Patents
Literature
326 results about "Isocyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group -N≡C. It is the isomer of the related nitrile (-C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.
Novel metal nanoparticle and formation of conductive pattern using the same
InactiveUS20080020317A1Easy to achieve nanoparticle arrangementOvercome problemsLiquid surface applicatorsPhotosensitive materialsSelf-assembled monolayerPhosphate
A metal nanoparticle which is prepared by forming a self-assembled monolayer including a terminal reactive group on the surface thereof, and introducing a functional group capable of being removed by the action of an acid or an base into the terminal reactive group wherein the self-assembled monolayer is built up of a thiol, an isocyanide, an amine, a carboxylate or a phosphate compound having the terminal reactive group, or built up of a thiol, an isocyanide, an amine, a carboxylate or a phosphate compound having no terminal reactive group followed by introducing the terminal reactive group thereto; and a method for forming a conductive pattern using the same are provided. Since the metal nanoparticle of exemplary embodiments of the present invention can easily form a high conductive film or a high conductive pattern through photo-irradiation and photo-degradation and randomly regulate its conductivity when occasions demand, it can be advantageously applied to an antistatic washable sticky film, antistatic shoes, a conductive polyurethane printer roller, an electromagnetic interference shielding, and the like.
Owner:SAMSUNG ELECTRONICS CO LTD
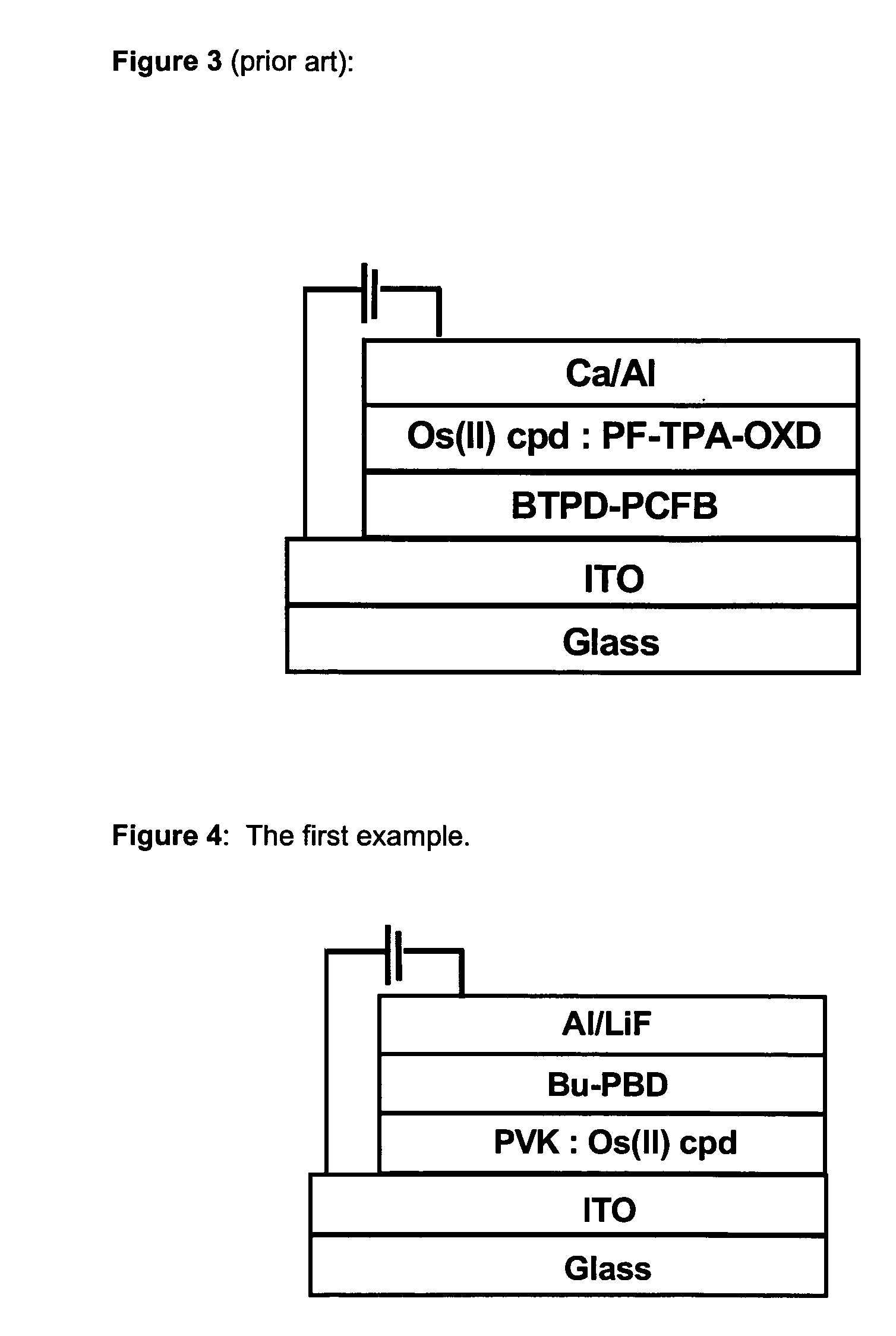
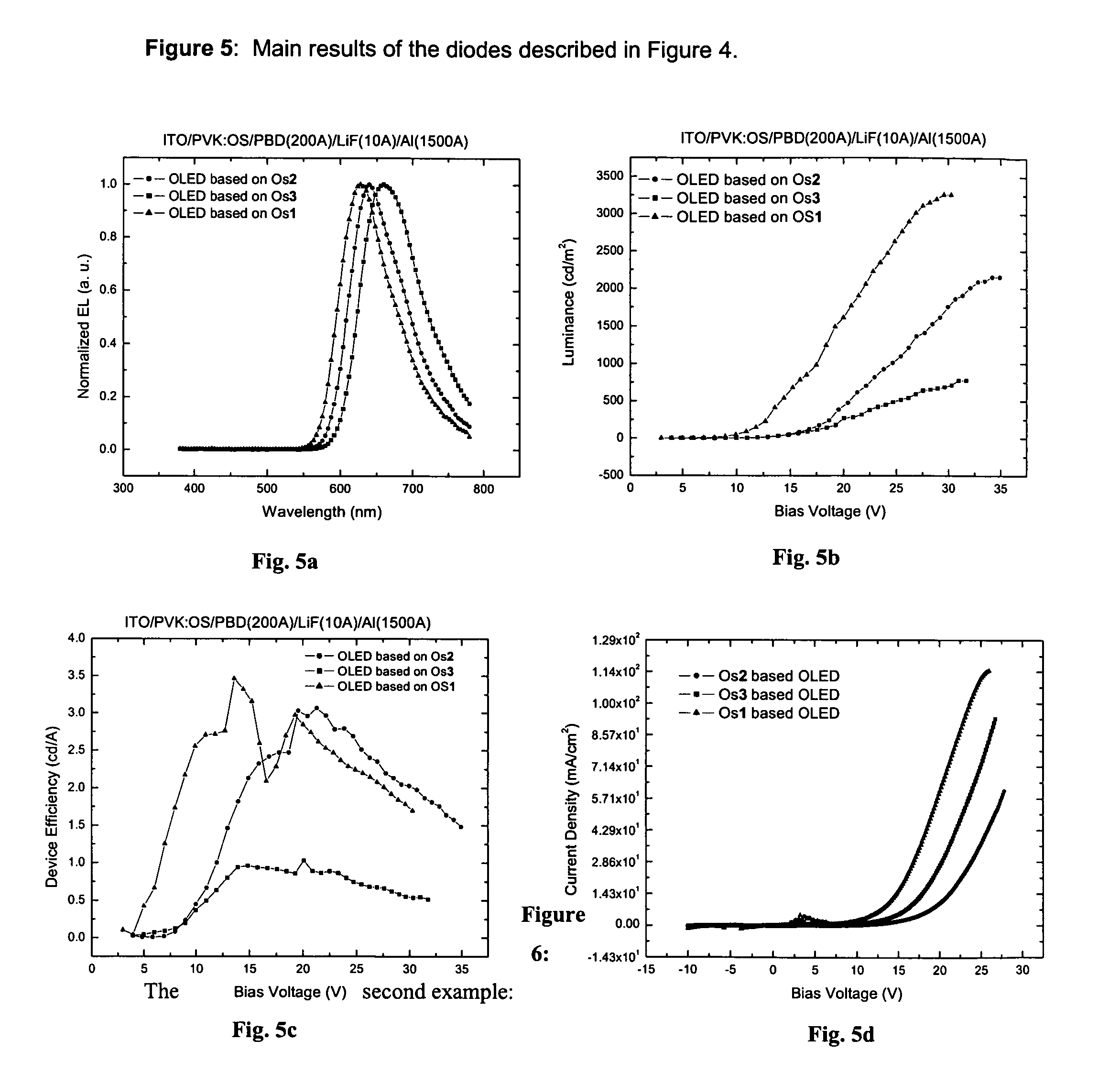
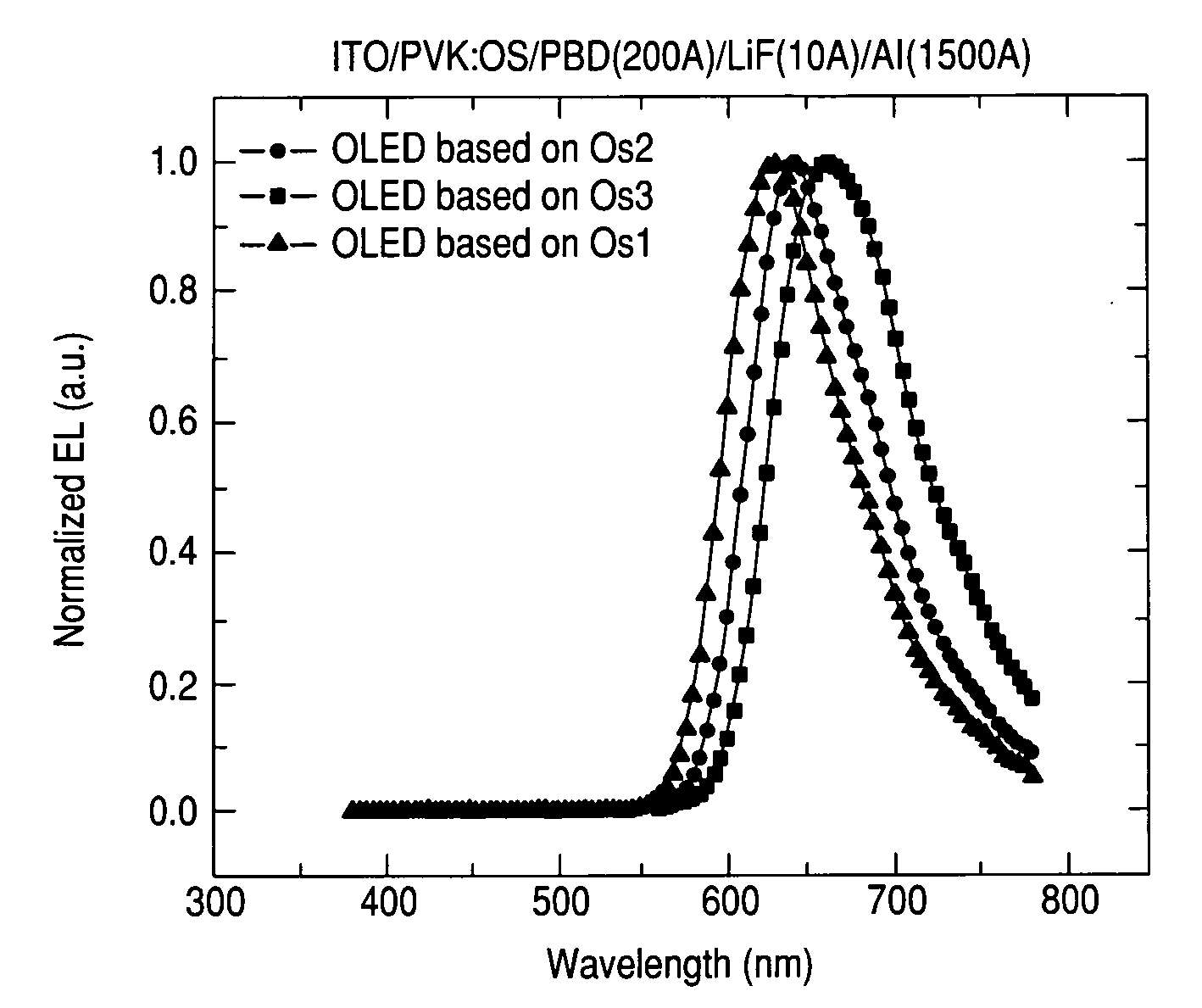
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20070001166A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores NˆN, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NˆN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NˆN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of NˆN chromophores residing opposite to the L. X,1 X2 and X3 independently are C or N; when X2 is N, R1 is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:TAO YE +3
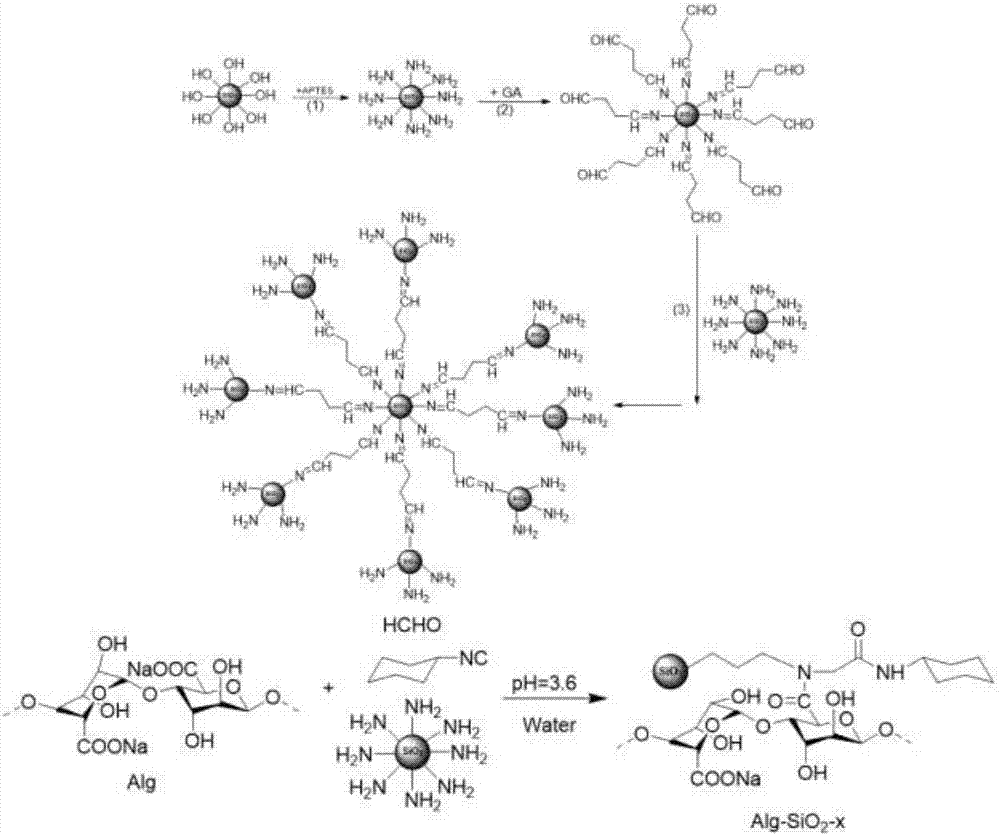
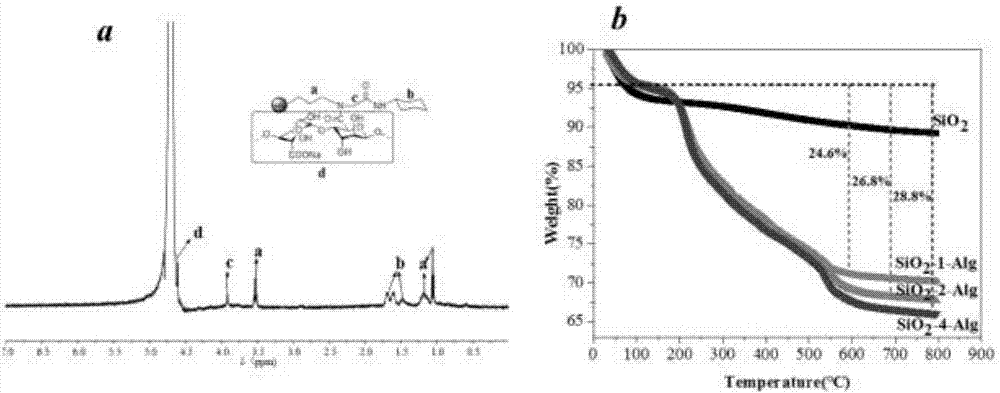
pH responsive drug-loading Pickering emulsion and preparation method thereof
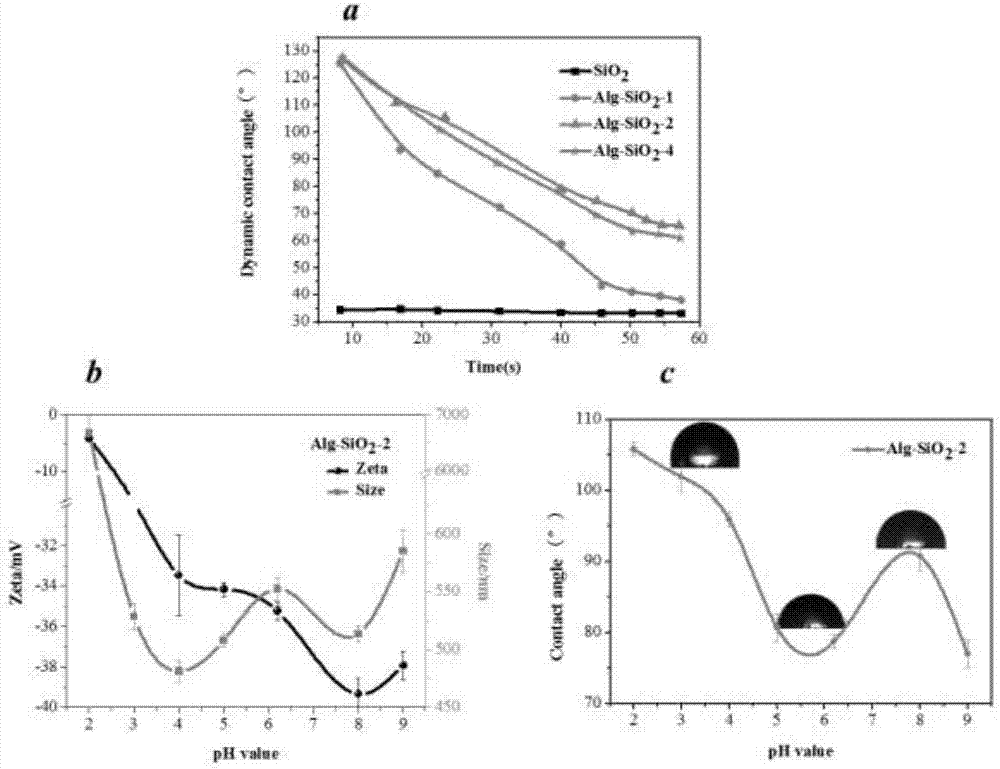
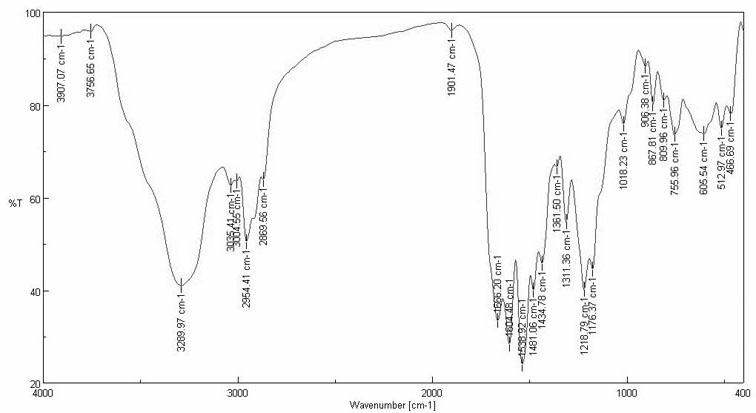
The invention provides a pH responsive drug-loading Pickering emulsion and a preparation method thereof. The method comprises the step of preparing sodium alginate modified silicon dioxide nanoparticles which are prepared from the Ugi reaction of sodium alginate, formaldehyde, cyclohexyl isocyanide and fractal silicon dioxide, wherein the fractal silicon dioxide is prepared in the manner of utilizing layering molecular imprinting to perform covalence assembling on amino blocked silicon dioxide and aldehyde blocked silicon dioxide and repeating the imprinting process according to the requirement till reaching the required layer number of the silicon dioxide. The invention also provides a Pickering emulsion prepared from the nanometer particle. The invention can prepare a novel pH responsive Pickering emulsion in the manner of grafting sodium alginate (Alg) onto the fractal SiO2 surface through Ugi condensation reaction, can enrich the application of the triggering emulsion in a pesticide release controlling system and can widen the application of the Pickering emulsion at the aspect of pesticide delivery.
