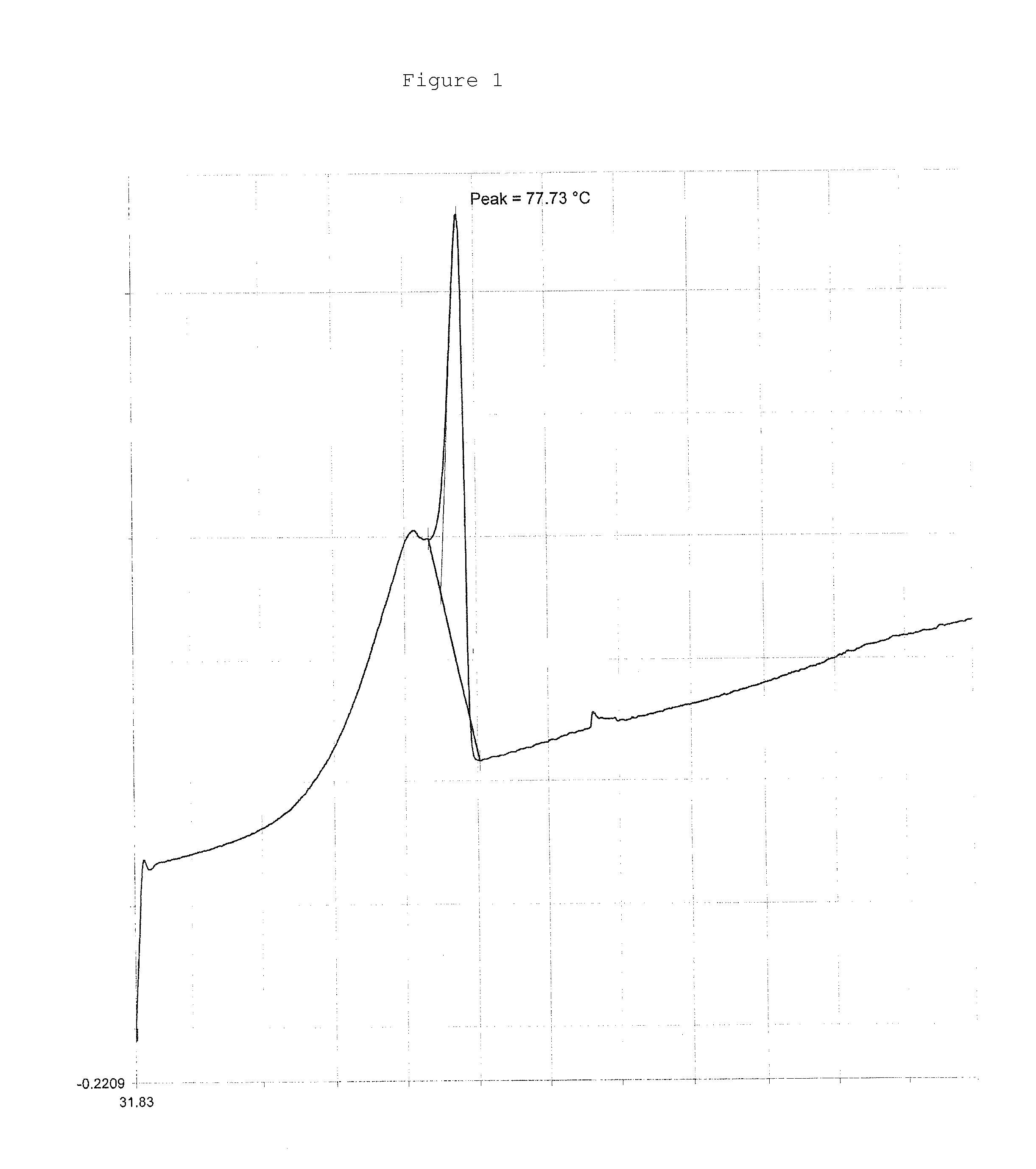
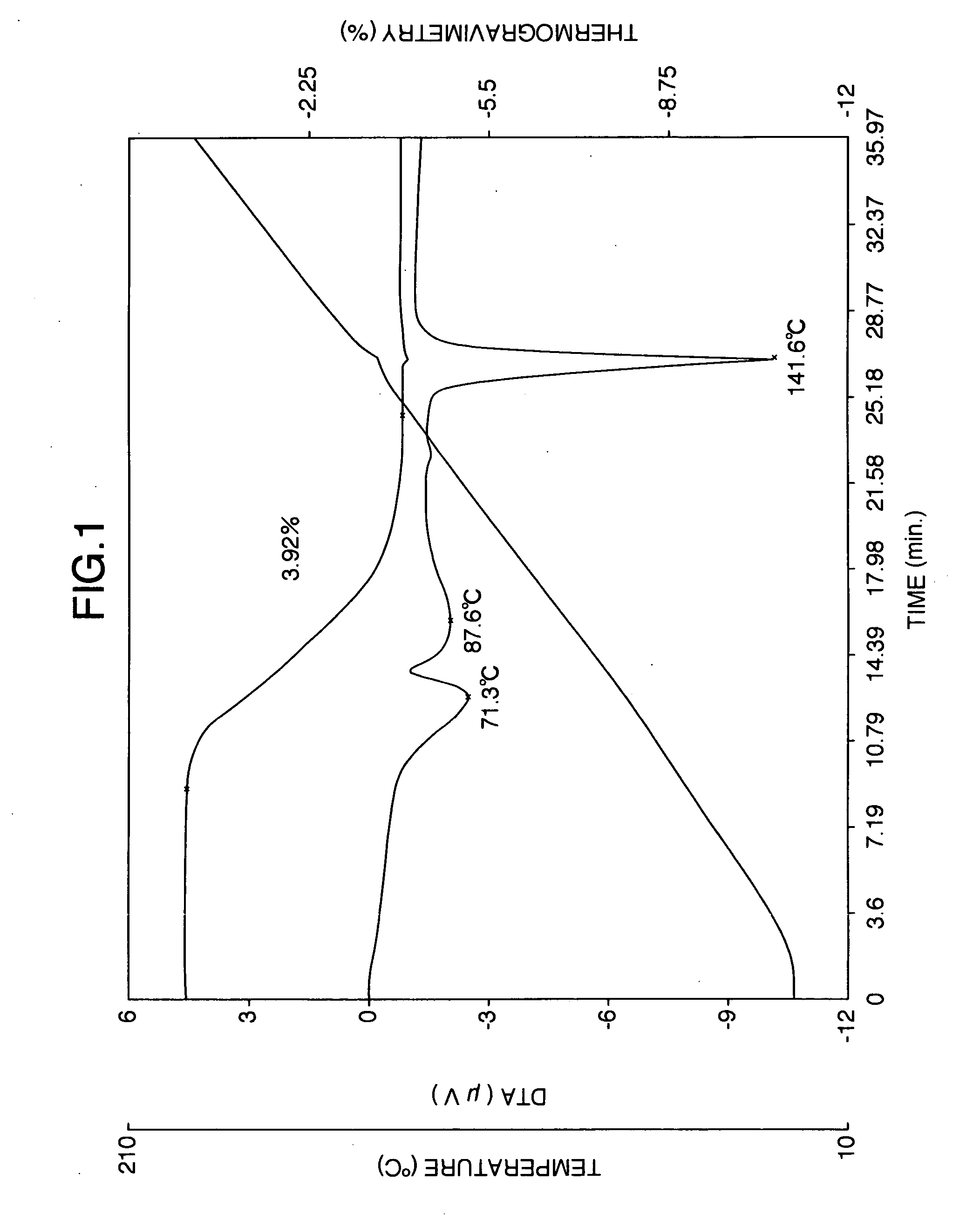
Patents
Literature
84 results about "Escitalopram" