Method for preparing benzofuran-pyrrole compounds by copper catalysis
A benzofuran and compound technology, applied in the field of fine chemical organic synthesis, can solve the problems of unfriendly environment, harsh reaction conditions, low product yield, etc., and achieve the effects of easy availability of raw materials and reagents, mild reaction conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of benzofuran-pyrrole derivative 3a
[0029]
[0030] DCE (4 mL), 1,6-diynol-3-phenol 1a (0.278 g, 1.0 mmol) and ethyl isocyanacetate 2a (0.16 mL, 1.5 mmol) were added to a 25 mL pressure-resistant tube with a magnetic stirring device ), add cuprous iodide (0.019g, 0.1mol) and stir evenly, then put it into an 80°C oil bath and continue stirring. TLC (developing agent is V 石油醚 :V 乙酸乙酯 =3:1) The detection substrate disappears, and the reaction ends. Pour the reaction solution into saturated brine (10mL), extract with dichloromethane (3×10mL), combine the organic phases, then backwash the organic phase with saturated sodium chloride water (3×10mL), pass through anhydrous calcium chloride Steps such as drying, suction filter, vacuum distillation obtain viscous solid, finally through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =3:1) A yellow solid was obtained, which was confirmed to be benzofuran-pyrrole derivative 3a by NMR a...
Embodiment 2
[0034] Replace 2a in Example 1 with 2b, and other conditions are the same as Example 1, and its yield is 86%.
[0035]
[0036] Spectrum analysis data 3b:
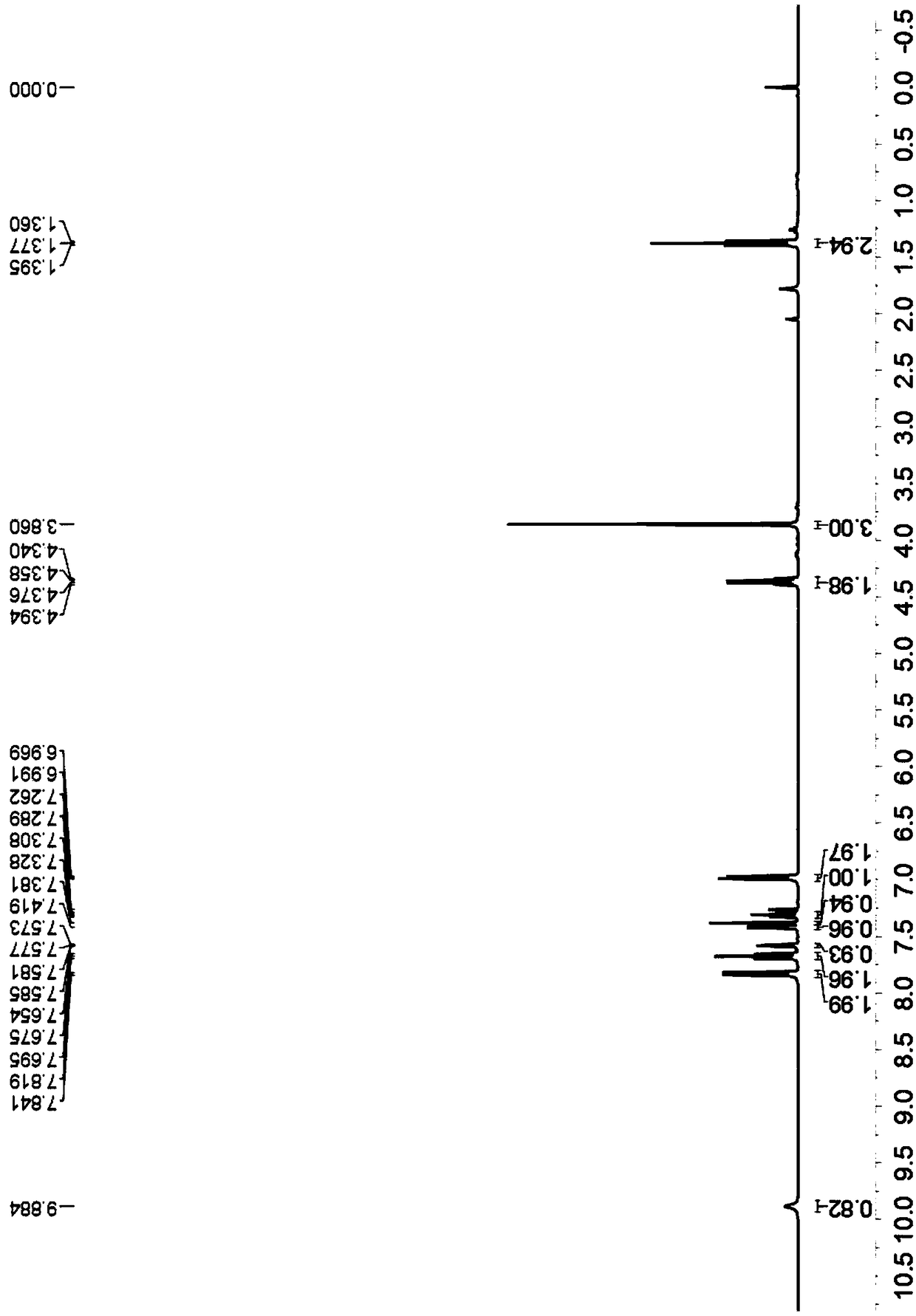
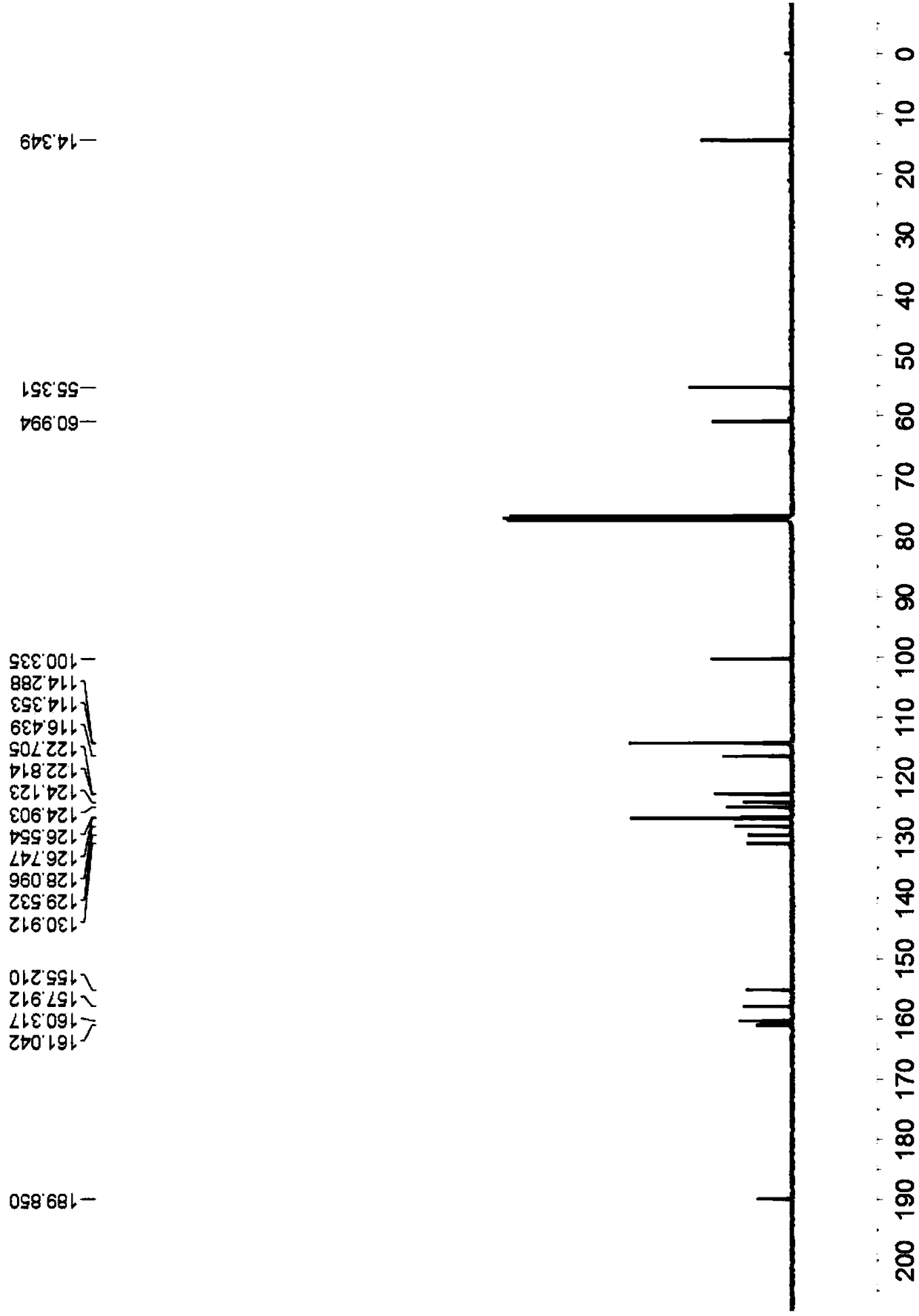
[0037] Yellow solid, melting point 249-250°C; 1 H-NMR (400MHz, CDCl 3 )δ10.32(s,1H),7.82(d,J=8.4Hz,2H),7.66(t,J=7.6Hz,2H),7.47(d,J=3.2Hz,2H),7.33-7.29( m, 2H), 7.11(s, 1H), 6.98(d, J=8.8Hz, 2H), 3.90-3.87(m, 7H), 3.78-3.76(m, 4H) (Figure 3); 13 C-NMR (CDCl 3 ( Figure 4 ); HRMS(ESI-TOF)m / zcalculated for C 25 h 23 N 2 o 5 [M+H] + :431.1596 found: 431.1607.
