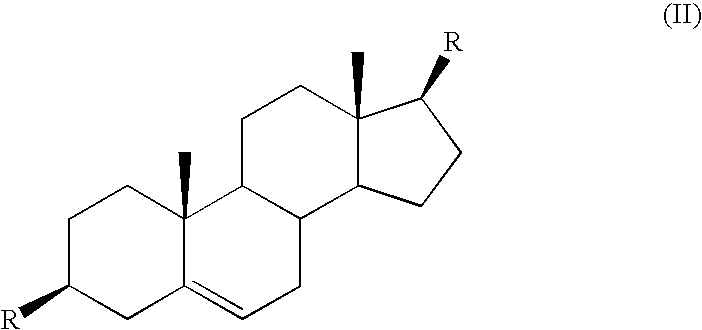
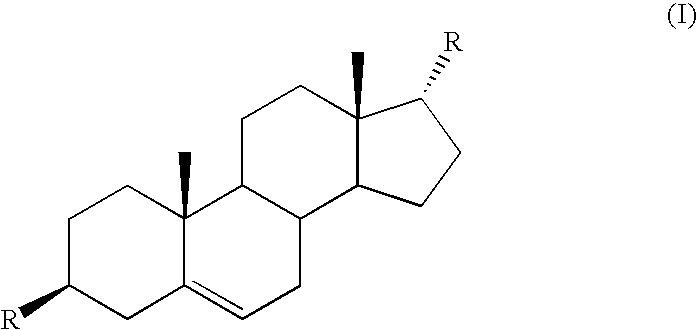
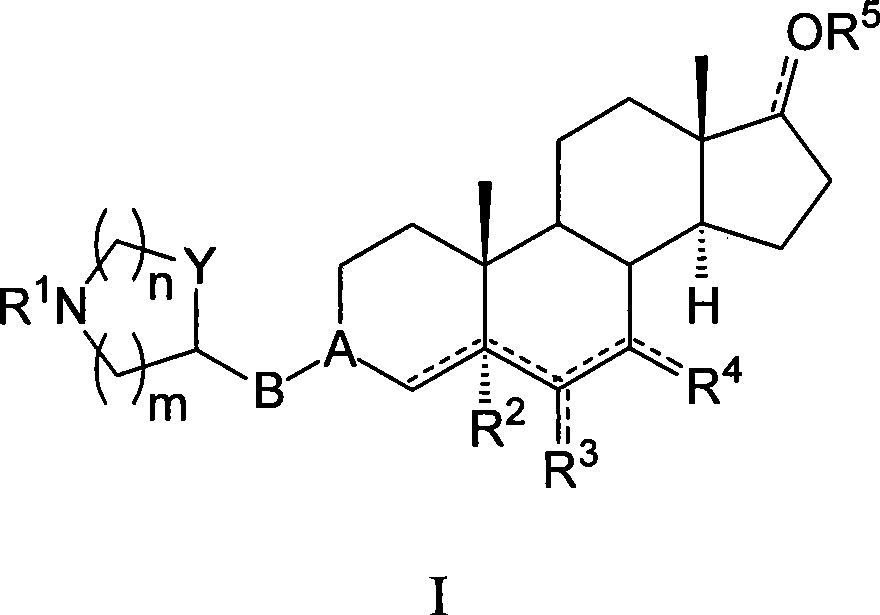
Azaheterocyclyl derivatives of androstanes and androstenes as medicaments for cardiovascular disorders
InactiveCN101466726AImprove efficacyGood cure rateOrganic active ingredientsSteroidsATPaseEndogenous ouabain
Compounds of formula (I) wherein: the groups are as defined in the description, are useful for the preparation of medicaments for the treatment of cardiovascular disorders, in particular heart failure and hypertension. The compounds are inhibitors of the enzymatic activity of the Na+,K+-ATPase. They are useful for the preparation of a medicament for the treatment of a disease caused by the hypertensive effects of endogenous ouabain, such as renal failure progression in autosomal dominant polycystic renal disease (ADPKD), preeclamptic hypertension and proteinuria and renal failure progression in patients with adducin polymorphisms.