Owner:HAINAN UNIVERSITY
Double-hindered phenol structure-contained hydrazides compound and preparation method thereof
ActiveCN102344389AHigh molecular weightEnhance thermal oxidation resistanceHydrazide preparationPtru catalystOrganosolv
The invention discloses a double-hindered phenol structure-contained hydrazides compound for a light stabilizer and a preparation method thereof. The double-hindered phenol structure-contained hydrazides compound has a double-hindered phenol and double-hydrazide structure, thus, the molecular weight is increased, and the migration resistance and thermal oxidation resistance capacity is strong. The preparation method comprises the following steps: performing addition reaction on hindered phenols and alpha, beta-acrylic ester under the action of a catalyst to obtain an addition product, wherein the molar ratio of the hindered phenols to the alpha, beta-acrylic ester is 1.0:(0.6-1.8), and the catalyst is alkali metal, an alkali metal hydroxide, an alkali metal hydride or an alkali metal alcoholate; then, performing carbonyl nucleophilic substitution reaction on the addition product and a hydrazine hydrate in an organic solvent A to obtain a hydrazides substitution product, wherein the molar ratio of the addition product to the hydrazine hydrate is 1.0:(0.8-5.0); performing the reaction between a diisocyanate compound and the hydrazides substitution product at the temperature of 0-10 DEG C; and carrying out recrystallization to obtain a goal product. By adopting the preparation method disclosed by the invention, the operation is simple, the raw materials are available, and the cost is low.
Owner:YANTAI RUILONG CHEM TECH CO LTD
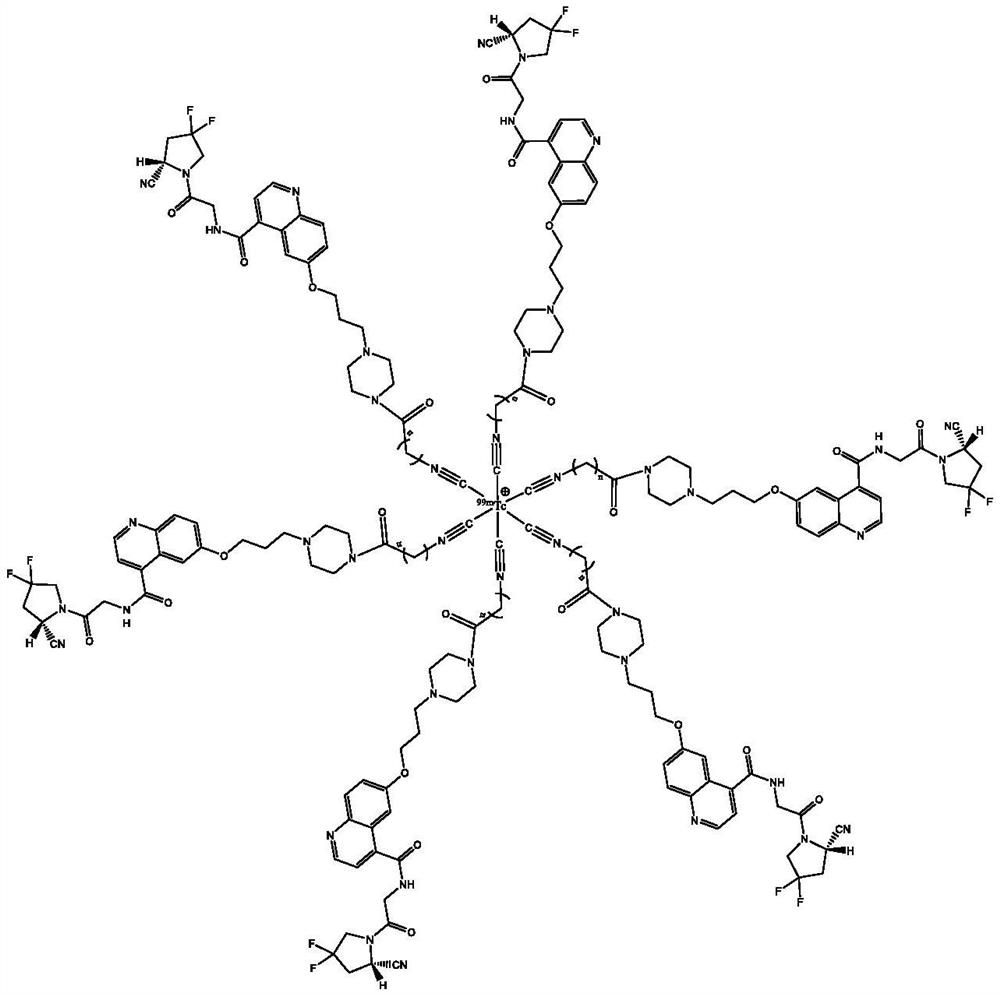
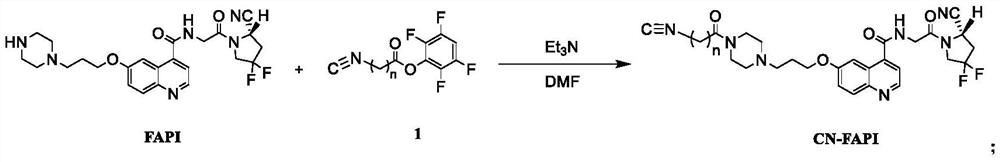
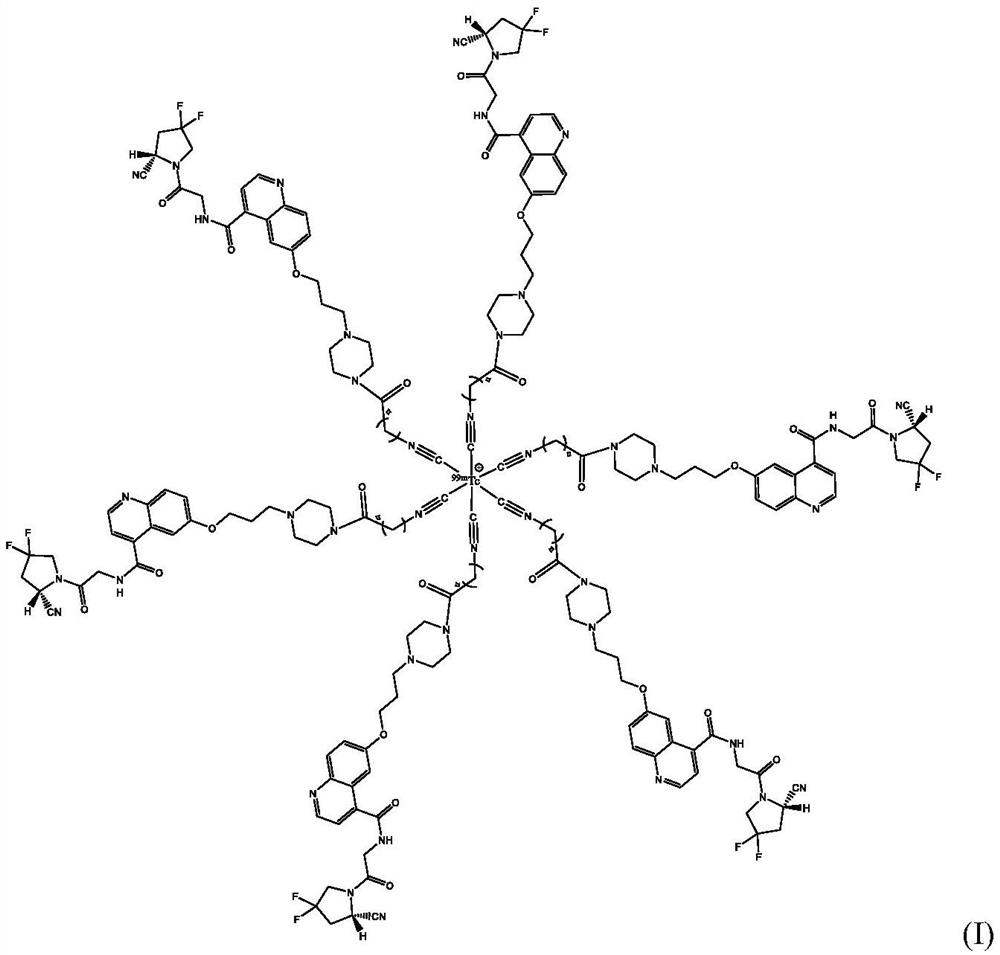
Technetium-99m labeled isonitrile-containing FAPI derivative and preparation method and application thereof
ActiveCN112409414AReduce intakeExcellent anti-tumor performanceOrganic chemistry methodsRadioactive preparation carriersMouse tumorPerylene derivatives
The invention discloses a technetium-99m labeled isocyanide-containing FAPI derivative with a structural general formula of [99mTc-(CN-FAPI) 6] < + > and a preparation method and an application thereof. The [99mTc (CNFAPI) 6] < + > complex is obtained through two steps of synthesis of a ligand CN-FAPI and preparation of [99mTc-(CNFAPI) 6] < + >. The complex is simple and convenient to prepare, high in radiochemical purity and good in stability, has relatively high uptake and good retention at tumor parts of tumor-bearing mice, has specificity in tumor uptake, and is a novel tumor imaging agentwith popularization and application values.
