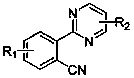
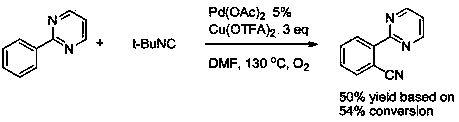
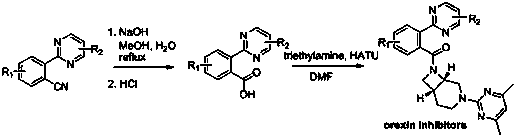
Aryl pyrimidine ortho-position monocyano compounds and synthesis method thereof
A technology of arylpyrimidine and compound, applied in the field of arylpyrimidine ortho-monocyano compound and its synthesis, can solve the problem that yield and conversion rate are not very high, arylpyrimidine ortho-monocyanation reaction has not been reported, etc. problems, to achieve the effect of moderate conditions, simple operation and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 2-(2-cyano-4-methoxyphenyl)pyrimidine
[0035] 2-(2-cyano-4-methoxyphenyl)pyrimidine adopts the following steps: 1. add 18.60 grams of 2-(4-methoxyphenyl)pyrimidine in 1000 milliliters of reactor, 1.23 grams of [RhCp * Cl 2 ] 2 , 2.75 grams of silver hexafluoroantimonate, 58.00 grams of copper trifluoroacetate, 16.60 grams of tert-butylisonitrile, 500 milliliters of 1,2-dichloroethane, heated to 130 ° C. Use thin layer chromatography to track the reaction until the raw materials disappear; ② After the reaction, add 3 M ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain 15.82 g of 2-(2-cyano-4-methoxyphenyl) with a yield of 75%. Melting point: 128-129°C.
[0036] –1 ): 3079, 2979, 2222, 1606, 1552, 1416, 1289, 1054, 889, 80...
Embodiment 2
[0041] Example 2: Preparation of 2-(2-cyano-4-chlorophenyl)pyrimidine
[0042] 2-(2-cyano-4-chlorophenyl)pyrimidine adopts the following steps: 1. add 15.24 grams of 2-(4-chlorophenyl)pyrimidine in 1000 milliliters of reactor, 0.49 grams of [RhCp * Cl 2 ] 2 , 1.10 g of silver hexafluoroantimonate, 46.40 g of copper trifluoroacetate, 13.28 g of tert-butylisonitrile, 400 ml of 1,2-dichloroethane, heated to 130°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add 3 M ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain 12.76 g of 2-(2-cyano-4-chlorophenyl) with a yield of 74%. Melting point: 212-214°C.
[0043] –1 ): 3083, 3032, 2231, 1573, 1415, 1383, 807, 634.
[0044] 1 H NMR (CDC...
Embodiment 3
[0048] Example 3: Preparation of 2-(2-cyano-4-p-toluenesulfonate phenyl)pyrimidine
[0049] 2-(2-cyano-4-p-toluenesulfonate phenyl) pyrimidine adopts the following steps: 1. add 22.82 grams of 2-(4-p-toluenesulfonate phenyl) pyrimidine in 1000 ml reactor, 0.861 g [RhCp * Cl 2 ] 2 , 1.93 g of silver hexafluoroantimonate, 60.90 g of copper trifluoroacetate, 17.43 g of tert-butylisonitrile, 350 ml of 1,2-dichloroethane, heated to 130°C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add 3 M ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③ The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain 20.64 g of 2-(2-cyano-4-p-toluenesulfonylphenyl) with a yield of 84%. Melting point: 135-137°C.
[0050] -1 3073, 3035, 2222, 1576, 1553, 1418, 1376, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com