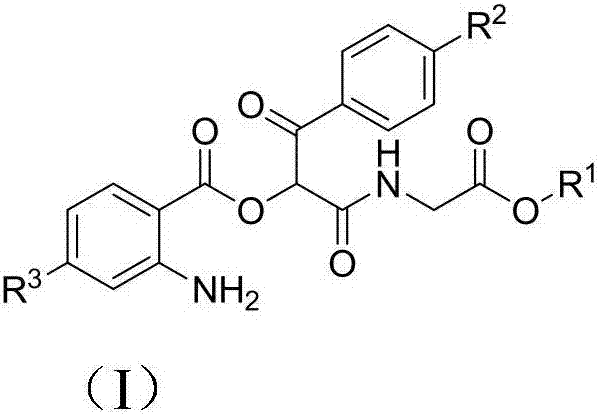
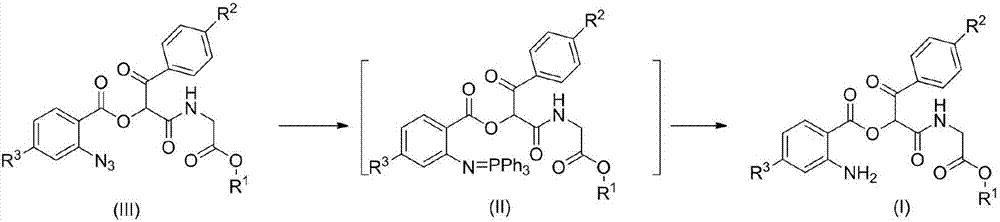
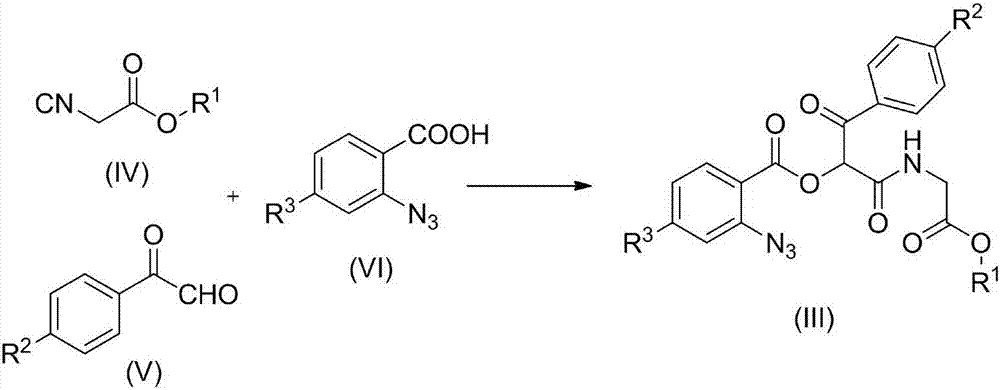
Aminodiacyloxylamide derivative, preparation method, and application thereof
A technology of aminobisacyloxyamide and derivatives is applied in the application field of active ingredients to achieve the effects of low preparation cost, simple operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] preparation of
[0024] Add 4-chloro-o-azidobenzoic acid (VI) (1mmol), p-chloroacetophenone aldehyde (V) (1mmol) and methyl acetate isonitrile (IV) (1mmol) to a 50mL flask, and react at 30°C , the reaction solvent is water, after reacting for 12 hours, add triphenylphosphine (1.2mmol), continue to stir at 30 ℃ for 3 hours, after the reaction is completed, the reaction solution is extracted with dichloromethane, dried, and then The solvent dichloromethane was removed, and the residue was subjected to column chromatography to obtain 0.264 g of white crystals, with a yield of 67%.
[0025] Elemental analysis: found value C% 51.73 H% 3.55 N% 7.40
[0026] Calculated value C% 51.59 H% 3.67 N% 6.38
[0027] 1 H NMR (CDCl 3 ,600MHz)δ(ppm)8.96(t,J=6.0Hz,1H,NH),8.11(d,J=8.4Hz,2H,Ar-H),8.04(d,J=8.8Hz,1H,Ar-H) H), 7.66(d, J=8.0Hz, 2H, Ar-H), 6.88(t, J=2.8Hz, 3H, NH 2 ,CH),6.67-6.60(m,2H,Ar-H),3.95(d,J=5.6Hz,2H,CH 2 ),3.61(s,3H,CH 3 ).
[0028] HRMS Calculated for [C 19...
Embodiment 2
[0030] preparation of
[0031] Add 4-chloro-o-azidobenzoic acid (VI) (1mmol), p-methylacetophenone aldehyde (V) (1mmol) and methyl acetate isonitrile (IV) (1mmol) in a 50mL flask, at 30°C Reaction, the reaction solvent is water, after 12 hours of reaction, add triphenylphosphine (1.2mmol), continue to stir at 30 ℃ for 3 hours, after the reaction is completed, the reaction solution is extracted with dichloromethane, dried, and then The dichloromethane was removed as the solvent, and the residue was subjected to column chromatography to obtain 0.260 g of white crystals, with a yield of 62%.
[0032] Elemental analysis: found value C% 57.59 H% 4.65 N% 6.80
[0033] Calculated C% 57.35 H% 4.57 N% 6.69
[0034] 1 H NMR (DMSO, 400MHz) δ (ppm) 9.06 (s, 1H, NH), 8.10 (t, J = 9.6Hz, 3H, Ar-H), 7.48 (d, J = 7.6Hz, 2H, Ar-H ),6.98(s,3H,NH 2 ,CH),6.72(s,2H,Ar-H),4.05(s,2H,CH 2 ),3.71(s,3H,CH 3 ),2.50(s,3H,CH 3 ).
[0035] HRMS Calculated for [C 20 h 19 ClN 2 o 6 +H]+:419.10...
Embodiment 3
[0037] preparation of
[0038] Add o-azidobenzoic acid (VI) (1mmol), p-isooctylacetophenone aldehyde (V) (1mmol) and ethyl acetate isonitrile (IV) (1mmol) in the 50mL flask, react at 30°C, The reaction solvent is water. After 12 hours of reaction, triphenylphosphine (1.2 mmol) was added and stirred for 3 hours at 30°C. After the reaction was completed, the reaction solution was extracted with dichloromethane, dried, and then removed under reduced pressure. The dichloromethane was removed from the solvent, and the residue was subjected to column chromatography to obtain 0.278 g of a light yellow oily liquid, with a yield of 56%.
[0039] Elemental Analysis: Found C% 67.93 H% 7.40 N% 5.77
[0040] Calculated value C% 67.72 H% 7.31 N% 5.64
[0041] 1 HNMR (CDCl 3 ,400MHz)δ(ppm)8.93(t,J=6.4Hz,1H,NH),8.13-6,69(m,11H,8Ar-H,NH 2 ,CH),4.09-0.80(m,24H,CH,7CH 2 ,3CH 3 ).
[0042] HRMS Calculated for [C 28 h 36 N 2 o 6 +H]+: 497.2652, Found: 497.2690.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com