Owner:BEIJING NORMAL UNIVERSITY
Method for synthesis of alpha-sulfonamido amide, carboxylic acid and hydroxamic acid derivatives
Owner:ADVANCED SYNTECH
Palladium catalyst, and preparation method and application thereof
InactiveCN103012785AImprove stabilityEasy to synthesizeGroup 8/9/10/18 element organic compoundsPolymer sciencePalladium catalyst
The invention discloses a palladium catalyst, and a preparation method and application thereof. The structure of the palladium catalyst is represented by the following general formula, wherein R is selected from -CH2CH3 and -Ph, and R1 is selected from -CH3, -CF3, -H, -F, -NO2, -OCH3 and -COOCH3. The palladium catalyst disclosed by the invention has favorable isonitrile polymerization catalyzing power, is easy to synthesize, has high stability, and can be used for active / controllable polymerization of isonitrile monomers; and the obtained polyisonitrile has high molecular weight and narrow molecular weight distribution.
Owner:HEFEI UNIV OF TECH
Furanocoumarin-Tr*ger's Base derivative as well as synthesis method and application thereof
ActiveCN110551145AMild reaction conditionsShort reaction timeOrganic chemistryFluorescence/phosphorescenceFluorescenceSynthesis methods
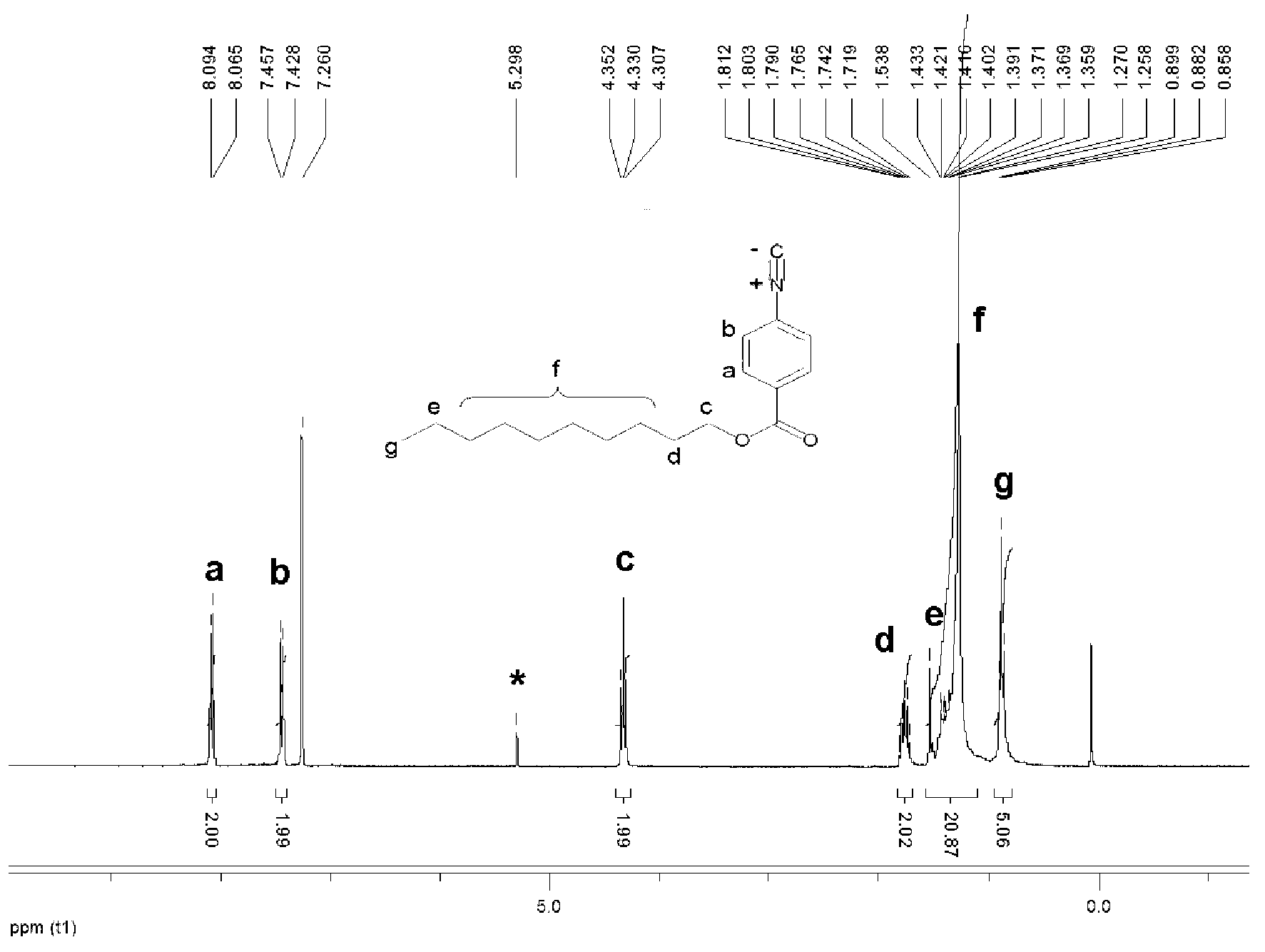
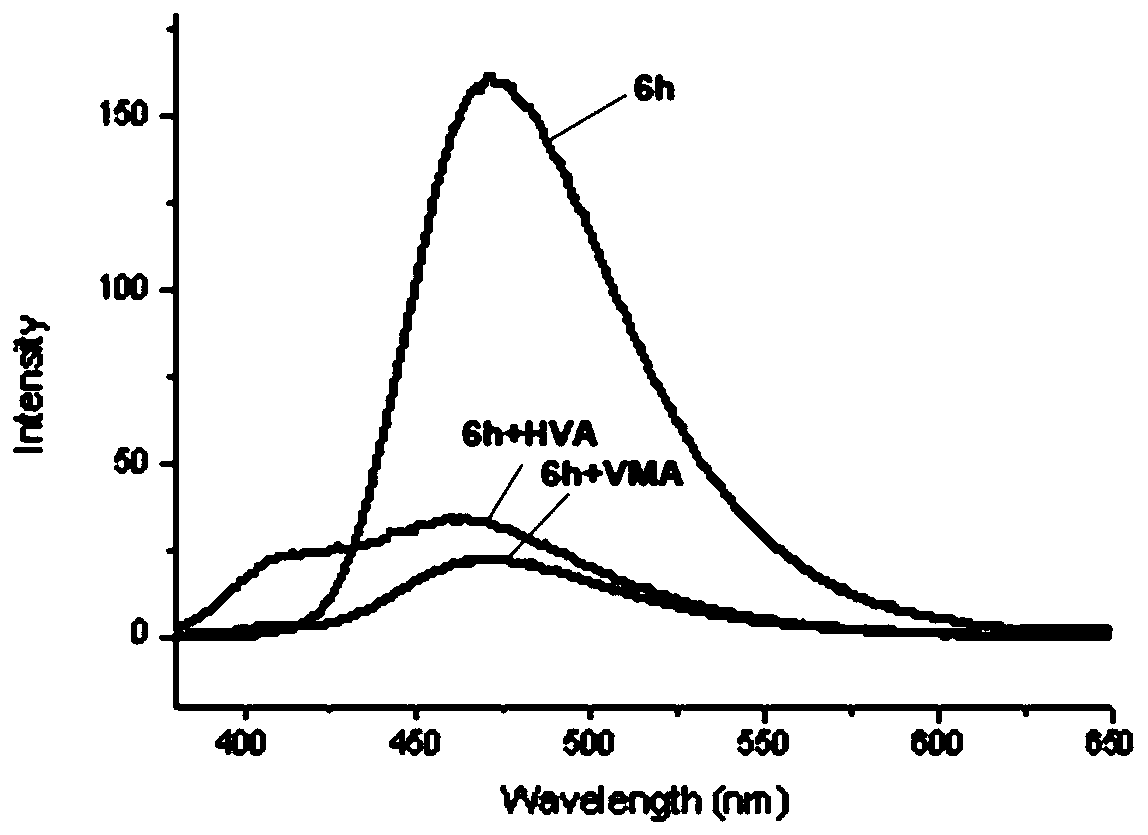
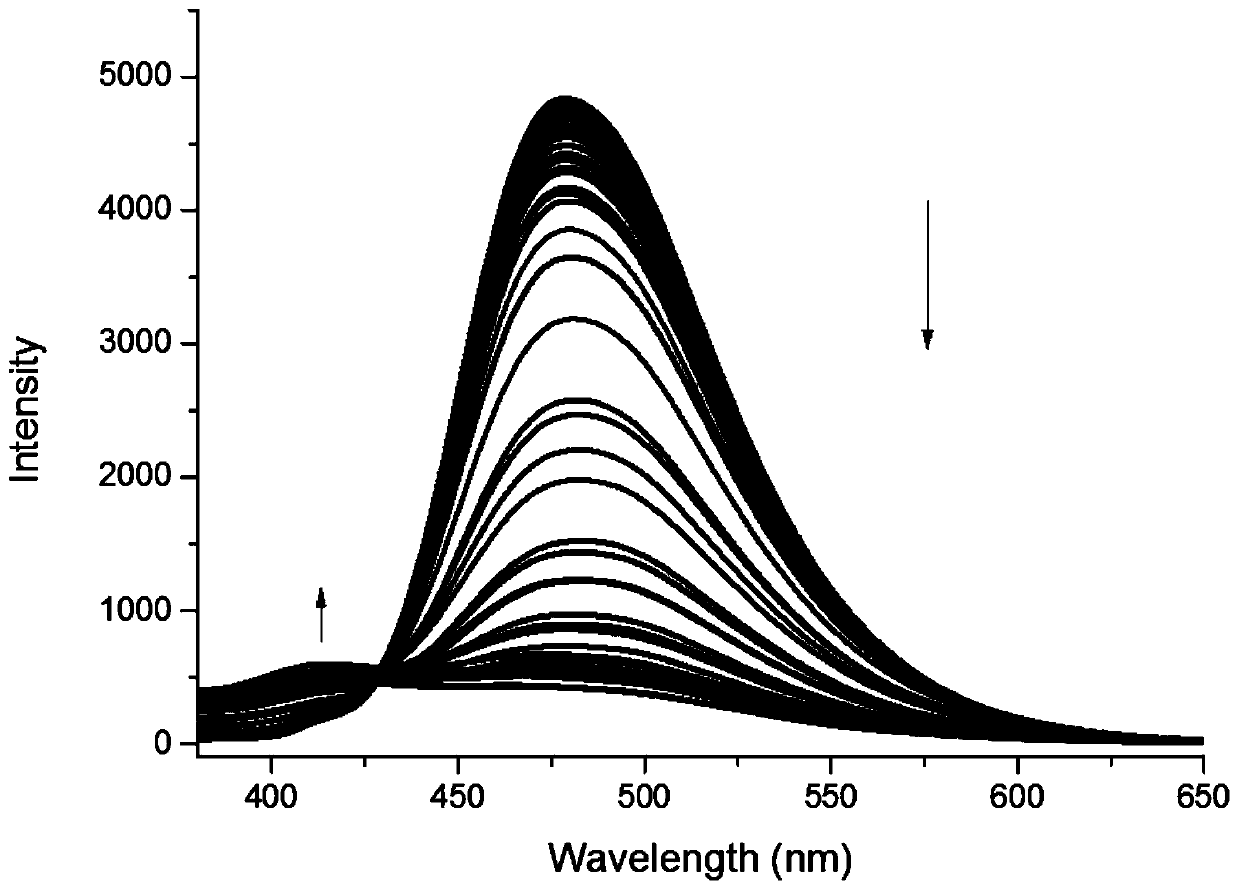
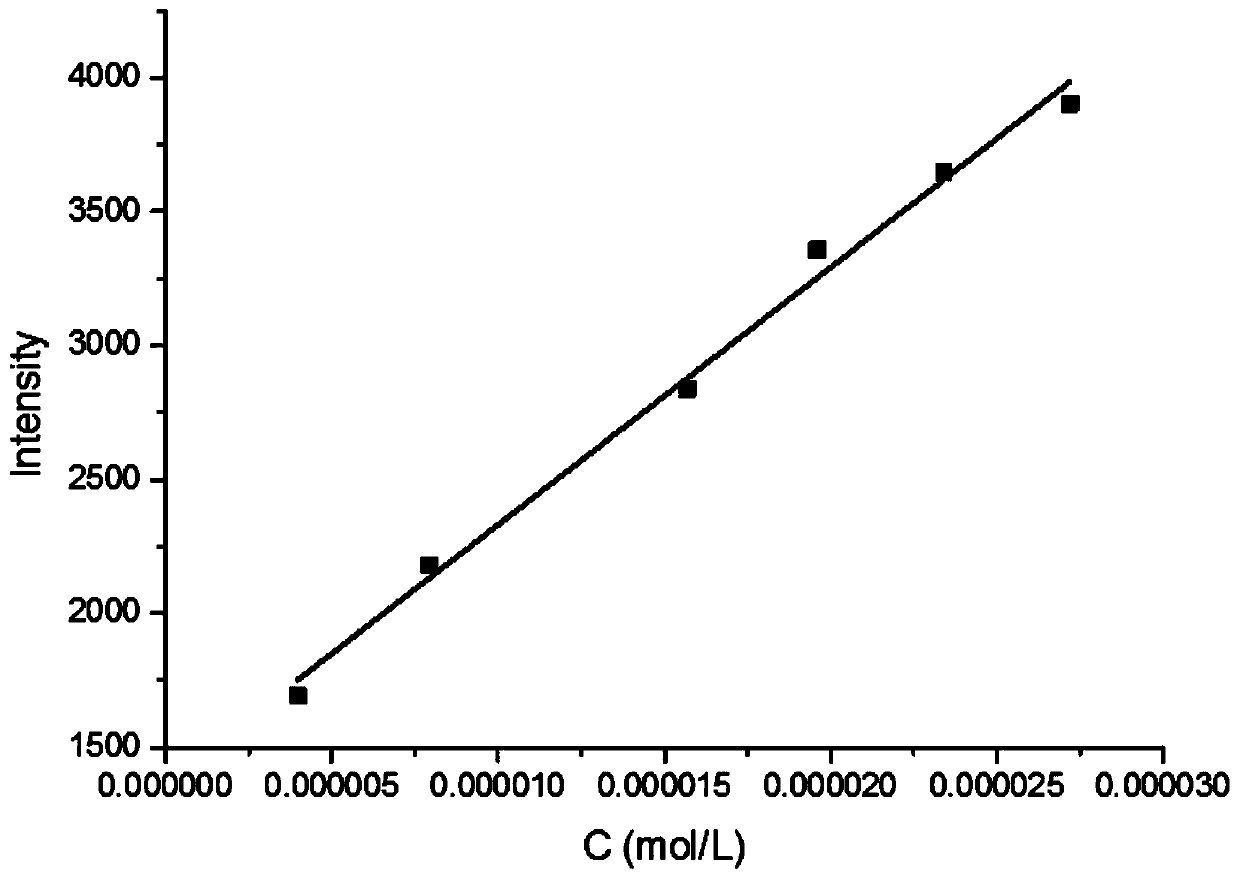
The invention provides a furanocoumarin-Base derivative, wherein the structural formula of the derivative is as shown in the following formula 6 or 7; the derivative is synthesized by performing ringformation and coupling reaction on parabromoaniline,paraformaldehyde, 4-hydroxycoumarin, isonitrile and n-butyllithium; and the synthesis process is mild in reaction condition, short in reaction timeand high in yield. The Base derivative containing a coumarin fragment has excellent luminescent property and high bioactivity; some of the products have anti-tumor activity, show high-selectivity inhibition on human triple negative breast cancer cells (MDA-MB-231) and have research value of being further developed into anti-tumor medicines; and the other products can be applied to synchronous detection on neuroblastoma metabolites homovanillic acid (HVA) and vanilmandelic acid (VMA) and have the potential of being developed into a high-efficiency fluorescent probe for human neuroblastoma earlywarning and definite diagnosis.
