Method for synthesizing finasteride
A technology of finasteride and androsteroid, applied in the field of compound synthesis, can solve the problems of high synthesis cost, low reaction yield, expensive raw material and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
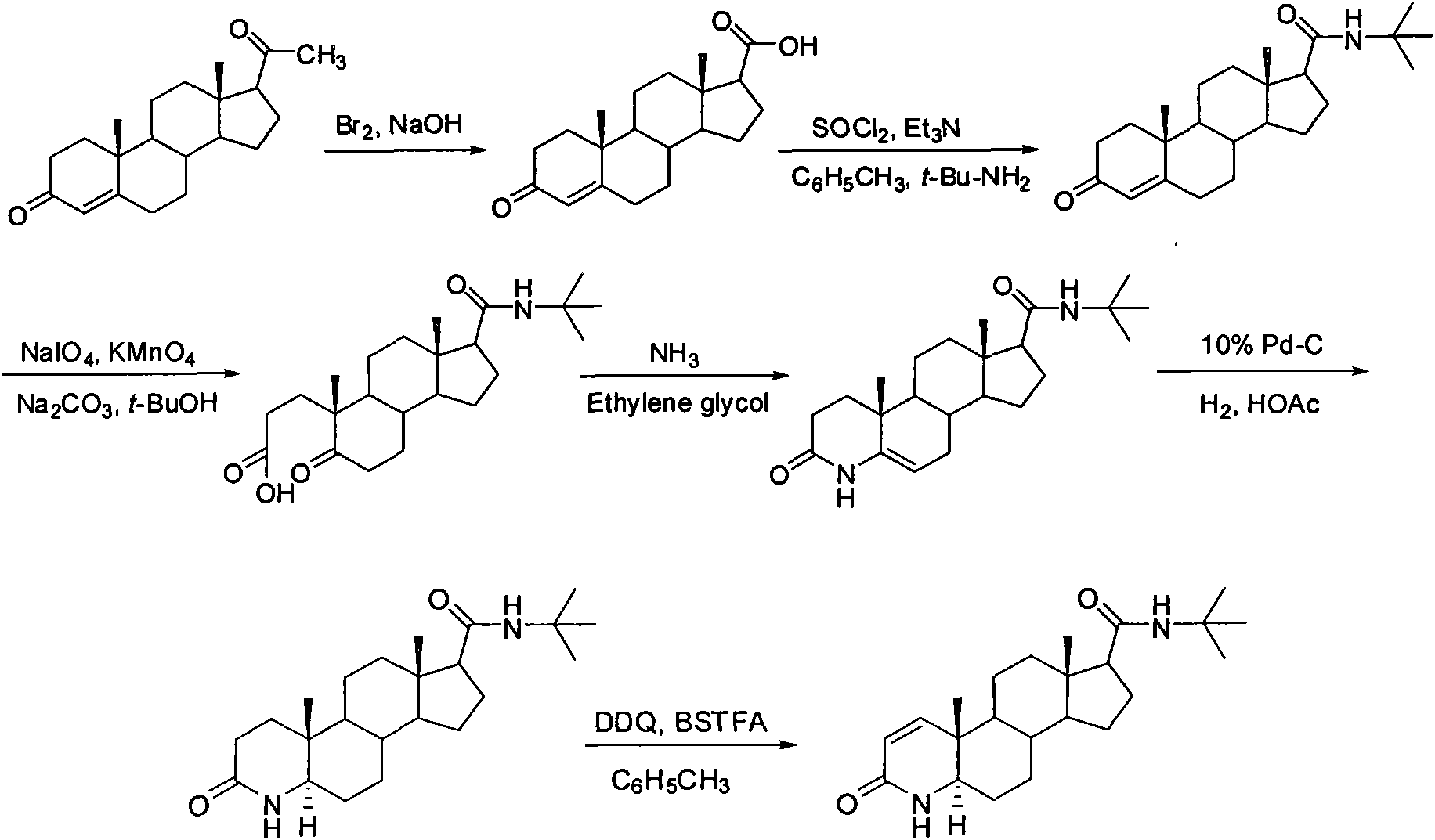
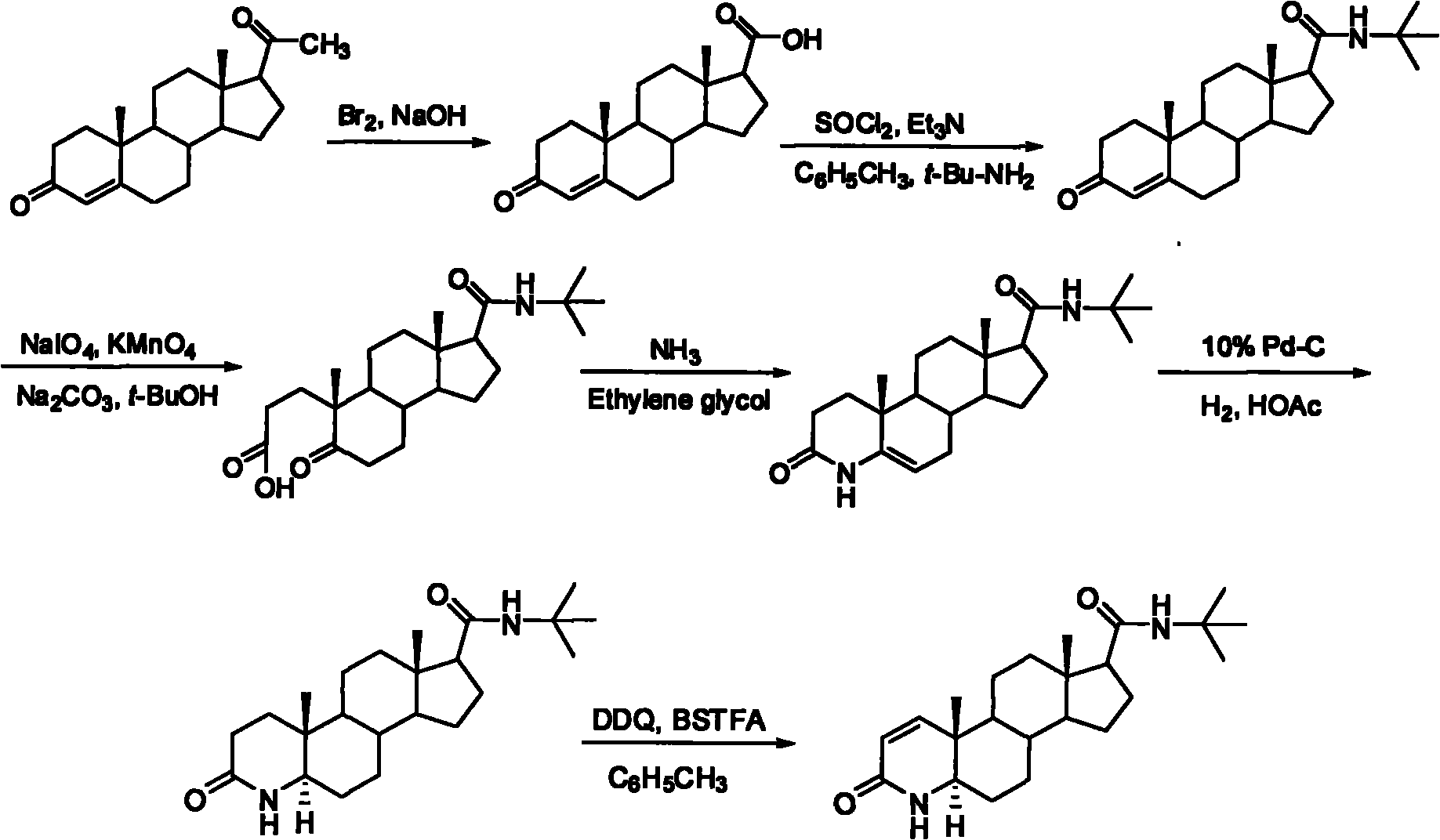
[0041] A kind of synthetic method of finasteride, its technical synthetic route schematic diagram is shown in figure 1 .
[0042] A synthetic method for finasteride, comprising the steps of:
[0043] (1), the synthesis of 3-carbonyl-4-androstene-17β-carboxylic acid
[0044] Progesterone (31.5 g, 100.0 mmol) was dissolved in dioxane (200 mL). At low temperature, top up with Br 2(15.78 g, 98.6 mmol) and 15% sodium hydroxide solution (40 mL). After dropping, react for 6 hours. Pour into ice water (70 mL), extract the aqueous layer with ethyl acetate (40 mL×3), combine the organic phases, wash with appropriate amount of saturated sodium bicarbonate solution and saturated sodium chloride solution, and dry over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized with ethyl acetate to obtain white crystal 3-carbonyl-4-androstene-17β-carboxylic acid (27.80 g, yield 88%), mp 246~247 ° C (document mp 245~248℃);...
Embodiment 2
[0058] A synthetic method for finasteride, comprising the steps of:
[0059] (1), the synthesis of 3-carbonyl-4-androstene-17β-carboxylic acid