Owner:CVIE THERAPEUTICS LTD
Butane diacid(5-androstene-17-ketone- 3beta -hydroxyl group ) diester solid dispersoid and method for making same and applications thereof
InactiveCN101081213AGood water solubilityEasy to preparePowder deliveryOrganic active ingredientsSolubilityButanedioic acid
The present invention discloses solid dispersoid of bis(5-androstene-17-one-3beta-hydroxy) succinate and its preparation process and application. The solid dispersoid consists of bis(5-androstene-17-one-3beta-hydroxy) succinate and polyvinyl pyrrolidone intelligent weight ratio of 1-20. It is prepared through heating the mixture of succinic acid and bis(5-androstene-17-one-3beta-hydroxy) succinate to form melt and prepare powdered bis(5-androstene-17-one-3beta-hydroxy) succinate, coating bis(5-androstene-17-one-3beta-hydroxy) succinate with polyvinyl pyrrolidone in water solution to obtain the solid dispersoid. The solid dispersoid of bis(5-androstene-17-one-3beta-hydroxy) succinate has over 80 times raised water solubility and obvious curative effect on drug liver injury.
Owner:SUN YAT SEN UNIV
Method for synthesizing 17alpha-hydroxyprogesterone
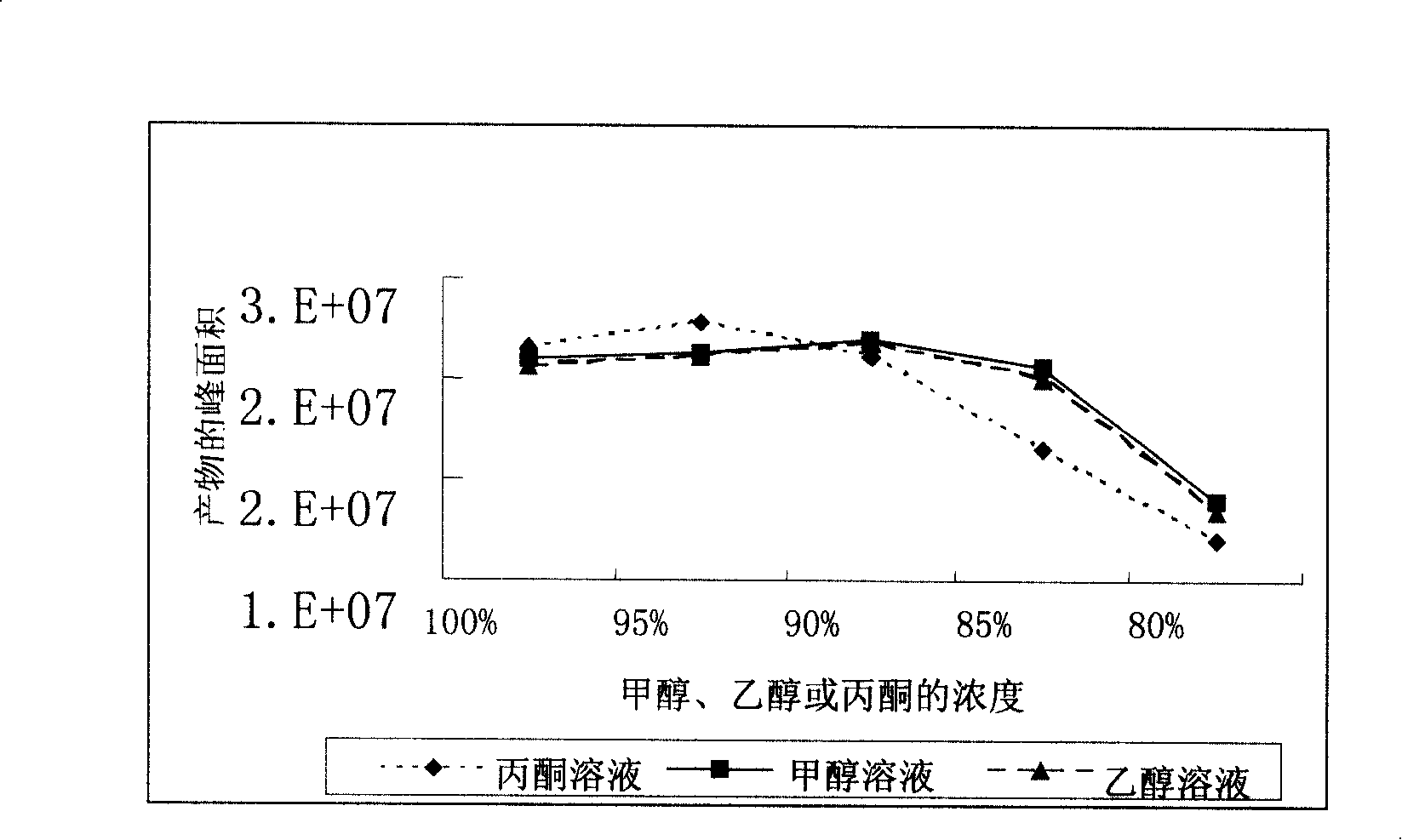
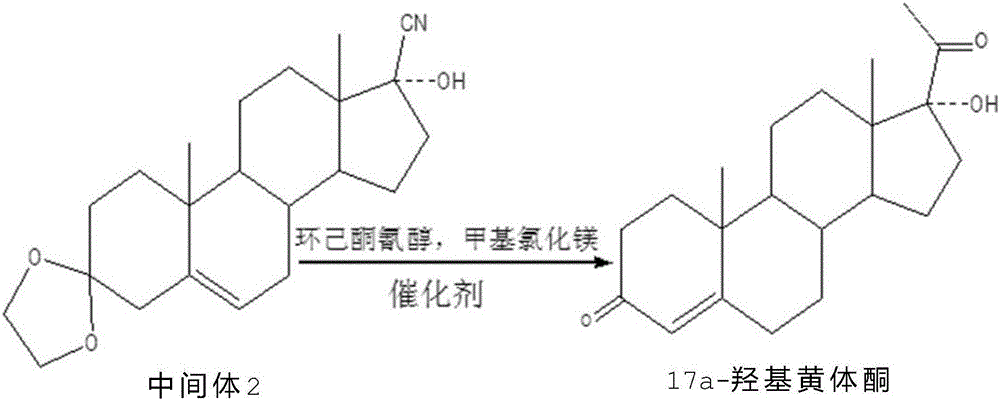
The invention provides a method for synthesizing 17alpha-hydroxyprogesterone.17beta-cyano-5-androstene-17-alcohol-3,3-ethylene dihydroxy ketal (an intermediate II for short) is adopted as a raw material, and cheap cyclohexanone cyanohydrin is adopted as an inhibitor of a main impurity methyltestosterone. Due to the fact that a cyclohexanone cyanohydrin structure is similar to a D-loop structure in the molecular structural formula of the intermediate II and can replace a D loop to react with OH<1> in a Grignard reaction system, generated methyltestosterone is greatly reduced. Under the catalytic