Owner:XUZHOU NORMAL UNIVERSITY
Method for tandem synthesis of dipyrrole and its derivatives through one-pot process
InactiveCN102351772AEasy to operateMild reaction conditionsOrganic chemistryOrganic solventAntibacterial activity
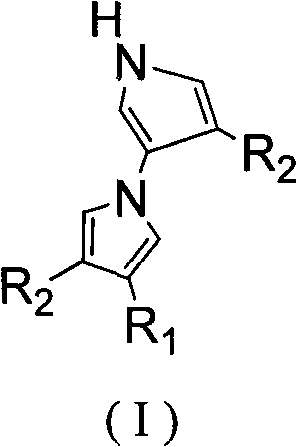
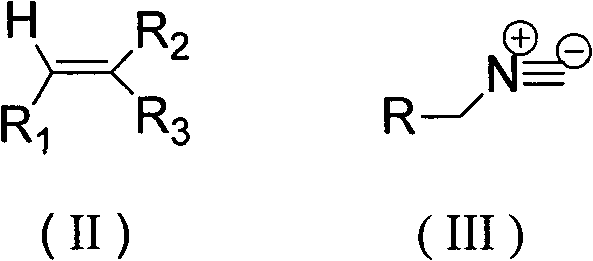
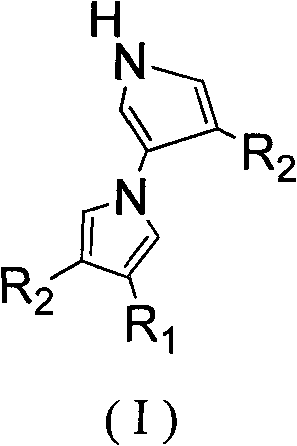
The invention relates to a method for tandem synthesis of dipyrrole and its derivatives through a one-pot process. Dipyrrole and its derivatives have a structural formula as shown in formula (I). Dipyrrole and its derivatives are prepared in this way: trisubstituted alkene shown in formula (II) and isocyanide shown in formula (III) are taken as the main raw materials which react with the catalyst alkali at a certain temperature in an organic solvent so as to obtain the target product. The preparation method of dipyrrole and its derivatives in the invention has mild reaction conditions, and the products have good antibacterial activity and antitumor activity, wherein, the (I), (II) and (III) have the following structural formulas.
Owner:邱方利
Method for preparing benzofuran-pyrrole compounds by copper catalysis
The invention discloses a method for preparing benzofuran-pyrrole compounds by copper catalysis. The method comprises the following steps: carrying out a stirring and refluxing reaction on 1,6-dialkynyl-3-ol compounds represented by formula (1) and isocyanides represented by formula (2) in an organic solvent system with a metal copper salt as a catalyst, carrying out TLC tracking detection until the reaction is completed, and post-treating the obtained reaction solution to obtain the benzofuran-pyrrole compounds represented by formula (3). The method has the advantages of simplicity in operation, easy availability of raw materials and reagents, mild reaction conditions, green and environmentally-friendly reaction system, and easiness in separation and purification of the product, is suitable for synthesizing various highly-functionalized benzofuran-pyrrole compounds, is particularly suitable for large-scale industrial production, and can realize high-efficiency and high-yield production of the benzofuran-pyrrole compounds.
Owner:XUZHOU NORMAL UNIVERSITY
Multi-responsive polymer nanocarrier with rapid cell penetrating characteristic as well as synthesis method and application of nanocarrier
InactiveCN106397758AEasy accessEfficient entryEnergy modified materialsPharmaceutical non-active ingredientsNanocarriersSynthesis methods
The invention relates to a multi-responsive polymer nanocarrier with a rapid cell penetrating characteristic. The nanocarrier is formed by self-assembling of a multi-responsive amphipathic triblock copolymer, the nanocarrier adopts an onion structure sequentially comprising an acid-responsive PLLA core, cyanine molecules with a near infrared absorption characteristic, a hydrogen-peroxide-responsive PPI (polyphenyl isocyanide) intermediate layer and a left-handed spiral hydrophilic PPI hydrophilic chain from inside to outside sequentially, wherein the structure of the multi-responsive amphipathic triblock copolymer is shown in the general formula in the specification, R1 is a group with stimulation responsiveness, and R2 is a chiral PEG hydrophilic chain; x ranges from 20 to 50, y ranges from 20 to 50, and z ranges from 30 to 80. The multi-responsive polymer nanocarrier can be used for transporting hydrophobic dye molecules and medicine molecules, can rapidly and efficiently penetrate cell membranes, has multiple stimuli-responsiveness, realizes programmable controlled release of medicines and has a chemical-photothermal collaborative treatment characteristic.
Owner:HEFEI UNIV OF TECH
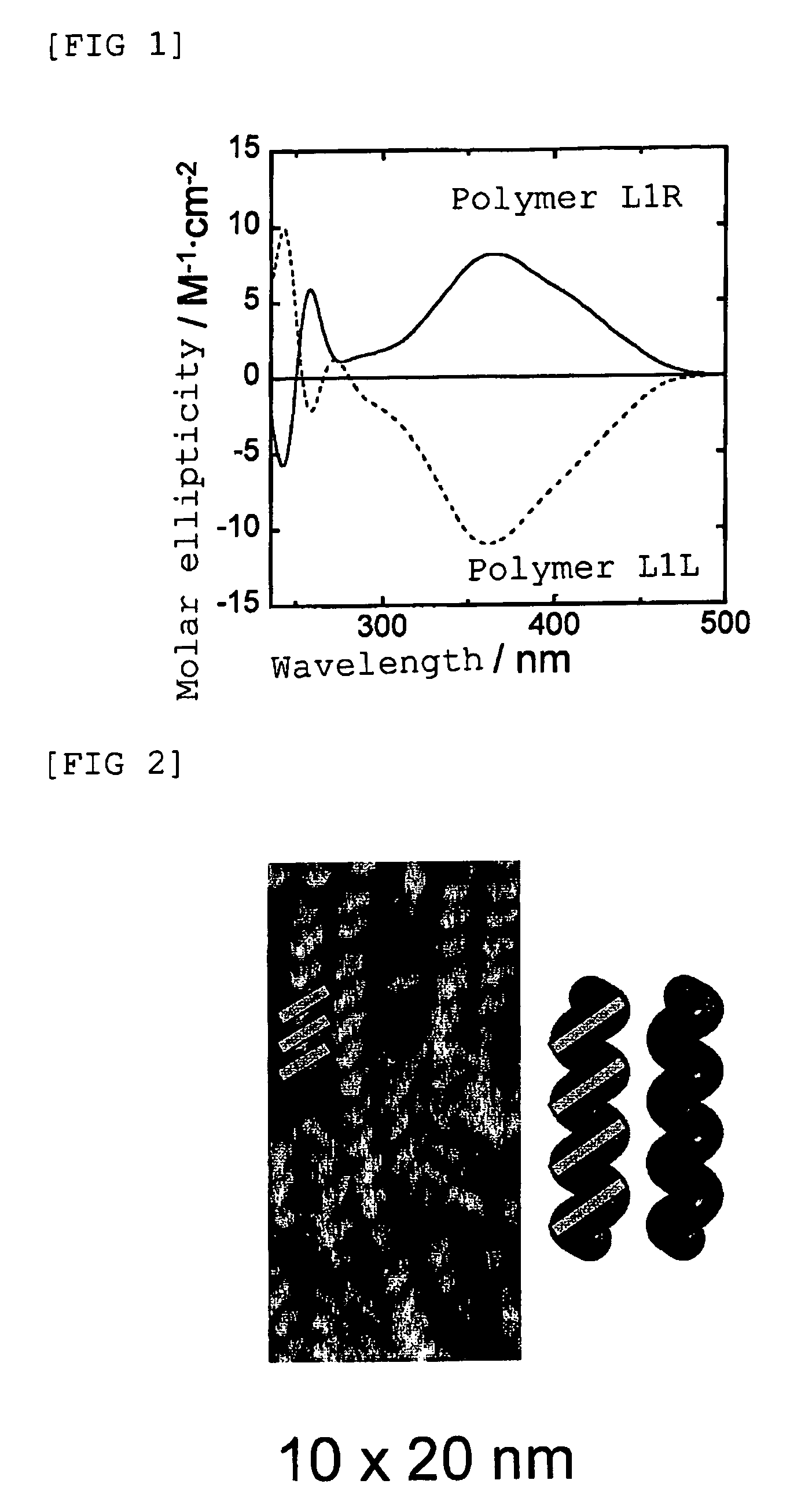
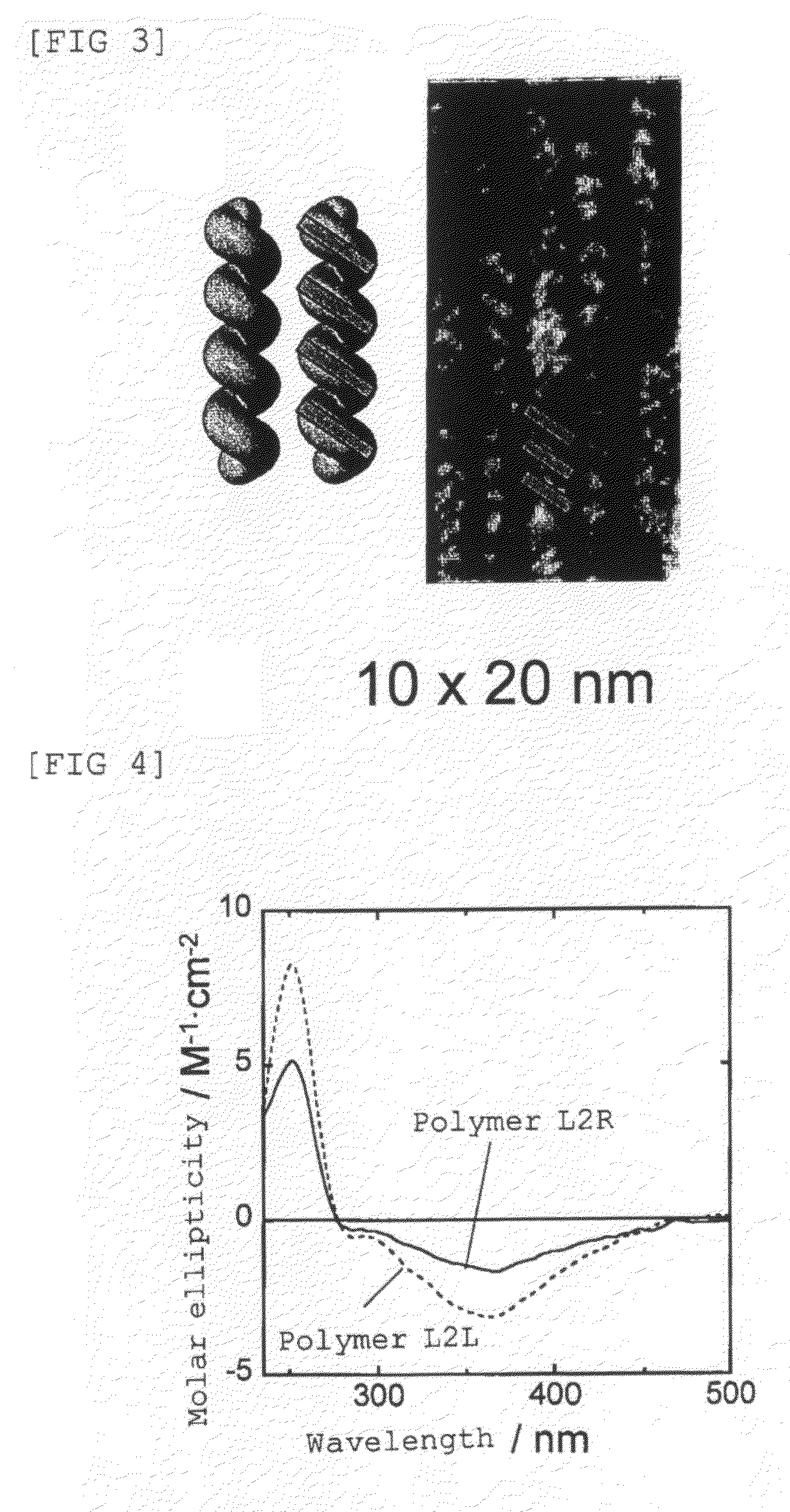
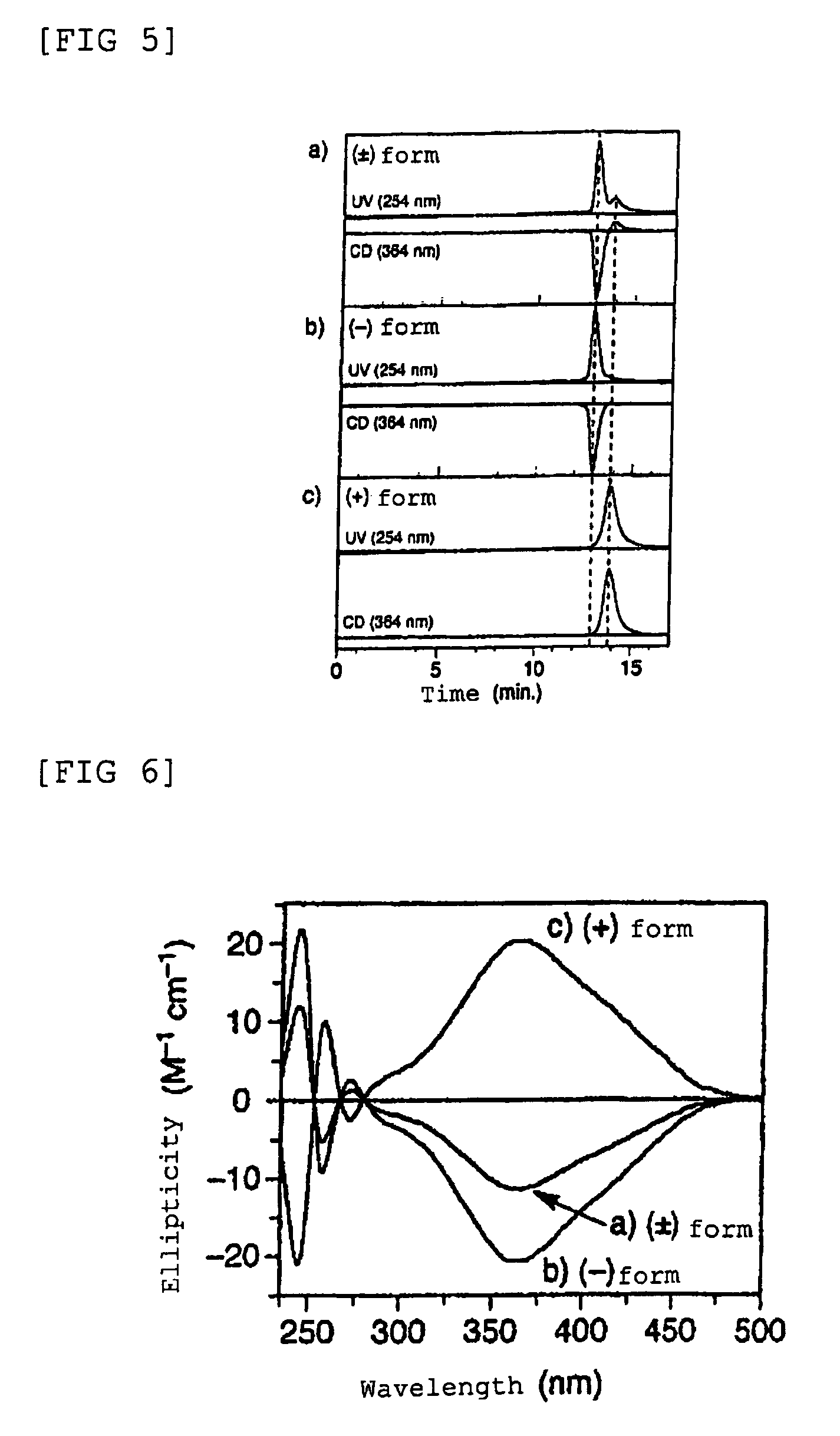
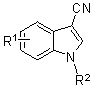
Optically-active helix chain poly(phenyl isocyanide) and polymerization method thereof
The invention discloses an optically-active helix chain poly(phenyl isocyanide) and a polymerization method thereof. The polymerization process is shown as the general formula in the description; in the formula, the structural formula of a chiral phosphine ligand is shown in the description. A palladium catalyst is adopted, the chiral phosphine ligand (S-or R-BINAP) is added to induce polymerization of achiral phenyl isocyanide, and the optically-active polymer with excessive one-handed helixes is obtained; obtained poly(phenyl isocyanide) has a high molecular weight and narrow molecular weight distribution.
