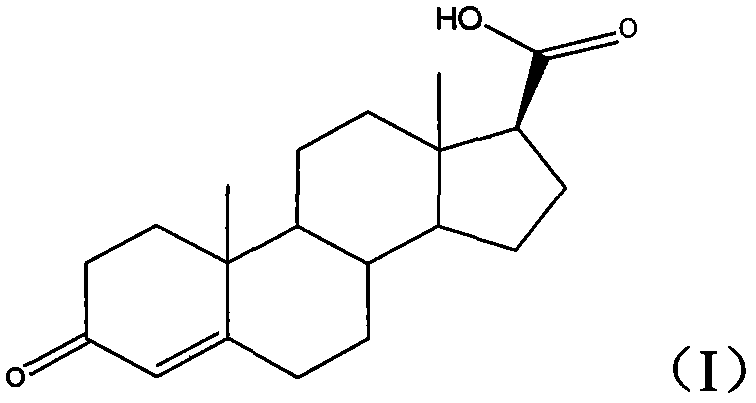
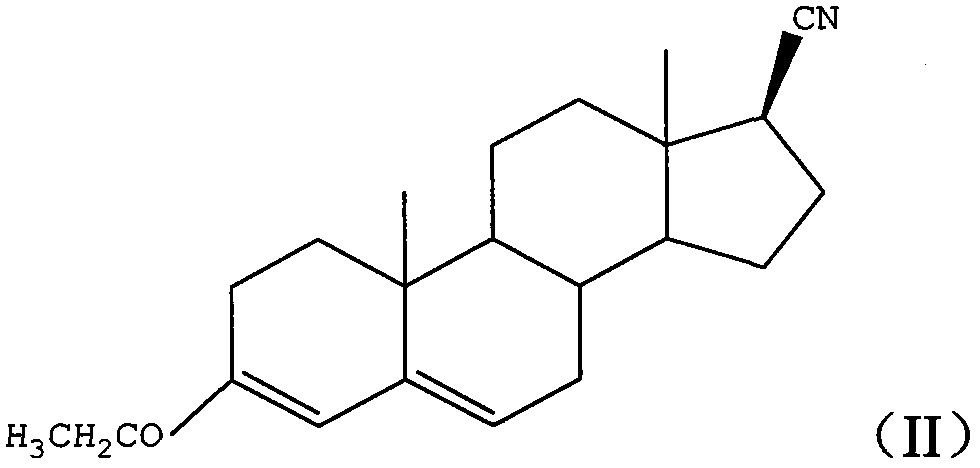
Method for preparing 17beta-carboxyl-4-androstene-3-ketone
A technology of androstene and carboxyl, which is applied in the field of preparation of 17β-carboxy-4-androstene-3-one, can solve the problems of low yield, high price, and difficult availability of raw materials, and achieve high yield and low price. Low cost, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The embodiment of the present invention discloses a preparation method of 17β-carboxy-4-androsten-3-one, which comprises the following steps:
[0025] a), under strong alkali conditions, 3-ethoxy-androst-3,5-dien-17-one and p-toluenesulfonylmethyl isonitrile react in an organic solvent to obtain formula (II) structure first intermediate product;
[0026]
[0027] b) The first intermediate is hydrolyzed under acidic conditions to obtain 17β-carboxy-4-androsten-3-one.
[0028] The preparation method of 17β-carboxy-4-androstene-3-one provided by the embodiment of the present invention is based on 3-ethoxyandrost-3,5-dien-17-one with lower cost and easier access to the market , first converting the 17-keto group of the starting material into a 17β-cyano group in one step (step a above), then converting the 17β-cyano group into a 17β-carboxyl group, and converting the 3-ethoxy group into a 3-keto group ( Step b) above.
[0029] The reaction principle of above-mentioned...
Embodiment 1
[0040] Example 1 Preparation of the first intermediate
[0041] Under the protection of nitrogen flow, 25g 3-ethoxy androst-3,5-dien-17-one and 45g potassium tert-butoxide were added to 1L ethylene glycol dimethyl ether and 300ml tert-butanol, and cooled to -5°C, add dropwise a solution made of 23.43g p-toluenesulfonylmethyl isonitrile and 140ml ethylene glycol dimethyl ether, after the dropwise addition is completed, return the temperature to room temperature, stir for 3h, and pour half-saturated chlorine In the sodium chloride aqueous solution, precipitates were precipitated, filtered, washed with water, and dried under reduced pressure to obtain 23.8 g of white crystals with a yield of 92%.
[0042] The proton nuclear magnetic resonance spectrum of product is as follows:
[0043] 1 H-NMR (CDCl 3 ) 0.95(s, 3H, 18-CH 3 )
[0044] 1.20(s, 3H, 19-CH 3 )
[0045] 5.71 (s, 1H, 4-H)
[0046] It can be seen that the first intermediate product prepared in this embodiment is ...
Embodiment 2
[0048] Embodiment two prepares the first intermediate
[0049] Under the protection of nitrogen flow, 25g 3-ethoxy androst-3,5-dien-17-one and 45g potassium tert-butoxide were added to 1L ethylene glycol dimethyl ether and 300ml tert-butanol, and cooled to -5°C, add dropwise a solution composed of 22g p-toluenesulfonylmethyl isonitrile and 140ml ethylene glycol dimethyl ether, after the dropwise addition is complete, return the temperature to room temperature, stir for 3h, pour into half-saturated chlorinated In the aqueous sodium solution, precipitates were precipitated, filtered, washed with water, and dried under reduced pressure to obtain 23.3 g of white crystals with a yield of 90%. The proton nuclear magnetic resonance spectrum of product is as follows:
[0050] 1 H-NMR (CDCl 3 )0.95(s, 3H, 18-CH 3 )
[0051] 1.20(s, 3H, 19-CH 3 )
[0052] 5.71 (s, 1H, 4-H)
[0053] It can be known that the first intermediate product prepared in this example is 3-ethoxy-17β-cyanoan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com