Method for processing dehydroepiandrosterone mother liquor objects
A technology of dehydroepiandrosterone and a treatment method, applied in the directions of steroids, organic chemistry, etc., can solve problems such as pollution of the environment, and achieve the effects of high utilization rate and pollution reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
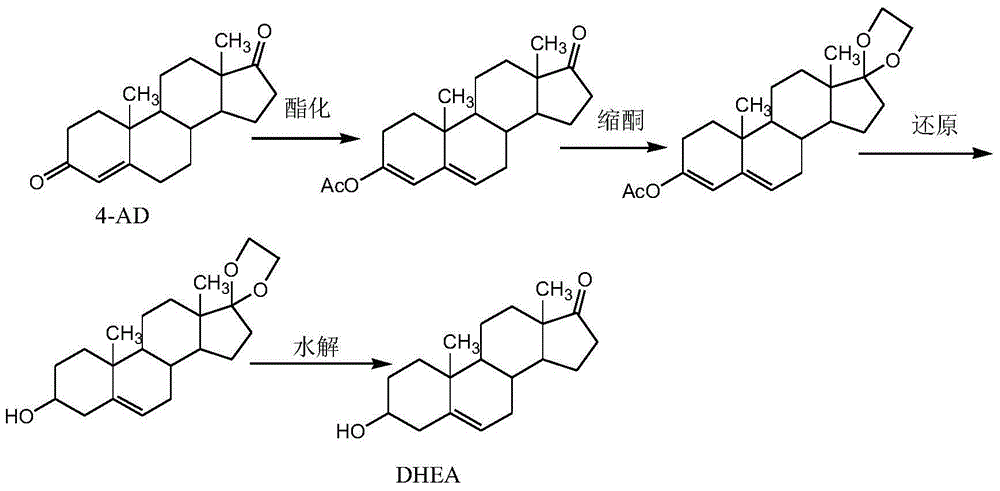
[0031] 100g of 4-AD sample was taken, refined through esterification, ketalization, reduction, and hydrolysis to obtain 66g of dehydroepiandrosterone (HPLC: 99.6%) and 30g of dehydroepiandrosterone mother liquor (dry weight). Stir and dissolve the mother liquor with 30ml of dichloromethane. The eluent is: petroleum ether: ethyl acetate = 3:1. The mother liquor is separated by column chromatography and collected in sections, monitored by TLC. Combine the same components, concentrate and dry the chromatographic solution, and dry the sample to obtain 10g dehydroepiandrosterone (HPLC: 99.6%), 8g 3α-hydroxyl-5-androsten-17-one, 5g3,17-bis Ketones.
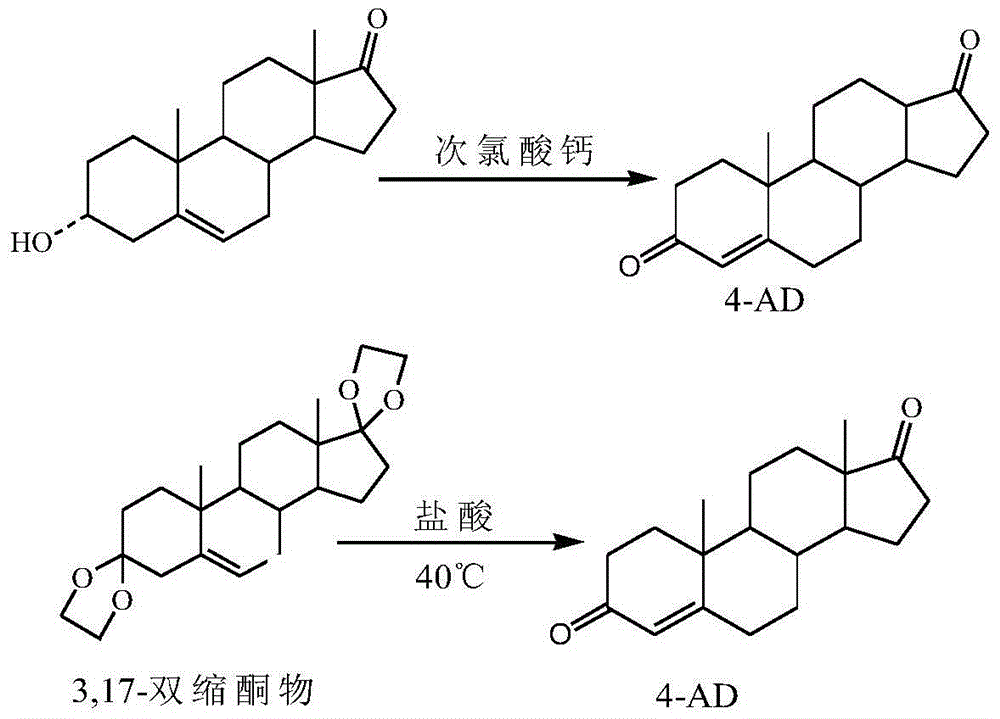
[0032] Accurately measure 32ml of ethyl acetate, add 24ml of glacial acetic acid into the reaction flask, add 8g of 3α-hydroxy-5-androsten-17-one obtained by passing through the column, stir and dissolve, add 2g of calcium hypochlorite to the reaction flask, 30 Temperature control reaction at ℃ for 1h, TLC spotting until the raw materi...
Embodiment 2
[0035] Take 200g 4-AD sample, undergo esterification, ketalization, reduction, and hydrolysis to obtain 130g dehydroepiandrosterone (HPLC: 99.7%) and 60g mother liquor (dry weight). Stir and dissolve the mother liquor with 90ml of dichloromethane, the eluent is: petroleum ether: ethyl acetate = 4:1, collect the chromatographic solution through the column, concentrate and dry the chromatographic solution, and dry the sample at 50-60°C to obtain 20g of Hydroepiandrosterone (HPLC: 99.5%), 18 g of 3α-hydroxy-5-androsten-17-one, 10 g of 3,17-bisketal.
[0036] Accurately measure 90ml of ethyl acetate, add 72ml of glacial acetic acid into the reaction flask, add to the reaction flask, add 18g of 3α-hydroxy-5-androsten-17-one obtained through the column, stir to dissolve, and add 5.04g of Calcium hypochlorite, react at 20°C for 2 hours, spot on TLC plate until the reaction of the raw materials is complete, add 3.6g of sodium bisulfite and stir for 15min, add 270ml of water and stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com