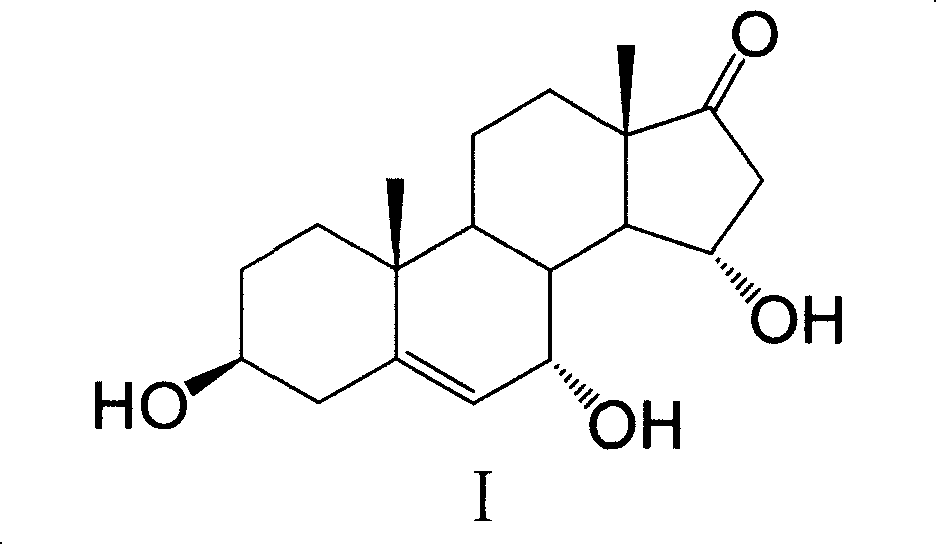
Extraction and purification method of 7 Alpha, 15Alpha-dihydroxy androstene alcohol ketone
A technology of hydroxyandrostenolone and purification method, applied in microorganism-based methods, biochemical equipment and methods, organic chemistry, etc., can solve problems such as inability to extract products, improve extraction efficiency, reduce production costs, and improve overall production. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
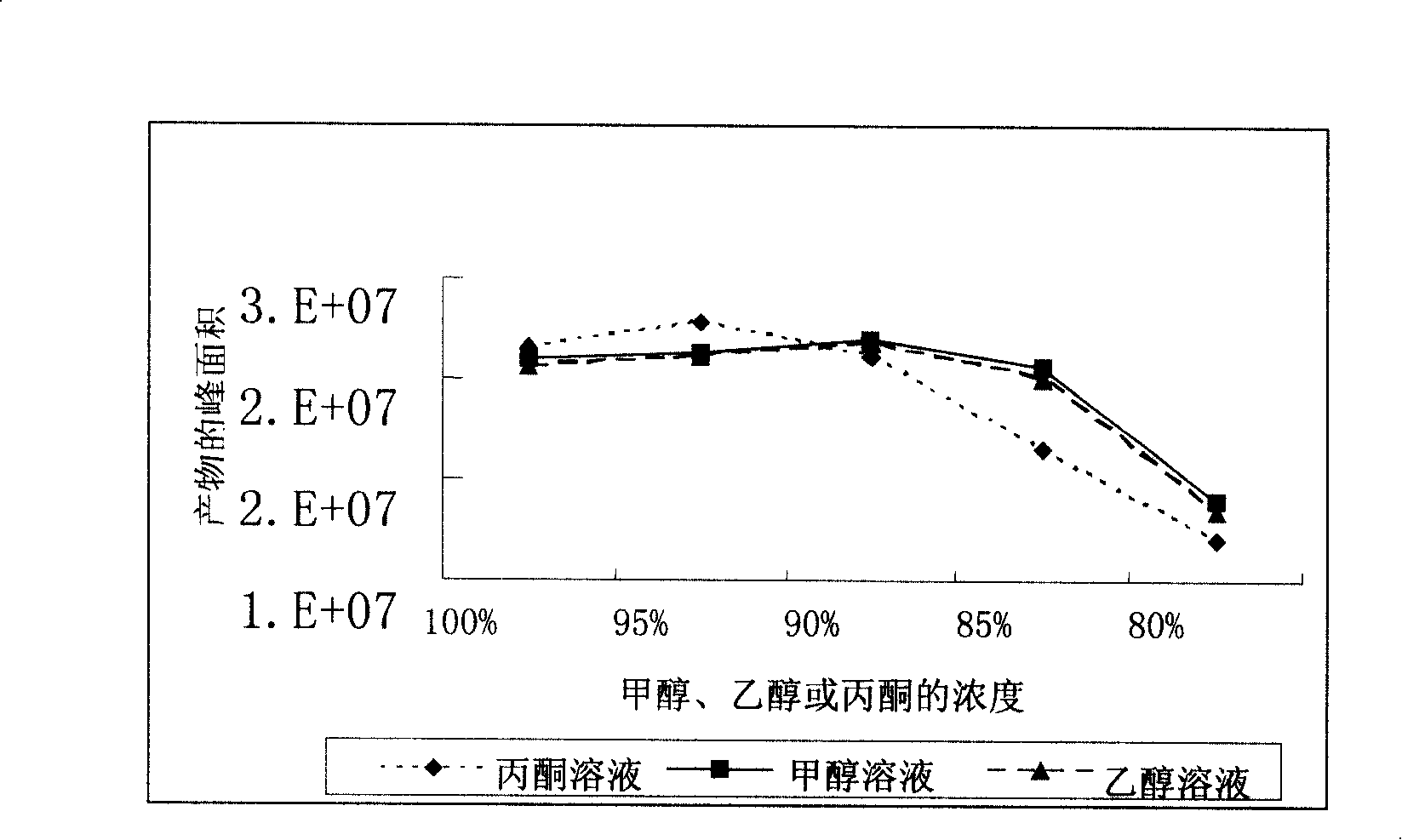
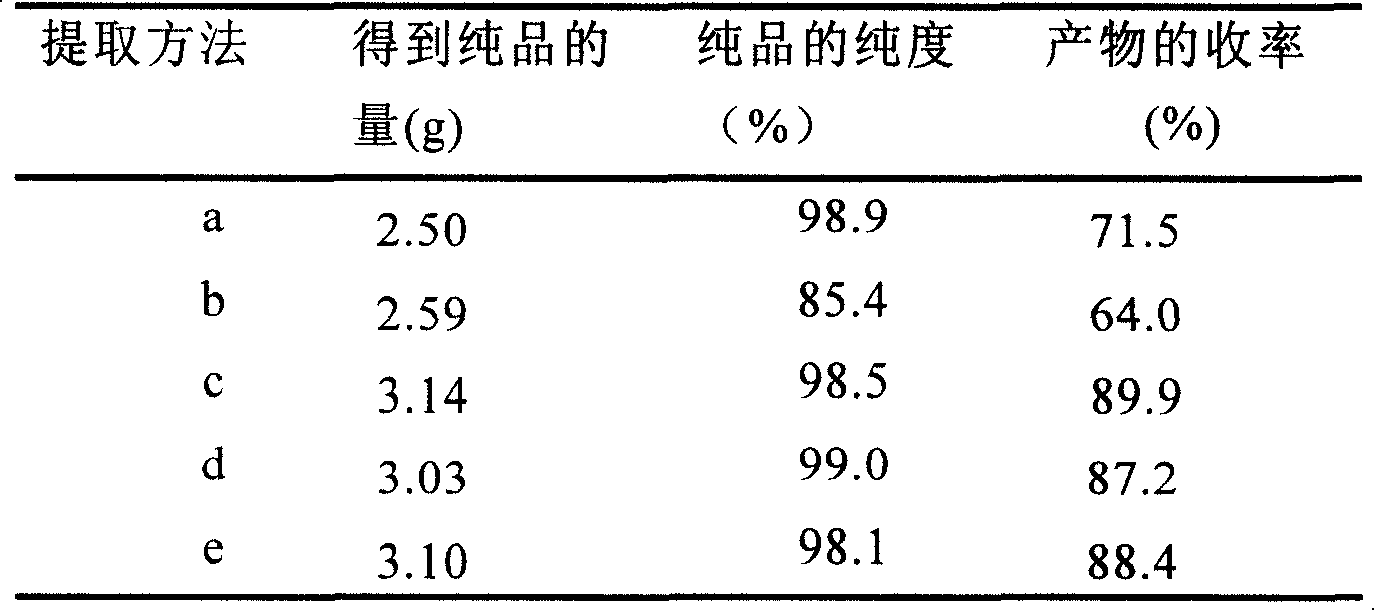
[0032] 1 L of fermentation broth with a substrate feeding concentration of 8 g / L and a conversion rate of 85.1% was centrifuged (rotating at 4000 rpm for 15 minutes). The supernatant was extracted with 1 L of propyl acetate; 0.24 kg of bacteria were first added to 1.2 L of 100% methanol at room temperature, stirred and soaked for 4 hours, then filtered, and the bacteria were extracted again with 0.8 L of 100% methanol in the same way. The methanol solutions obtained from the two extractions were mixed, and the organic solvent in the solution was distilled off, and then extracted with 3 L and 2 L of propyl acetate successively. All extracts were combined, and 10 g of anhydrous sodium sulfate was added to remove water. Then distill off most of the ethyl acetate to about 200ml, cool to room temperature and let stand for 2 hours, then filter the obtained solid to wash with 20ml of pre-cooled propyl acetate, then heat to dissolve with 30ml of methanol, and then add 200ml of 4-methy...