Owner:HEFEI UNIV OF TECH
Organic fluorine-silicon light-cured resin and preparation method therefor and application thereof
ActiveCN105061770AReduce surface tensionImprove self-cleaning abilityAntifouling/underwater paintsPaints with biocidesPolymer scienceAcrylate ester
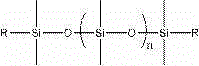
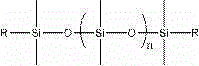
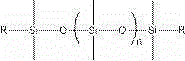
The invention relates to organic fluorine-silicon light-cured resin, the molecular weight of the organic fluorine-silicon light-cured resin is 2000-15000. The organic fluorine-silicon light-cured resin is prepared by the following steps: S1, putting hydroxylalkyl polysiloxane, diisocyanate and a catalyst in a reaction container, heating and stirring the mixture under nitrogen protection to 30-60 DEG C, and insulating the mixture to react for 1.0-3.0 hours; and S2, adding fluorocarbon resin into a polysiloxane prepolymer obtained in the S1 at 30-65 DEG C, and insulating the mixture to react for 1.0-5.0 hours; and then adding hydroxyl acrylate and a polymerization inhibitor and insulating the mixture to react for 1.0-5.0 hours to prepare the organic fluorine-silicon light-cured resin, wherein the dosage ratio of hydroxylalkyl polysiloxane and fluorocarbon resin is 0.1-2, and hydroxylalkyl polysiloxane has the structure shown in the formula, wherein the formula is shown in the description; the group R is -CH2OH, -CH2CH2OCH2OH, -CH2CH2CH2OCH2OH, -CH2CH2CH2OH or -CH2CH2CH2CH2OH and n is an integer from 0 to 100; the dosage of diisocyanate accounts for 10-30% of all components in total mass; the dosage of the hydroxyl acrylate accounts for 10-30% of all components in total mass.
Owner:GUANGDONG BOSSIN NOVEL MATERIALS TECH CO LTD
Polyisocyanide derivative having controlled helical main chain structure
InactiveUS7619109B2Easy to fixLow costOptically-active compound separationOrganic racemisationSingle typeBackbone chain
Owner:JAPAN SCI & TECH CORP
3-cyan substituted indole compound and synthetic method thereof
InactiveCN102718694AReduce usageRaw materials are easy to getOrganic chemistryCyanide compoundCombinatorial chemistry
The invention relates to a 3-cyan substituted indole compound and a synthetic method thereof. The constitutional formula of the compound is that R1 is a methoxy group, and R2 is phenyl (Ph), benzyl (Bn), allyl or n-butyl. According to the synthetic method of the 3-cyan substituted indole compound, raw materials are easy to obtain, novel tertiary butyl isonitrile is firstly used as a source of cyan, and high toxic metal cyanide is avoided to be used. During the reaction, conventional reaction solvents are used, the operation is simple, the operation condition is moderate, the reaction is environment-friendly, the top yield can reach 74%, and the 3-cyan substituted indole compound has a good application prospect in industrial production.
Owner:SHANGHAI UNIV
Aminodiacyloxylamide derivative, preparation method, and application thereof
ActiveCN106916081AEnhanced inhibitory effectReduce manufacturing costBiocideOrganic compound preparationCarboxylic acidEthyl Chloride
The invention provides an aminodiacyloxylamide derivative which has bactericidal activity and contains an amino group. The general formula of the aminodiacyloxylamide derivative is represented as (I), wherein R1 is a methyl group, an ethyl group and an isohexyl group; R2 is chlorine, an isooctyl group and an n-heptyl group; and R3 is hydrogen or chlorine. A preparation method includes the steps of: 1) performing a reaction to azide-carboxylic acid and keto-aldehyde and isonitrile at 30 DEG C, reaction solvent being water; 2) after the reaction is carried out for 24 h, adding triphenylphosphine, continuously performing the reaction for 2-5 h; and 3) after the reaction is finished, extracting the reaction liquid with dichloromethane, drying the reaction liquid and removing the solvent, dichloromethane, under reduced pressure, and performing column chromatography to the residual substance to produce the target compound (I). The compound has good inhibition activity on penicillium digitatum and penicillium italicum, and can be used as a bactericide.
Owner:CHINA THREE GORGES UNIV
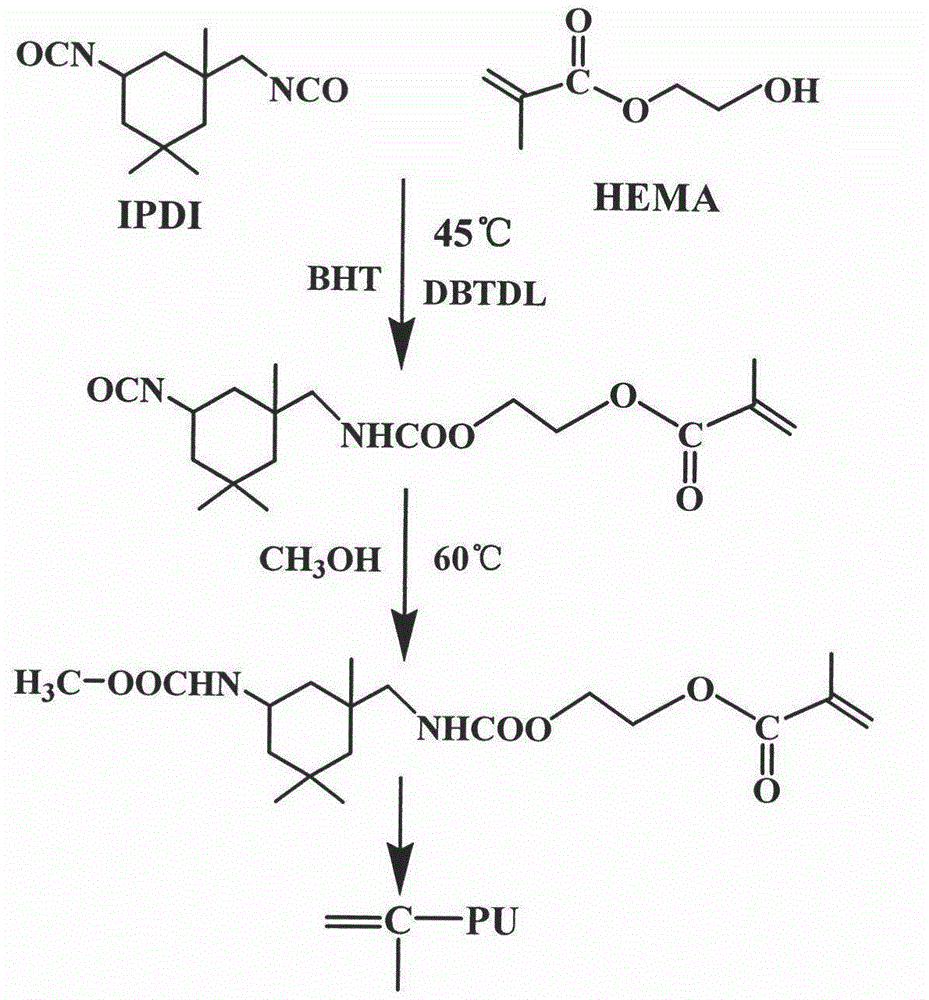
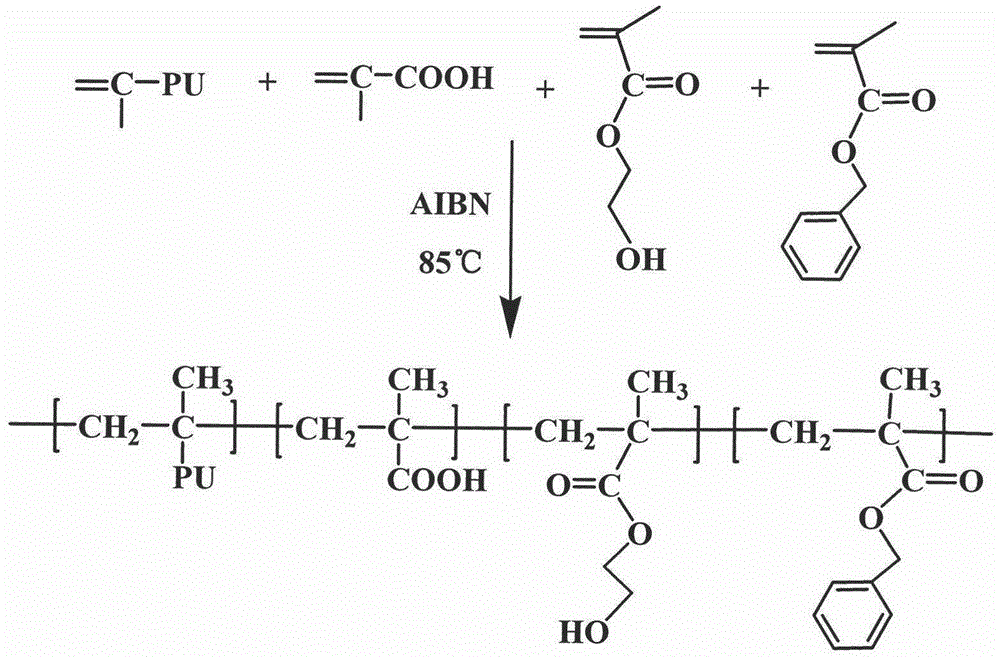
Urethane-acrylate copolymer and photoresist composition thereof
ActiveCN104817656AExcellent etch resistanceAdjustable acid valuePhotosensitive materials for photomechanical apparatusSolventPrepolymer
The invention provides a urethane-acrylate copolymer and a photoresist composition thereof and belongs to the field of photoresists. A hydroxyl-containing acrylate monomer and a diisocyanate compound undergo a reaction to produce an NCO-terminiated prepolymer, the NCO-terminiated prepolymer and saturated monobasic alcohol undergo an end-capping reaction to produce a urethane-acrylate polymer monomer containing tail end double bonds, and the urethane-acrylate polymer monomer undergoes a free radical copolymerization reaction to produce the urethane-acrylate copolymer. Through use of annular diisocyanate, photoresist corrosion resistance is improved. Through use of acrylic acid or methacrylic acid, the copolymer has the characteristics of adjustable acid value and dissoluble alkali lye. Through use of other rigid acrylate monomers, copolymer heat resistance and hardness are improved. Through use of the urethane acrylate and acrylic acid monomers, the copolymer has excellent adhesion. The photoresist is prepared by mixing the resin, an active diluent, a solvent, a photoinitiator and a pigment, can produce images with good comprehensive properties and high resolution and can be used in the field of photoresists.