action of anhydrous lithium chloride or CuCl, the reaction temperature is reduced to -15 DEG C to -10 DEG C, and other generated impurities can be reduced at the low temperature. Therefore, the product quality is improved by controlling Grignard reaction conditions, the content of crude HPLC can reach 98% or above, and the total weight yield ranges from 84% to 88% and is improved by 10% to 14% compared with a traditional process.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +2
Preparation method of 5alpha-androstane-3,17-dione
The invention relates to a preparation method of 5alpha-androstane-3,17-dione. The method specifically comprises the following steps: 1, carrying out an etherification reaction on 4-androstenedione, triethyl orthoformate and anhydrous ethanol in the presence of an etherification catalyst, and performing post-treatment after the etherification reaction is completed in order to prepare a compoundwet 3-ethoxy-3,5-androstadien-17-one; 2, adding a the wet 3-ethoxy-3,5-androstadien-17-one into a methanol-dichloromethane mixed solvent, performing uniform stirring, adjusting the pH value of the obtained solution, carrying out a catalytic hydrogenation reaction under the action of a palladium-carbon catalyst, and filtering out the catalyst after the reaction is finished in order to obtain a 3-ethoxy-3-androstene-17-one solution; and 3, carrying out a hydrolysis reaction on the 3-ethoxy-3-androstene-17-tone solution and an acid, removing the solvent after the hydrolysis reaction is finished,and carrying out filtering, water washing and drying to obtain the 5alpha-androstane-3,17-dione. The method has the advantages of short process route, easily controlled production process, environmental friendliness, low production cost, and suitableness for industrial large-scale production.