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
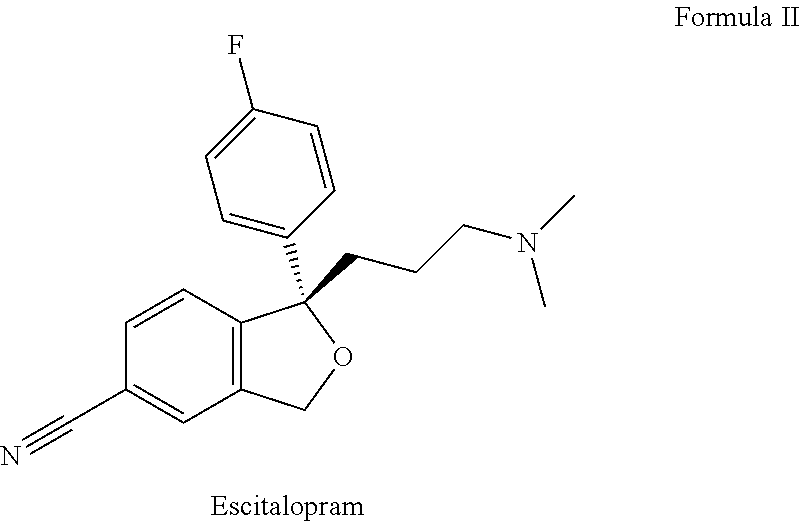
Escitalopram is used to treat depression and anxiety.