Owner:SUZHOU RUIHONG ELECTRONIC CHEM CO LTD +1
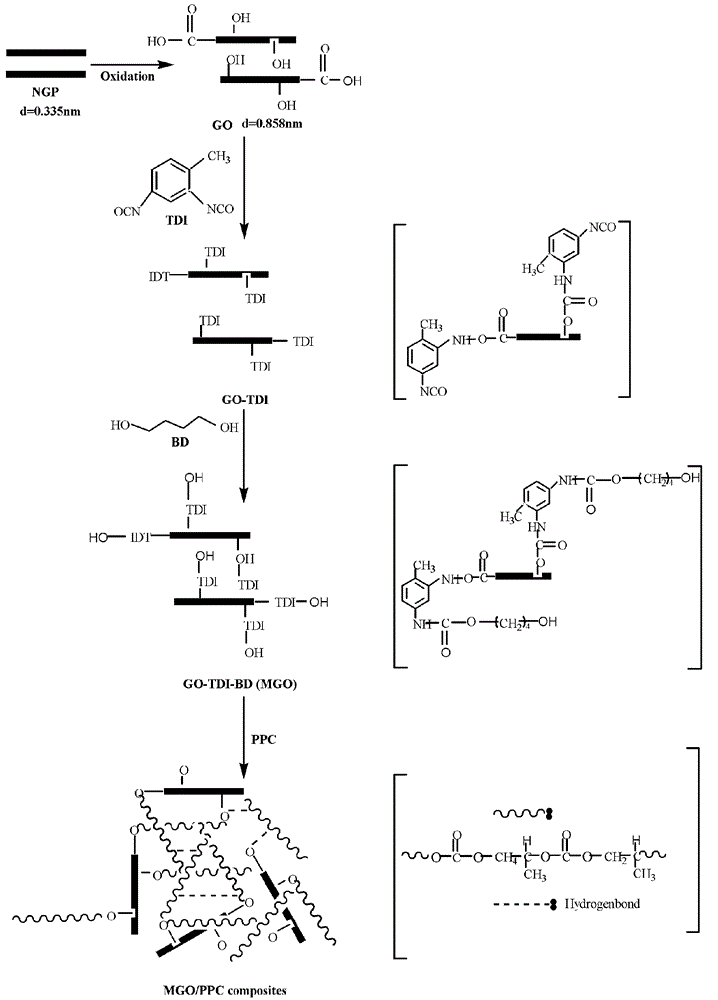
Preparation process of novel poly propylene carbonate nano-composite
The invention discloses a preparation process of a novel poly propylene carbonate (PPC) nano-composite. The composite is prepared by taking functionalized graphite and carbon nanotubes as packing, taking degradable PPC polymer as a matrix and then fusing or mixing in solution. The preparation process includes steps (1), synthesis and surface treatment of functionalized packing: oxidizing the packing by strong oxidizers and then enabling the packing to react with isocyanate and polyatomic alcohol to obtain the functionalized packing; and (2), preparation of PPC-matrix composite: mixing the functionalized packing with polymer in solution or by means of fusing. By the preparation process, dispersion effect of the packing can be improved, interface bonding strength of the packing and the matrix can be improved, and mechanical and thermal performance of the composition can be improved. The composite prepared by the preparation process is excellent in performance and low-cost and is a full-degradable environment-friendly material, and further, the applicable range of PPC is expanded effectively and good application prospect can be achieved.
Owner:XIHUA UNIV
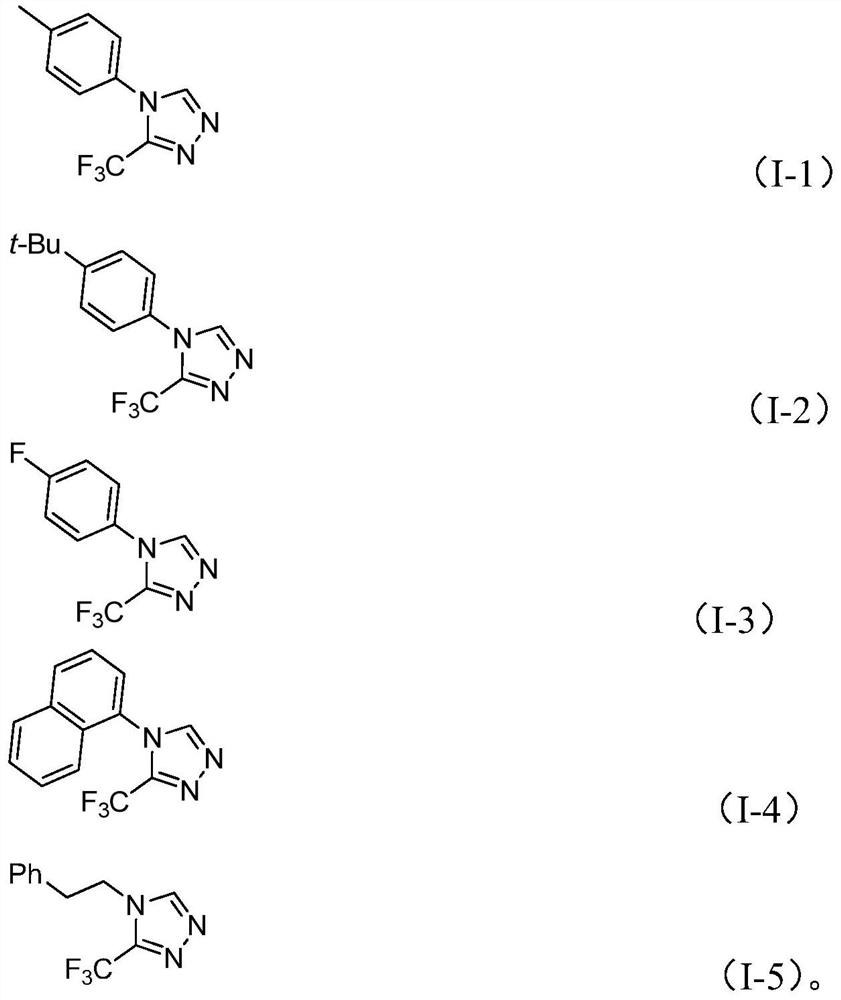
Preparation method of 3-trifluoromethyl substituted 1,2,4-triazole compound
InactiveCN113307778AStrong designabilityWide range of toleranceOrganic chemistryOrganosolvEthyl group
The invention discloses a preparation method of a 3-trifluoromethyl substituted 1,2,4-triazole compound. The preparation method comprises the following steps: adding molybdenum hexacarbonyl, cuprous acetate, triethylamine, a molecular sieve, trifluoroethylimidoacyl chloride and functionalized isonitrile (NIITP) into an organic solvent, conducting reacting at 70-90 DEG C for 18-30 hours, and after the reaction is completed, performing post-treatment to obtain the 3-trifluoromethyl substituted 1,2,4-triazole compound. The preparation method has the advantages of mild conditions, simple operation, cheap and easily available initial raw materials and high reaction efficiency, can be expanded to gram-level reaction, can synthesize trifluoromethyl-containing 1,2,4-triazole compounds substituted by different functional groups through substrate design, and is convenient to operate and widened in applicability.
Owner:HANGZHOU VOCATIONAL & TECHN COLLEGE
Ablative black film for directly making plate by flexographic plate computer and preparation method of ablative black film
ActiveCN102591137AGood film formingReduced laser ablation energyOriginals for photomechanical treatmentPhotosensitive materials for photomechanical apparatusPolymer sciencePolymer adhesive
The invention relates to an ablative black film for directly making a plate by a flexographic plate computer and a preparation method of the ablative black film. The ablative black film is characterized by comprising at least one type of nano carbon black particles, one infrared laser ablative dye and two polymer adhesives, a polyether polyurethane compound modified by the first polymer adhesive polyamide and the second polymer adhesive single isocyanate, and a super dispersing agent with the structural formula of R1-X-(CH2CH2O)p-(CHCH3CH2O)q-R2. The laser ablative black film prepared by the method has high sensitivity and good film forming property and is difficult to wrinkle.
Owner:LUCKY HUAGUANG GRAPHICS
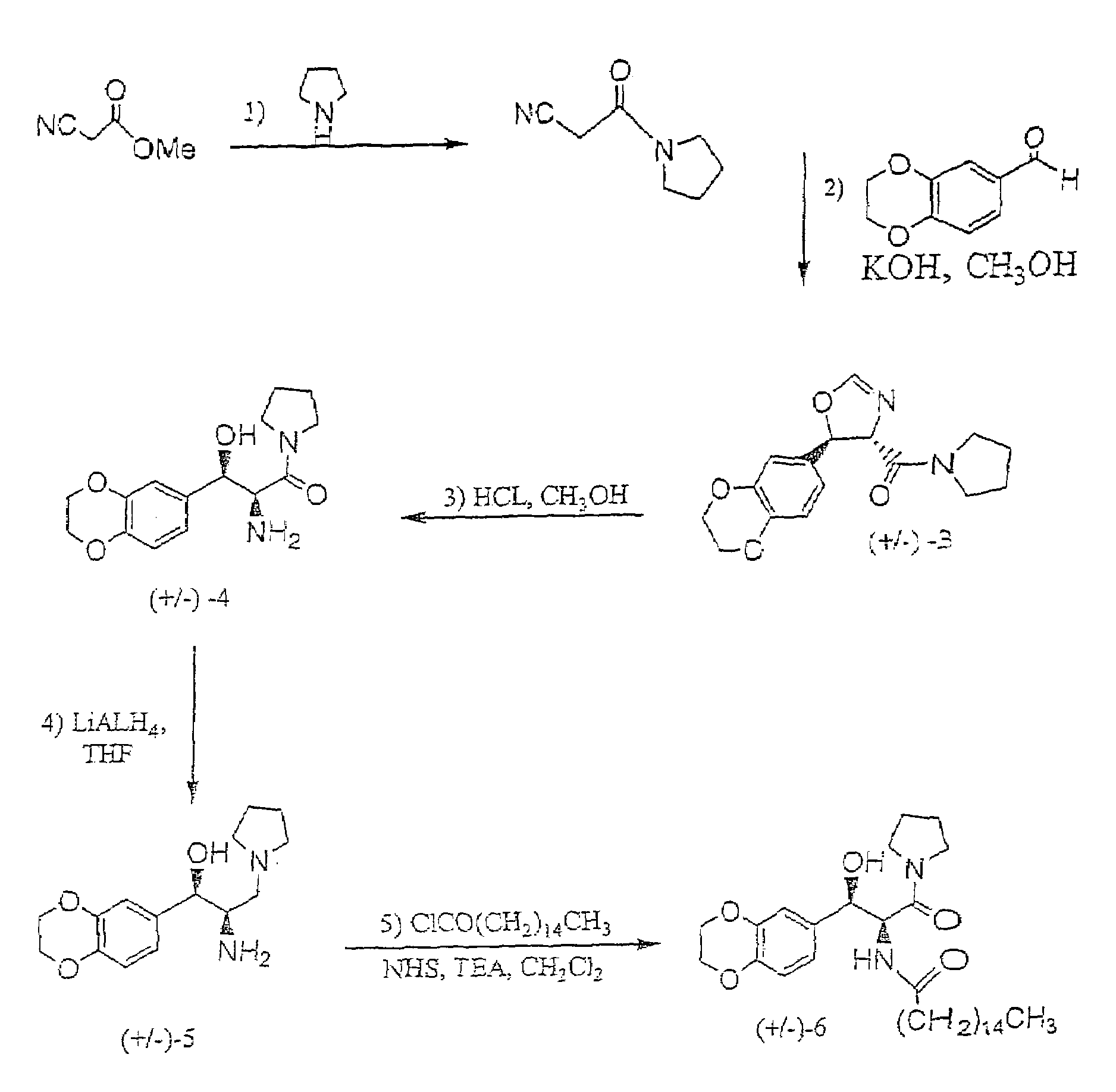
Diastereoselective synthesis of UDP-glucose: N-acylsphingosine glucosyltransferase inhibitors
InactiveUS7041831B2Efficient and highly diastereoselectiveBiocideOrganic chemistryN-Acyl SphingosineGlycosyltransferase inhibitor
Disclosed is a method of preparing a composition comprising a compound represented Structural Formula (I): The method comprises the step of reacting an aldehyde compound R10CHO with an isonitrile compound represented by Structural Formula (II):
Owner:GENZYME CORP
Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound
The invention relates to a simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound. The spiro[oxoindole-3,5'-oxazoline] heterocyclic compound in the invention has a structural formula as represented by Fig. I. According to the invention, isatin or substituted isatin is used as a raw material, DABCO is used as alkali, and tetrahydrofuran and water (in a ratio of 1:2) are used as a mixed solvent, and the spiro[oxoindole-3,5'-oxazoline] heterocyclic compound substituted by a plurality of functional groups can be obtained in one step through Aldol condensation reaction and a cyclization reaction among the above-mentioned raw materials and isonitrile (wherein, the weight ratio of isatin to isonitrile is 1.2:1). According to the method, the reactions are simple, high yield is obtained, and a water / organics solvent is used to substitute commonly used organic solvents, being in accordance with requirements of green chemistry. Furthermore, a product of chiral spiro[oxoindole-3,5'-oxazoline] with a high d.r value and ee value can be obtained by using a chiral catalyst. The method provided in the invention enables rapid and large-scale synthesis of a library of various substituted spiro[oxoindole-3,5'-oxazoline] heterocyclic compounds to be realized and allows discovery of drug lead compounds to be accelerated.
Owner:EAST CHINA UNIV OF SCI & TECH
Phosphorescent Osmium (II) complexes and uses thereof
ActiveUS20090058281A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds.Compounds of interest include:wherein A is Os or RuThe anionic chelating chromophores N̂N, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage.L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene.L can occupy either cis or trans orientation.When L occupies the trans position, the preferred structure contains both the hexagonal fragment of N̂N as well as its pentagonal fragment located at the trans position respect to their counterparts of the second N̂N chromophore.When L occupies the cis position, the preferred structure consists of the pentagonal unit of N̂N chromophores residing opposite to the L.X,1 X2 and X3 independently are C or N;when X2 is N, R1 is omitted,when X3 is N, R2 is omitted,R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl,R2 is H, F or cyano substituent,X4 is either C or N;X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4,R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:SAMSUNG DISPLAY CO LTD
Preparation method of cefcapene diisopropylamine salt
InactiveCN104072518ALow costThorough responseOrganic compound preparationAmino compound preparationChlorosulfuric acidSide chain
The invention relates to a preparation method of cefcapene diisopropylamine salt. The preparation method comprises the following steps: (1) obtaining acylate 3 by using cefcapene side chain acid 2 in the presence of an acylation reagent namely SOCl2 and an acid-binding agent namely triethylamine; (2) obtaining a condensation product 5 by using acylate 3 and a 7-HACA raw material 4 in the presence of triethylamine and a catalyst namely 4-dimethylaminopyridine; (3) enabling the condensation product 5 to react with chlorosulfonic acid isocyanate to obtain a 3-bit aminoacyl ester compound 6; (4) performing salt formation on the compound 6 and diisopropylamine to obtain cefcapene diisopropylamine salt 1. According to the preparation method disclosed by the invention, thionyl chloride is used as the acylation reagent, and triethylamine is used as the acid-binding agent so as to reduce the cost; 4-dimethylaminopyridine with the catalyst amount is added in the step (2) to ensure that the reaction is performed more completely and the reaction time is shortened; the preparation method is simple and convenient in whole route operation, and is convenient for large-scale production.