Owner:HUAZHONG PHARMA
Processes for preparing C-7 substituted 5-androstenes
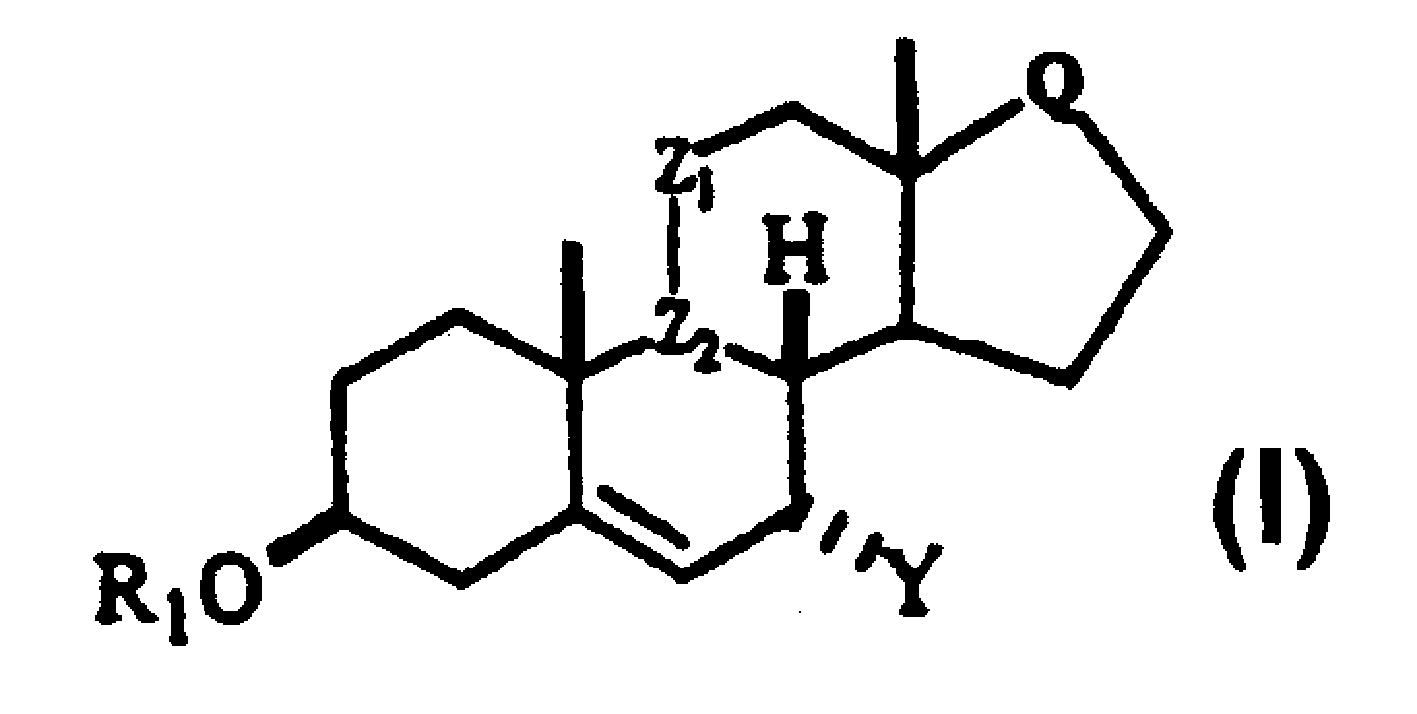
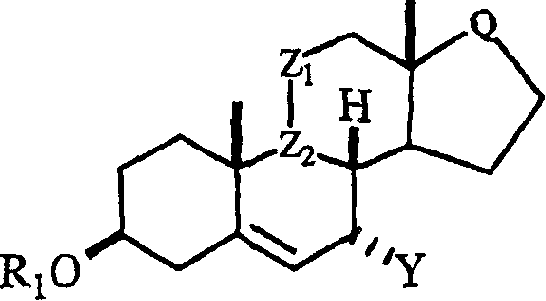
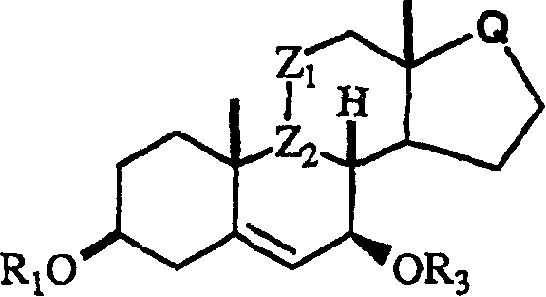
This invention relates to processes for the preparation of novel , substituted steroid compounds of Formula 1, wherein R1 is H or -COR2; R2 is C1-C6 alkyl or Ct-C6 alkoxy; Z1 is CH2, or wherein OR3 is in the alpha configuration; R3 is H or -COR2; Z2 is -CH-; or Z1 and Z2 may be taken together to form a carbon-carbon double bond; Q is or Y is -CN, -CH2-CH=CH2, C1-6- Alkyl C1-6 alkyl CHR4C (O)Ar, or CHR4 C (O)XC1-6 alkyl; CHR4(O)XAr, or CHR4 HC(O)XC1-6 alkyl; where R4 = O C1-6 alkyl or aryl X=O or S.
