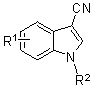
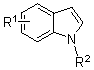
3-cyan substituted indole compound and synthetic method thereof
A technology of indole compound and cyano group substitution, which is applied in the direction of organic chemistry, can solve the problems of high substrate requirements, complicated reaction steps, and difficulty in obtaining 3-position cyanolated products, so as to avoid metal cyanides and conditions Moderate, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: 1-phenyl-1 H - Preparation of indole-3-cyano
[0045] 1-phenyl-1 H -Indole-3-cyano group adopts the following steps: 1. Add 9.7 grams of 1-phenyl-1 in a 250 ml round bottom flask H - Indole, 560 mg of palladium acetate, 43 g of additives, 12.5 g of tert-butylisonitrile, 150 ml of N,N-dimethylformamide, heated to 130°C. Track the reaction with thin layer chromatography, to the reaction raw material 1-phenyl-1 H -Indole disappears; 2. After the reaction is finished, add 3 N ammonia solution to the system, extract the product with ethyl acetate, remove the solvent with a rotary evaporator after drying, and obtain the crude product; 3. Column chromatography (petroleum ether) for the crude product : ethyl acetate = 6 : 1) purification to obtain 8.1 g of 1-phenyl-1 H -Indole-3-cyano, yield 74%. Melting point: 120-121°C.
[0046] IR (KBr, cm -1 ): 2223, 1599, 1539, 1501, 1458, 1224, 736, 694.
[0047] 1 H NMR (CDCl 3 , 500 MHz): δ = 7.85-7....
Embodiment 2
[0050] Example 2: 1-(4-fluoro-phenyl)-1 H - Preparation of indole-3-cyano
[0051] 1-(4-fluoro-phenyl)-1 H -Indole-3-cyano uses the following steps: ①Add 10.5 grams of 1-(4-fluoro-phenyl)-1 to a 250 ml round bottom flask H - Indole, 560 mg of palladium acetate, 43 g of additives, 12.5 g of tert-butylisonitrile, 150 ml of N,N-dimethylformamide, heated to 130°C. Track the reaction with thin layer chromatography until the reaction raw material 1-(4-fluoro-phenyl)-1 H -Indole disappears; 2. After the reaction is finished, add 3 N ammonia solution to the system, extract the product with ethyl acetate, remove the solvent with a rotary evaporator after drying, and obtain the crude product; 3. Column chromatography (petroleum ether) for the crude product : Ethyl acetate = 6: 1) purification to obtain 7.4 g of 1-(4-fluoro-phenyl)-1 H -Indole-3-cyano, yield 63%. Melting point: 164-167°C.
[0052] IR (KBr, cm -1 ): 2227, 1539, 1515, 1460, 1215, 837, 740.
[0053] 1 H N...
Embodiment 3
[0058] Example three: 1-(4-methoxy-phenyl)-1 H - Preparation of indole-3-cyano
[0059] 1-(4-Methoxy-phenyl)-1 H -Indole-3-cyano uses the following steps: ①Add 11.1 grams of 1-(4-methoxy-phenyl)-1 to a 250-ml round-bottomed flask H - Indole, 560 mg of palladium acetate, 43 g of additives, 12.5 g of tert-butylisonitrile, 150 ml of N,N-dimethylformamide, heated to 130°C. Track the reaction with thin-layer chromatography until the reaction raw material 1-(4-methoxy-phenyl)-1 H -Indole disappears; 2. After the reaction is finished, add 3 N ammonia solution to the system, extract the product with ethyl acetate, remove the solvent with a rotary evaporator after drying, and obtain the crude product; 3. Column chromatography (petroleum ether) for the crude product : Ethyl acetate = 6: 1) purification to obtain 4.6 grams of 1-(4-methoxy-phenyl)-1 H -Indole-3-cyano, yield 36%. Melting point: 126-129°C.
[0060] IR (KBr,cm –1 ): 2219, 1535, 1513, 1458, 1245, 1219, 1028, 836,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com