[0060] Progesterone (3.2 g, 10.0 mmol) was dissolved in dioxane (20 mL). At low temperature, top up with Br 2 (1.52g, 9.5mmol) and 15% sodium hydroxide solution (4mL). After dropping, react for 6 hours. Pour into ice water (7 mL), extract the aqueous layer with ethyl acetate (10 mL×3), combine the organic phases, wash with appropriate amount of saturated sodium bicarbonate solution and saturated sodium chloride solution, and dry over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethyl acetate to give white crystals of 3-carbonyl-4-androstene-17β-carboxylic acid (2.62g, yield 83%), mp 246~247°C (document mp 245~248℃);
[0061] (2), the synthesis of N-tert-butyl-3-carbonyl-4-androstene-17β-carboxamide
[0062] 3-carbonyl-4-androstene-17β-carboxylic a...
Embodiment 3
[0074] A synthetic method for finasteride, comprising the steps of:
[0075] (1), the synthesis of 3-carbonyl-4-androstene-17β-carboxylic acid
[0076] Progesterone (31.5 g, 100.0 mmol) was dissolved in dioxane (200 mL). At low temperature, top up with Br 2 (16.00 g, 100.0 mmol) and 15% sodium hydroxide solution (40 mL). After dropping, react for 6 hours. Pour into ice water (70 mL), extract the aqueous layer with ethyl acetate (40 mL×3), combine the organic phases, wash with appropriate amount of saturated sodium bicarbonate solution and saturated sodium chloride solution, and dry over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain white crystal 3-carbonyl-4-androstene-17β-carboxylic acid (28.10 g, yield 89%), mp 246~247 ° C (document mp 245~248℃);
[0077] (2), the synthesis of N-tert-butyl-3-carbonyl-4-androstene-17β-carboxamide
[0078] 3-carbonyl-4-androstene-17β...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com