Owner:PHARMACIA & UPJOHN CO
Method for preparing 19-nor-4-androstene-3,17-dione
InactiveCN106589033AEasy to operateImprove operational safetyOrganic compound preparationSteroidsHydrogenation reactionHydrolysis
Owner:ZHEJIANG XIANJU PHARMA
Bacterial strain for microbial transformation phytosterin as yield per unit androstane diene diketone
InactiveCN101402928AAchieve reuseImprove stabilityBacteriaMutant preparationBiotechnologyGrowth phase
The invention discloses phytosterol of microbial transformation, which is a strain which only produces androstene dione, pertaining to the fermentation technology field in biological engineering. The screened strains are classified and named as starch degrading bacillus (Bacillus a myloiquefaciens.) ST 06-95 and preserved in the China Center for Type Culture Collection with a preservation number of CCTCC No. M208135. Samples are collected from the nature separated for primary screening and put in slant preservation; wild strains ST 06 are taken as starting strains and screened by ultraviolet mutagenesis to obtain strains ST06-95 which mainly produce androstenone; and the growth of strains enters a logarithmic growth phase at the temperature of 30 DEG C, the pH value of 7.0 and a rotation speed of 220r / min and after culture lasting for 60h. Thallus in the growth phase are inoculated into a fermentation culture medium according to inoculation amount of 12 percent (V / V) and fermented for 6 days to 7 days at the temperature of 30 DEG C, the pH value of 7.0 and a rotation speed of 220r / min. When quantitative detection is carried out on the fermented products, the product content of ADD exceeds 1500mg / l, and ADD accounts for 98 percent of the total amount of AD and ADD. The microbial transformation provides a basis for the industrialization of the microbial transformation of steroidal medicaments.
Owner:JIANGNAN UNIV
Method for the preparation of highly pure 1-androstene derivatives
A method for preparing a 1-androstene derivative which comprises reacting a 2-iodo-androstane derivative with an oxidizing agent while maintaining the pH of the reaction mixture at a specific range gives the 1-androstene derivative with high purity and yield.
Owner:HANMI PHARMA
Method for preparing 17beta-carboxyl-4-androstene-3-ketone
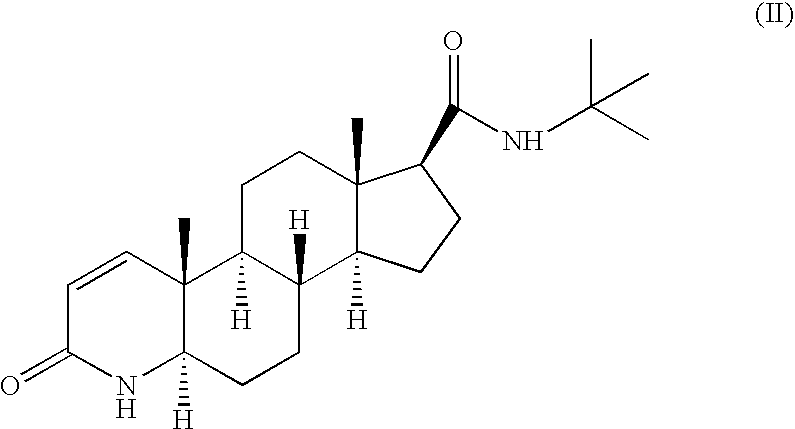
The invention discloses a method for preparing 17beta-carboxyl-4-androstene-3-ketone. The method comprises a step (a) of enabling 3-ethoxy androstane-3,5-diene-17-ketone to react with tosylmethyl isocyanide in an organic solvent under the conditions of strong base, and obtaining a first intermediate product with a formula (II) structure; a step (b) of hydrolyzing the first intermediate product under the acidic conditions, and obtaining 17beta-carboxyl-4-androstene-3-ketone. The preparation method has the advantages of being low in cost and high in yield; and the formula (II) is as follows.