Embodiment 3
[0039] Replace 2a in example 1 with 2c, other conditions are the same as example 1, and its yield is 77%.
[0040]
[0041]Spectral analysis data 3c:
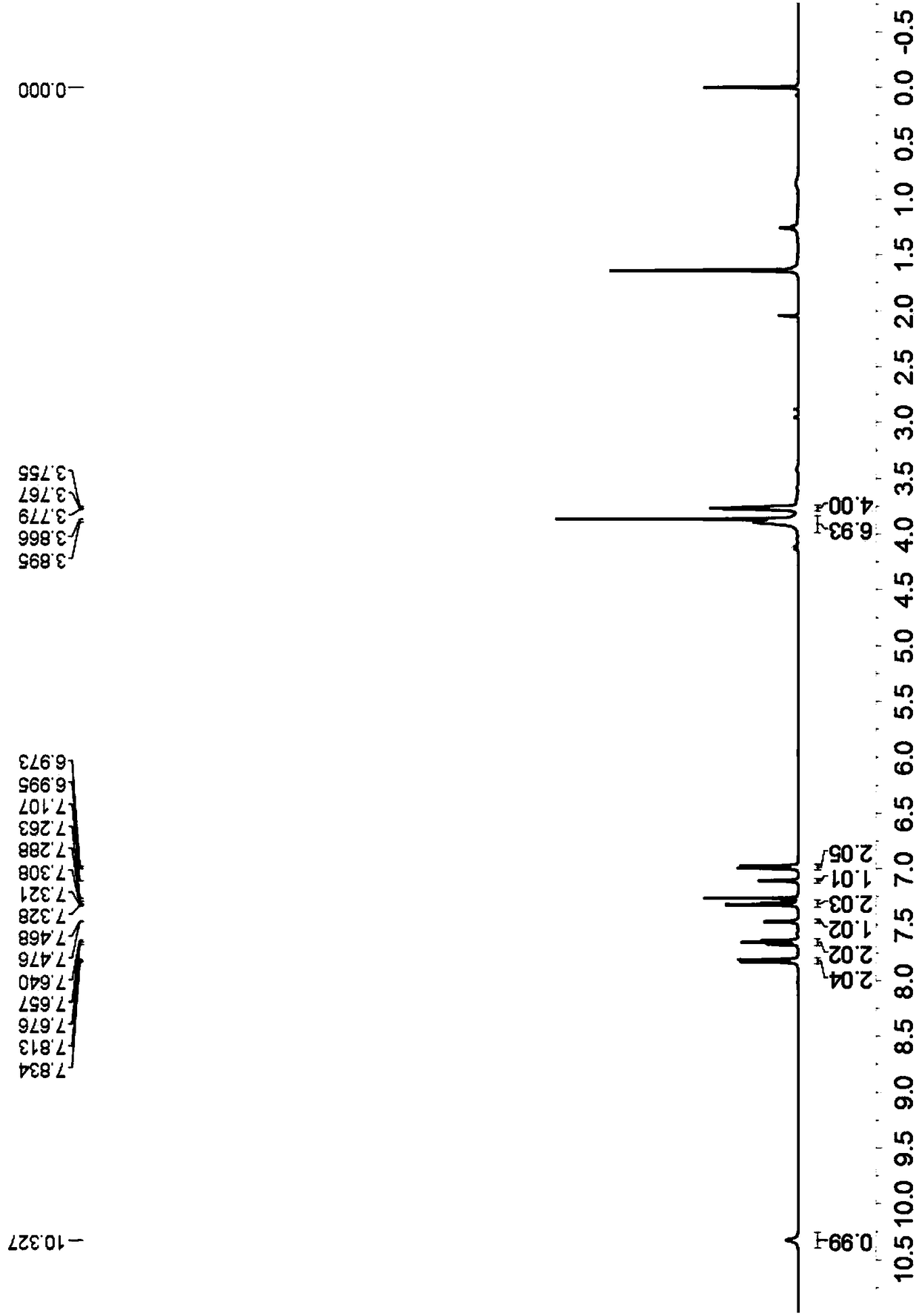
[0042] Yellow solid, melting point 264-266°C; 1 H-NMR (400MHz, CDCl 3 )δ10.54(s,1H),7.83(d,J=8.4Hz,2H),7.68-7.65(m,2H),7.50-7.49(d,J=2.4Hz,1H),7.33-7.29(m , 2H),7.20(s,1H),6.98(d,J=8.8Hz,2H),3.87(s,3H),3.83(t,J=6.8Hz,2H),3.70 (t,J=6.8Hz ,2H),2.11-2.04(m,2H),1.97-1.91(m,2H)( Figure 5 ); 13 C-NMR (CDCl 3 , 100MHz) δ190.2,160.3,159.8,157.7,155.2,131.5,129.4,127.4,127.3,126.7, 126.4,124.6,122.9,122.8,114.3,114.1,113.1,100.3,55.6,49.3,48. 6); HRMS (ESI-TOF) m / z calculated for C 25 h 23 N 2 o 4 [M+H] + :415.1652 found: 415.1661.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com