Embodiment 3
[0034]1 L of fermentation broth with a substrate feeding concentration of 8 g / L and a conversion rate of 85.1% was centrifuged (rotating at 4000 rpm for 15 minutes). The supernatant was extracted with 1 L of propyl acetate; 0.24 kg of bacteria were first added with 1.2 L of 100% ethanol at room temperature, stirred and soaked for 4 hours, then filtered, and the bacteria were extracted again with 0.8 L of 100% ethanol. The ethanol solutions obtained from the two extractions were mixed, and the organic solvent in the solution was distilled off, and then extracted with 3 L and 2 L of propyl acetate successively. All extracts were combined, and 10 g of anhydrous sodium sulfate was added to remove water. Then distill off most of the ethyl acetate to about 200ml, cool to room temperature and let stand for 2 hours, then filter the obtained solid to wash with 20ml of pre-cooled propyl acetate, then heat to dissolve with 30ml of methanol, and then add 200ml of 4-methyl-2- For pentanon...
Embodiment 4
[0036] 1 L of fermentation broth with a substrate feeding concentration of 8 g / L and a conversion rate of 85.1% was centrifuged (rotating at 4000 rpm for 15 minutes). The supernatant was extracted with 1 L of ethyl acetate; the cells were first extracted with 2 L of 85% acetone solution, and then extracted with 0.6 L of 85% acetone solution. Others were the same as in Example 1, and finally 5.89 g of a product with a purity of 98.5% was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com