Owner:赵云现
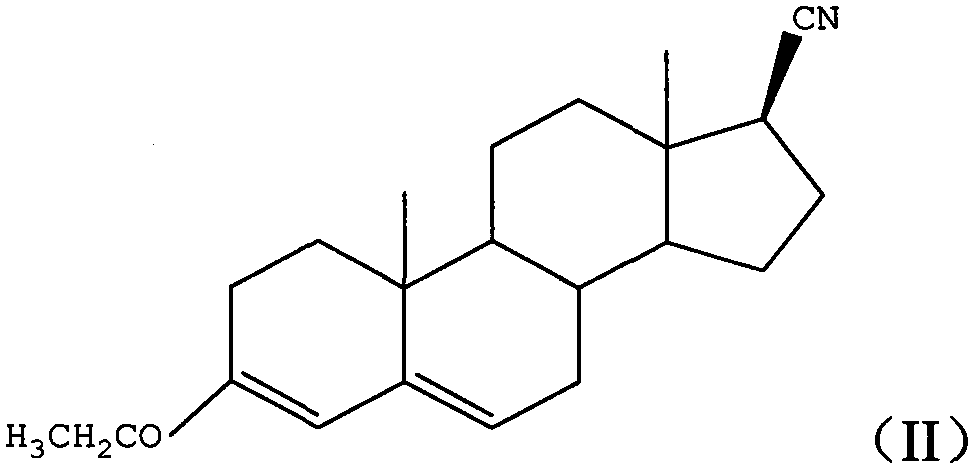
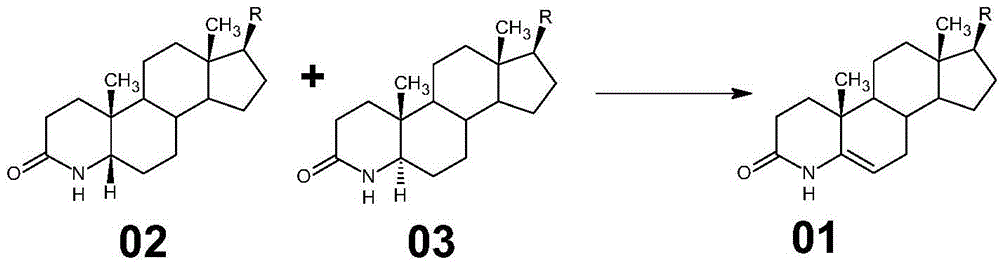
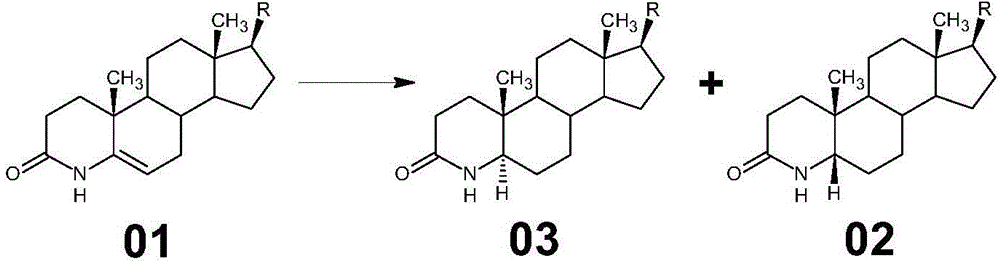
Method for preparing 3-carbonyl-4-aza-5-androstene-17 beta carboxylic acid derivative from mother solution reclaimed materials of hydrogenation reaction
The invention provides a method for preparing 3-carbonyl-4-aza-5-androstene-17 beta carboxylic acid derivative 01 from mother solution reclaimed materials of a hydrogenation reaction. The method comprises the following steps: (1) dissolving the mother solution reclaimed materials, which are obtained by preparing 3-carbonyl-4-aza-5 alpha-androstane-17 beta carboxylic acid derivative 03 by performing hydrogenation reaction on 3-carbonyl-4-aza-5-androstene-17 beta carboxylic acid derivative 01, into glacial acetic acid, and adding a palladium-carbon catalyst, introducing oxygen, pressurizing and heating to perform dehydrogenation reaction; (2) cooling the reaction system, filtering to obtain a filter cake, washing the filter cake by using glacial acetic acid, and performing suction filtration until the filter cake is dried, thereby obtaining a waste palladium-carbon filter cake; and (3) performing vacuum concentration on a filtrate until the filtrate is almost dried, adding methanol into the system, performing freezing crystallization, performing swinging filtration to obtain a filter cake, washing the filter cake by using methanol, performing swinging filtration until the filter cake is dried, and drying to obtain the 3-carbonyl-4-aza-5-androstene-17 beta carboxylic acid derivative 01. By adopting the method provided by the invention, wastes are recycled to synthesize an important medical intermediate, the cost is reduced, and pollution is reduced. Original auxiliary materials are simple, easily available and recyclable, and the process is simple and is suitable for industrial implementation.