Owner:WEIHAI HAOTONG MEDICAL SCI & TECH
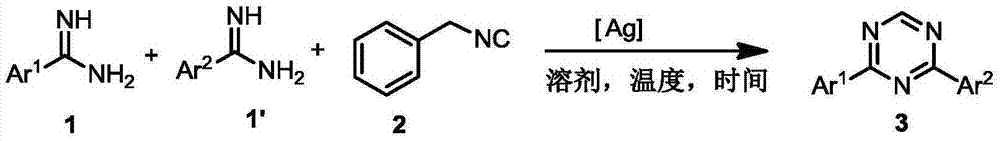
Preparation method for 2,4-disubstituted-1,3,5-triazine derivatives
ActiveCN106866563AEasy to synthesizeSimple reaction systemOrganic chemistryTriazine derivativeReaction system
The invention relates to a preparation method for symmetric and asymmetric 2,4-disubstituted-1,3,5-triazine derivatives. Specifically, substituted amidine reacts with benzyl isocyanide under the catalysis of a silver salt or copper salt so as to produce the symmetric and asymmetric 2,4-disubstituted-1,3,5-triazine derivatives. The method is simple in reaction system, mild in reaction condition and wide in substrate range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
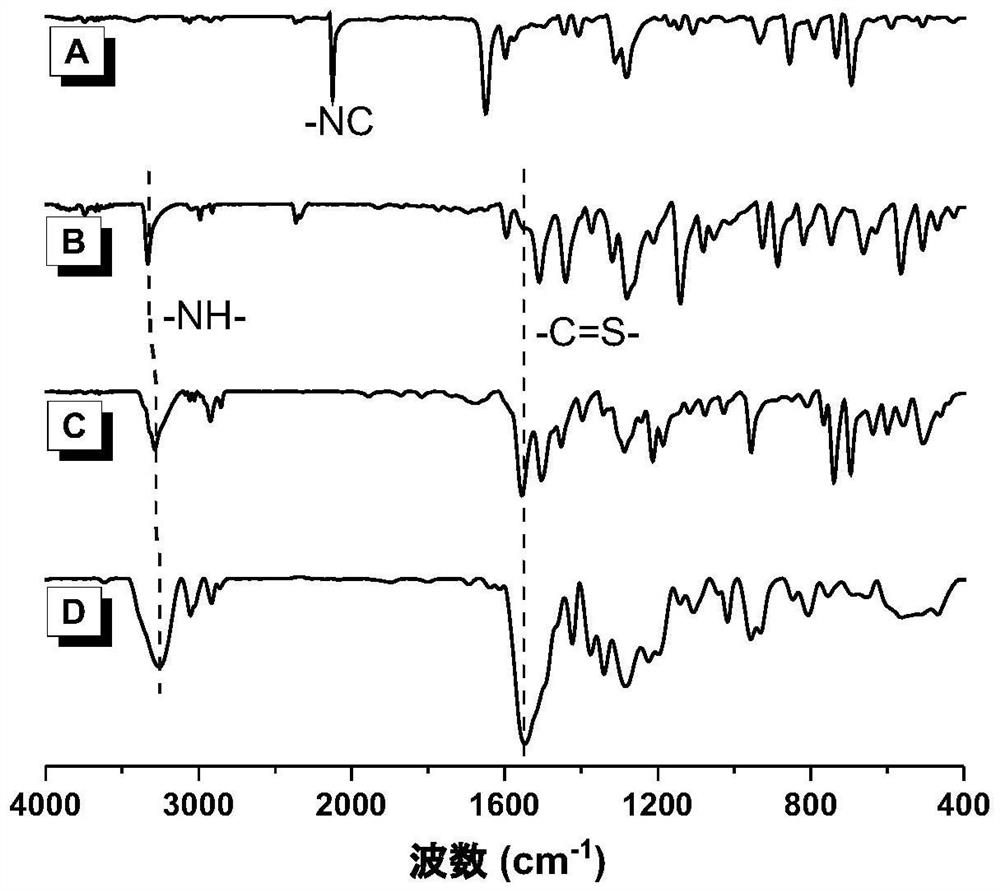
Polythiourea compound ands preparation method and application thereof
The invention discloses a polythiourea compound and a preparation method and application thereof. The method comprises the following steps: reacting an amine compound monomer, an isonitrile compound monomer and carbon disulfide in an organic solvent, and carrying out post-treatment to obtain the polythiourea. The monomers used in the invention are all industrial raw materials, the cost is low, and large-scale gram-level production can be carried out; the utilization rate of reaction atoms is 100%, and no toxic or harmful by-product is generated; the reaction can be carried out under the conditions of room temperature and air, the operation is simple, and the polymerization reaction yield is high; the product is easy to separate, and a polythiourea product and another thioformamide product can be obtained in one pot; symmetrical polythiourea, asymmetrical polythiourea with a controllable sequence and polythiourea with a disordered structure can be prepared by adjusting the feeding type, mode and proportion; the obtained series of polythiourea also has excellent self-repairing performance and luminescence performance, can be applied to the field of self-repairing and the field of photoelectric devices, and promotes further development of polythiourea compounds.
Owner:SOUTH CHINA UNIV OF TECH
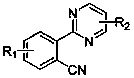
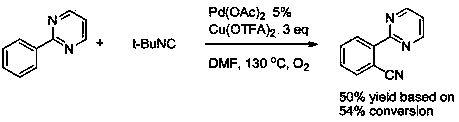
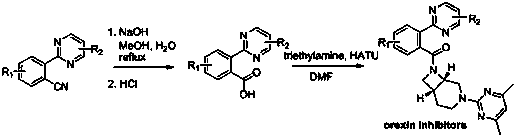
Aryl pyrimidine ortho-position monocyano compounds and synthesis method thereof
The invention relates to aryl pyrimidine ortho-position monocyano compounds and a synthesis method thereof. The structural formula of the compounds is shown in the specification, wherein R1=CH3, OCH3, Cl or OTs, and R2=H, C2H5 or Ph. The aryl pyrimidine ortho-position monocyano compounds provided by the invention are important intermediates for organic synthesis, and have potential pharmaceutical activity and material characteristics. The method has the advantages of accessible raw materials, conventional reaction solvent, moderate conditions and environment-friendly reaction, and is very simple to operate; the tert-butyl isonitrile used as the cyano source has the best reaction activity under catalytic action of rhodium; the maximum yield is up to 90%; and thus, the method has favorable development prospects in industrial production.
Owner:SHANGHAI UNIV
Continuous preparation method of bis(fluorosulfonyl)imide
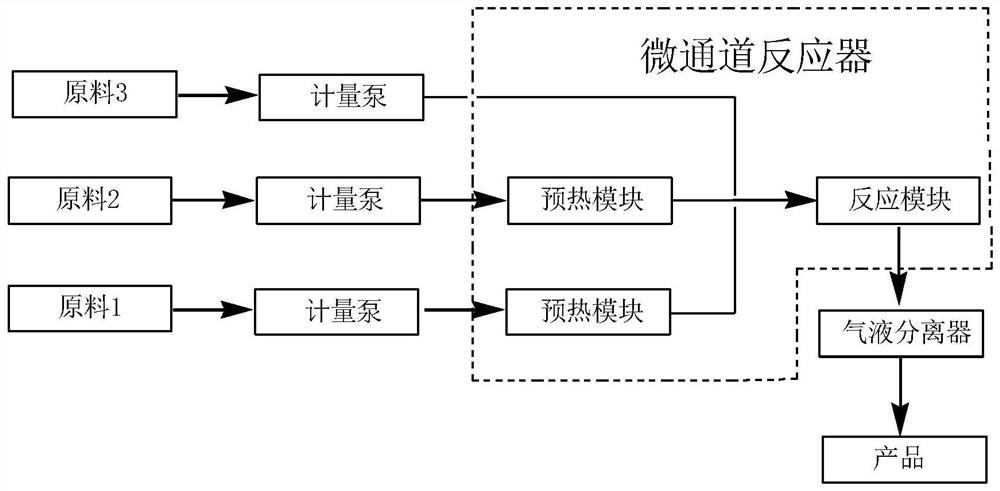
The invention discloses a continuous preparation method of bis(fluorosulfonyl)imide. The continuous preparation of bis(fluorosulfonyl)imide is carried out in a microchannel reactor, and comprises the following steps: A1, preheating a first raw material chlorosulfonic acid and a second raw material chlorosulfonyl isocyanate in a preheating module at the preheating temperature of 30-150 DEG C; A2, feeding a third raw material hydrogen fluoride, the preheated first raw material chlorosulfonic acid and the preheated second raw material chlorosulfonyl isocyanate into a reaction module for mixed reaction, wherein the molar ratio of the hydrogen fluoride to the chlorosulfonic acid to the chlorosulfonyl isocyanate is (2-2.5):1:1, the flow rate of the chlorosulfonic acid is 1-100g / min, the flow rate of the chlorosulfonyl isocyanate is 1-100g / min, the reaction temperature is 30-150 DEG C, the reaction pressure is 0-0.5 MPa, and the retention time is 1-100s; and A3, carrying out gas-liquid separation on the product at the outlet of the reaction module to obtain bis(fluorosulfonyl)imide. The method has the advantages of simple process, low equipment loss, high yield and the like.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
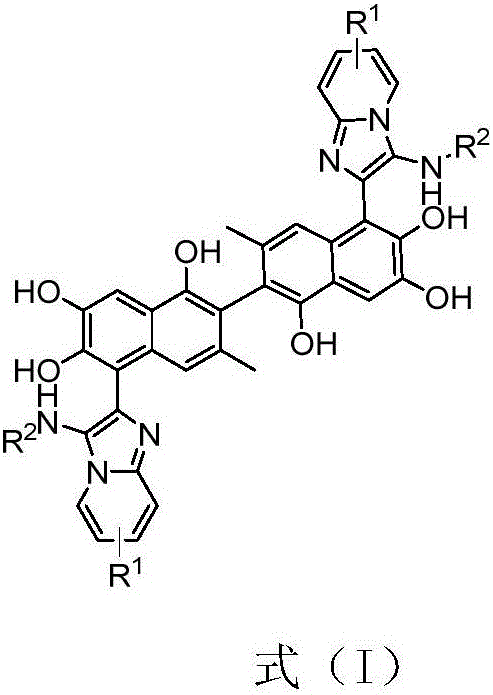
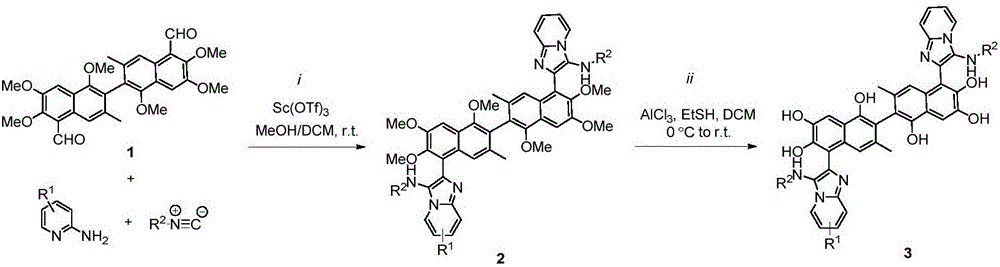
Apogossypol derivatives and preparation method thereof, and application of apogossypol derivatives in antitumor and immunoregulation
ActiveCN106632409AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryFiltrationOrganic synthesis
The invention belongs to the technical field of organic synthesis, particularly discloses a preparation method and application of apogossypol derivatives. The preparation method comprises the following steps: stirring apogossypol and isonitrile used as raw materials in a polar solvent at room temperature, and carrying out column chromatography separation to obtain a reaction product which is used as the raw material for the next reaction; under waterless oxygen-free conditions, adding ethanethiol into anhydrous aluminum chloride, cooling to 0 DEG C, dissolving the reaction product in the first step in anhydrous dichloromethane, and dropwisely adding into the solution; and after the reaction finishes, adding water to quench the reaction, regulating the pH value to 3-5, standing, carrying out vacuum filtration, washing the filter residue with dichloromethane, and carrying out vacuum drying, thereby obtaining the solid which is the target product. The multicomponent synthesis process centrally modifies the isopropyl of the apogossypol, thereby constructing series derivatives of apogossypol. The derivatives have favorable antitumor activity, have favorable inhibiting actions on IL-17A, and have wide application prospects.