Owner:HUNAN KEREY BIOTECH
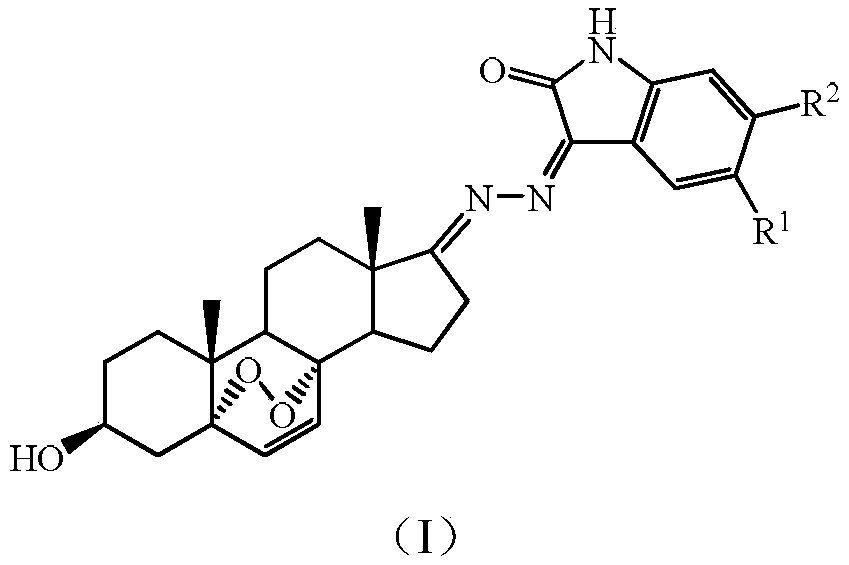
3beta-hydroxyl-5alpha,8alpha-peroxy-androstane-6-alkene-17-(isatin substituted) hydrazone derivative, as well as preparation and application thereof
InactiveCN108707180AMild reaction conditionsSimple stepsSteroidsAntineoplastic agentsHydrazine compoundKetone
The invention discloses a 3beta-hydroxyl-5alpha,8alpha-peroxy-androstane-6-alkene-17-(isatin substituted) hydrazone derivative, as well as preparation and application thereof, belonging to the technical field of medicinal chemistry. The structural formula of the 3beta-hydroxyl-5alpha,8alpha-peroxy-androstane-6-alkene-17-(isatin substituted) hydrazone derivative is as shown in the description, wherein R1 represents -H, -F, -Cl, -Br, -I, -NO2, -OCH3, -OCF3 or -CH3; R2 represents -H, -Cl or -Br. The preparation comprises the following steps: preparing 3beta-acetoxyl-5-androstene-17-ketone by using dehydroepiandrosterone as a raw material; performing bromination reaction and debromination reaction to obtain 3beta-acetoxyl-5,7-diene androstane-17-ketone intermediate, and hydrolyzing to reduce hydroxyl; constructing a 5alpha, 8alpha-peroxide bridge by illuminating; reacting with hydrazine hydrate to prepare 3beta-hydroxyl-5alpha, 8alpha-peroxy-androstane-6-alkene-17-hydrazine intermediate; and finally performing a condensation reaction to generate the 3beta-hydroxyl-5alpha,8alpha-peroxy-androstane-6-alkene-17-(isatin substituted) hydrazone derivative. The compound has an effect of preventing and treating cancers such as liver cancer, breast cancer and the like.
Owner:QIQIHAR MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com