Owner:江苏度未生物工程科技有限公司
2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof
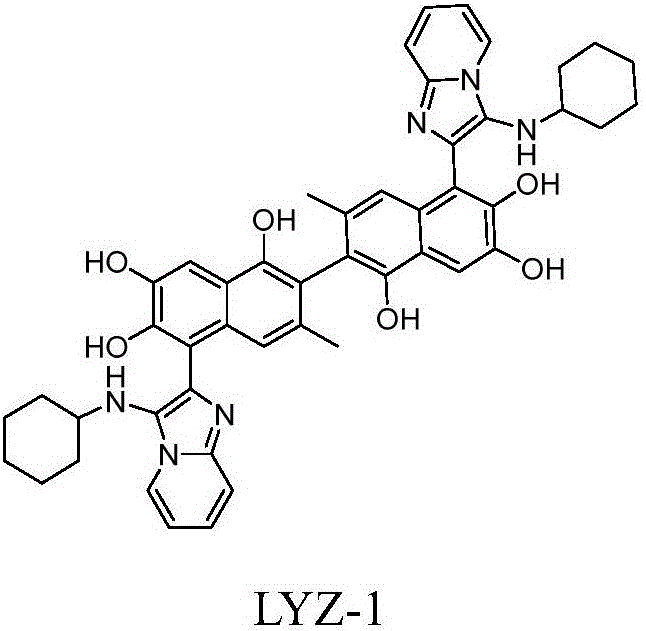
The invention discloses a 2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and a preparation method thereof. The preparation method comprises the following steps: adding 2-phenyl-imidazo [1,2-a] pyridine compound and p-tolylsulfonylmethyl isocyanide into a reaction tube in an argon environment, adding ferric trichloride, then adding a green solvent, and reacting for 18 to 48 hours at 80 to 120 DEG C; and after the reaction, extracting, chromatographically separating, and drying to obtain a target product. In the reaction, cheap metal ion (III) is used as a catalyst, water and polyethylene glycol 400 are used as reaction solvents, the sulfonylmethylation reaction of the imidazo [1,2-a] pyridine compound and p-tolylsulfonylmethyl isocyanide is realized, sulfonyl of the obtained compound is used as a leaving group, and the further functional group reaction is realized. The method has important significance on the research and application of the sulfonylmethylation reaction of the imidazo [1,2-a] pyridine compound.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
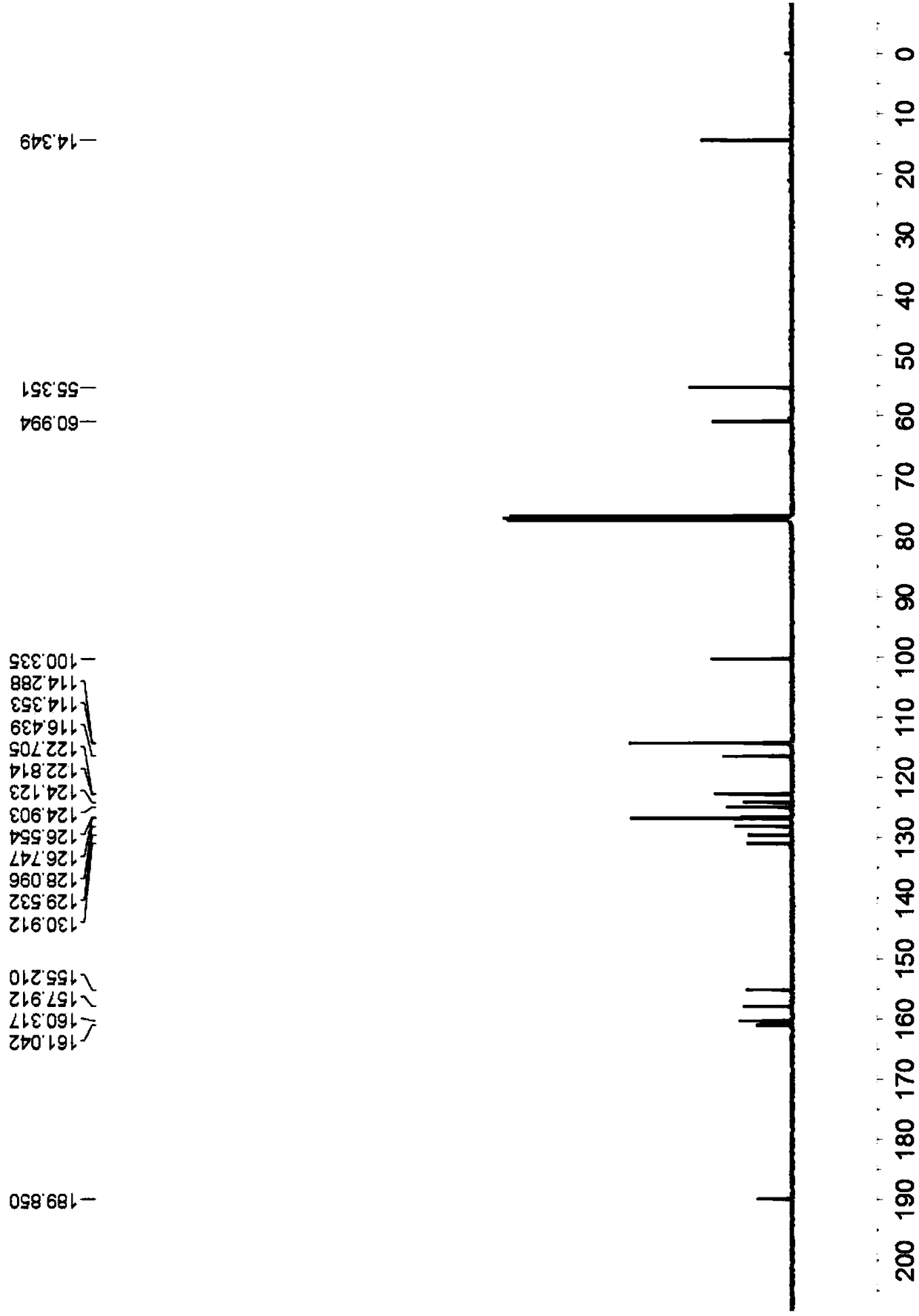
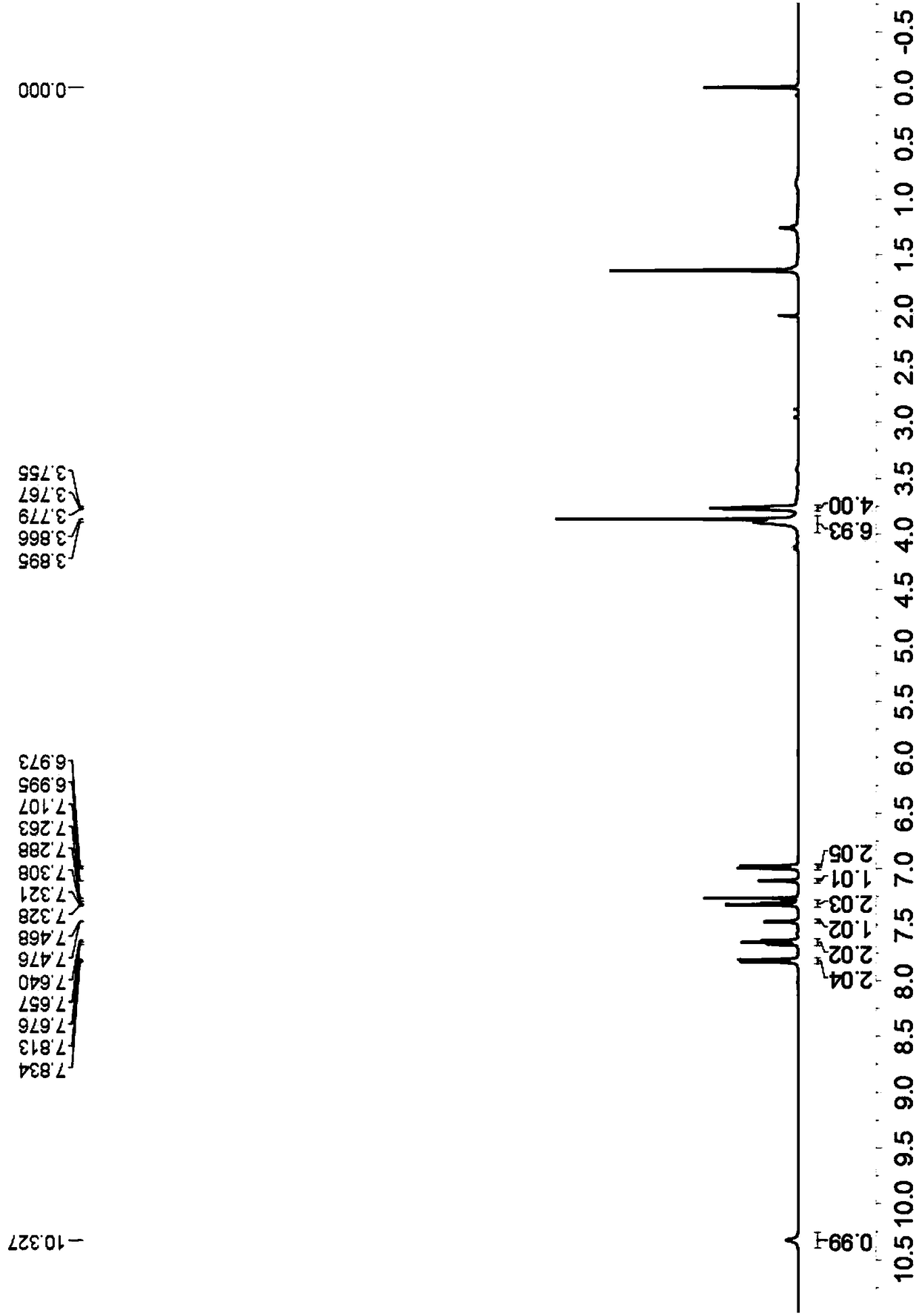
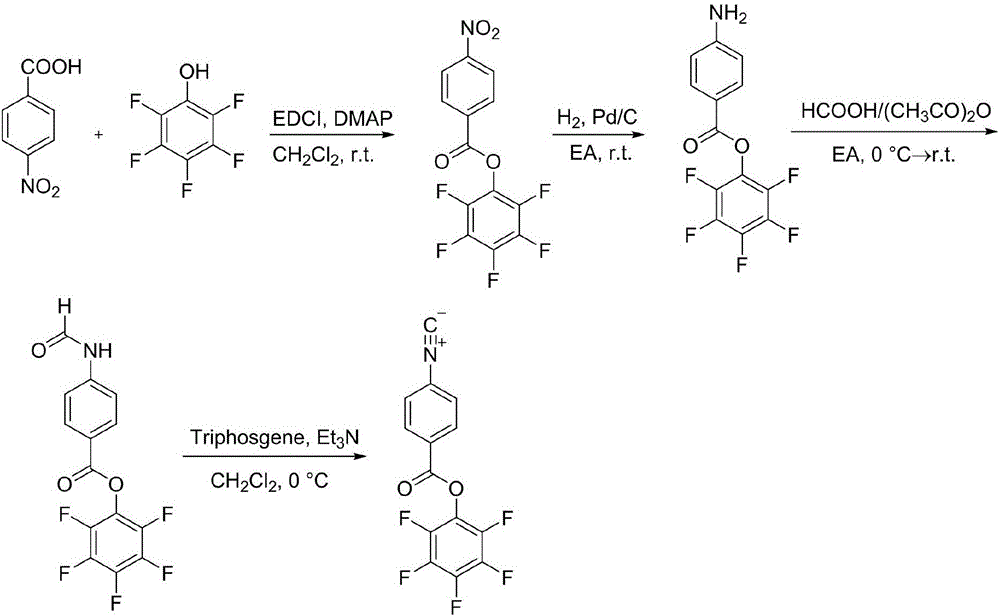
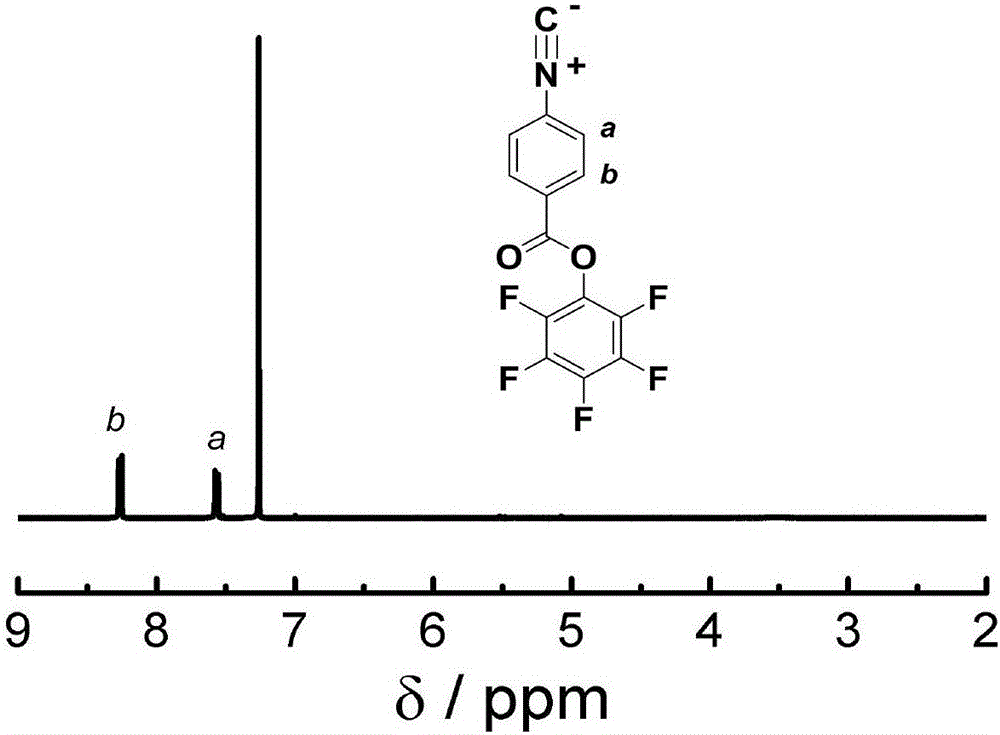



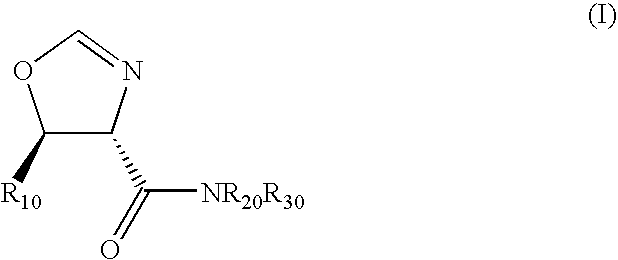
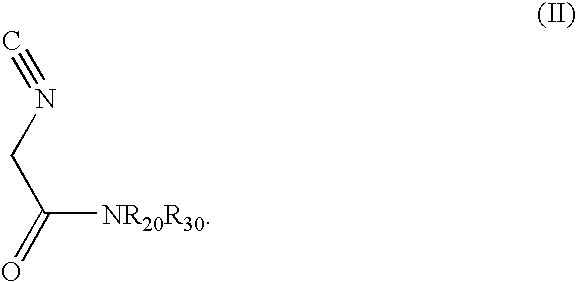
![Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound](https://images-eureka.patsnap.com/patent_img/5fad1adb-0495-47e7-aa6c-4aebe550e5c6/FSA00000531600400011.png)
![Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound](https://images-eureka.patsnap.com/patent_img/5fad1adb-0495-47e7-aa6c-4aebe550e5c6/FSA00000531600400012.png)
![Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound Simple and novel method for synthesizing spiro[oxoindole-3,5'-oxazoline] heterocyclic compound](https://images-eureka.patsnap.com/patent_img/5fad1adb-0495-47e7-aa6c-4aebe550e5c6/BSA00000531600500021.png)
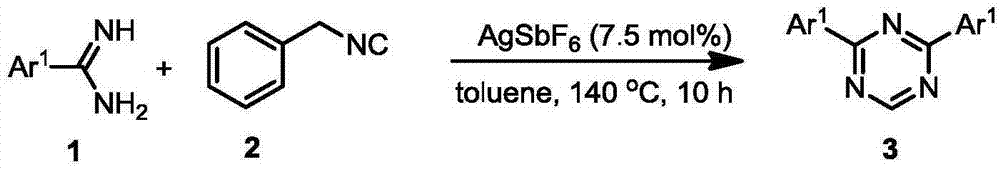
![2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof 2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/974c6cbf-ae85-4f66-bb5b-e1f15276c54d/BDA0001078459480000021.png)
![2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof 2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/974c6cbf-ae85-4f66-bb5b-e1f15276c54d/BDA0001078459480000022.png)
![2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof 2-phenyl-3-(toluenesulfonylmethyl) imidazo[1,2-a] pyridine compound and synthetic method thereof](https://images-eureka.patsnap.com/patent_img/974c6cbf-ae85-4f66-bb5b-e1f15276c54d/BDA0001078459480000023.png)