Dispersible tablet containing antipsychotic medicines and application thereof
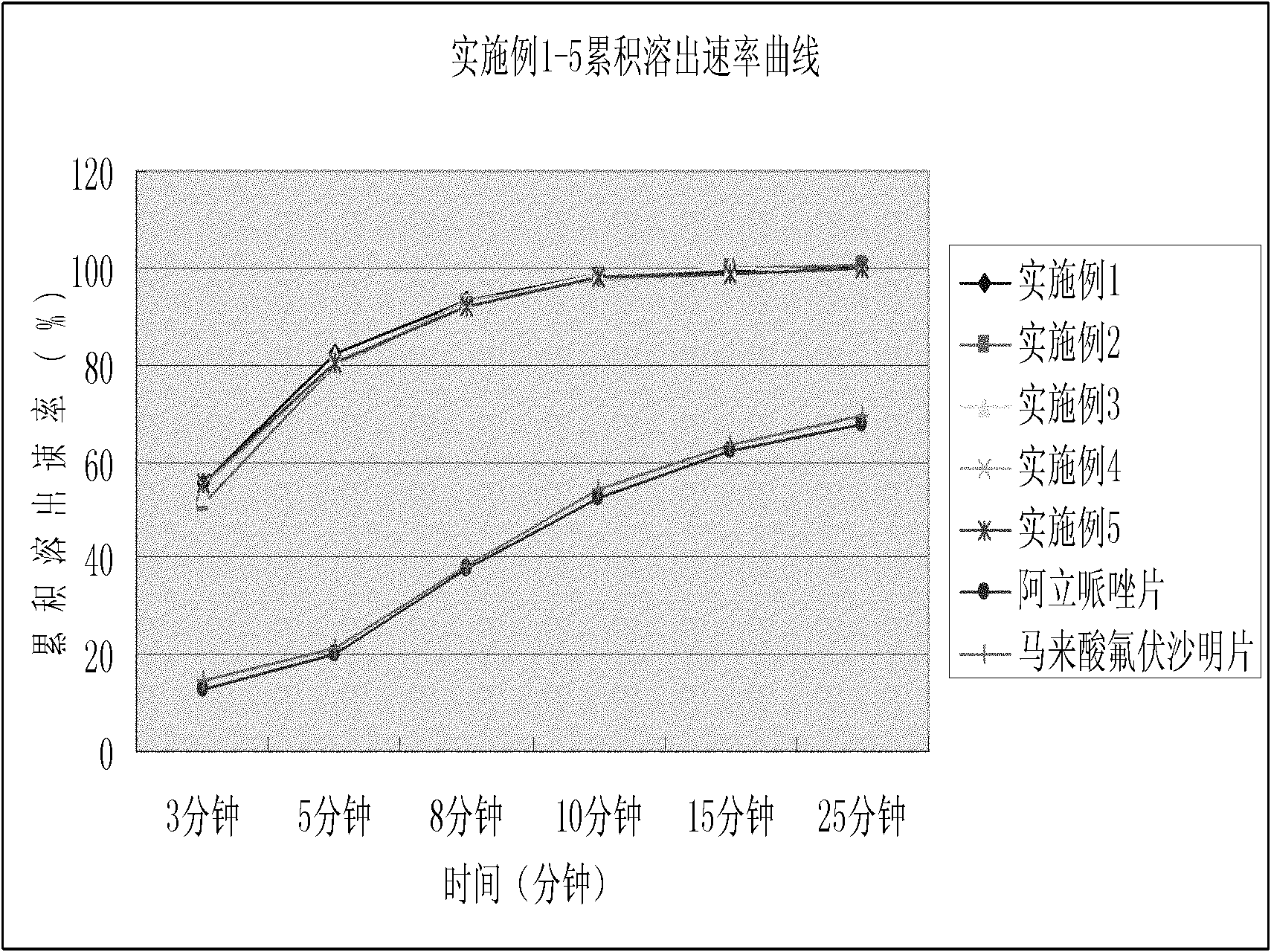
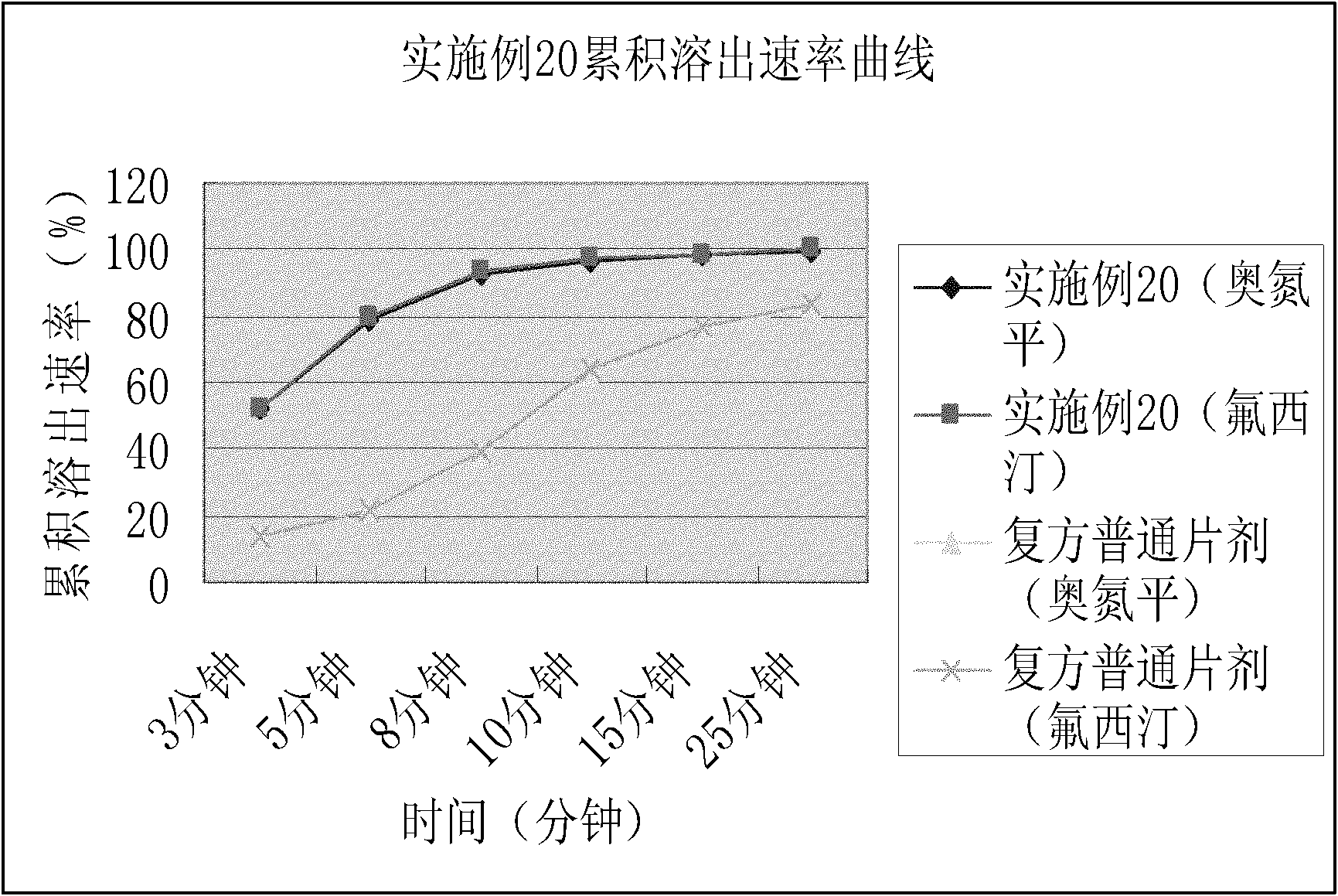
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
Combination of an NMDA receptor antagonist and a selective serotonin reuptake inhibitor for the treatment of depression and other mood disorders
The present invention provides a method for the treatment of depression, including treatment-resistant depression, and other mood disorders using a combination of an NMDA receptor antagonist and a SSRI that is citalopram or escitalopram. It has unexpectedly been shown that the combination has a synergistic and potentiated effect of either compound as monotherapy, resulting in an enhanced therapeutic effect at lower doses.
Owner:FOREST LAB HLDG LTD +1
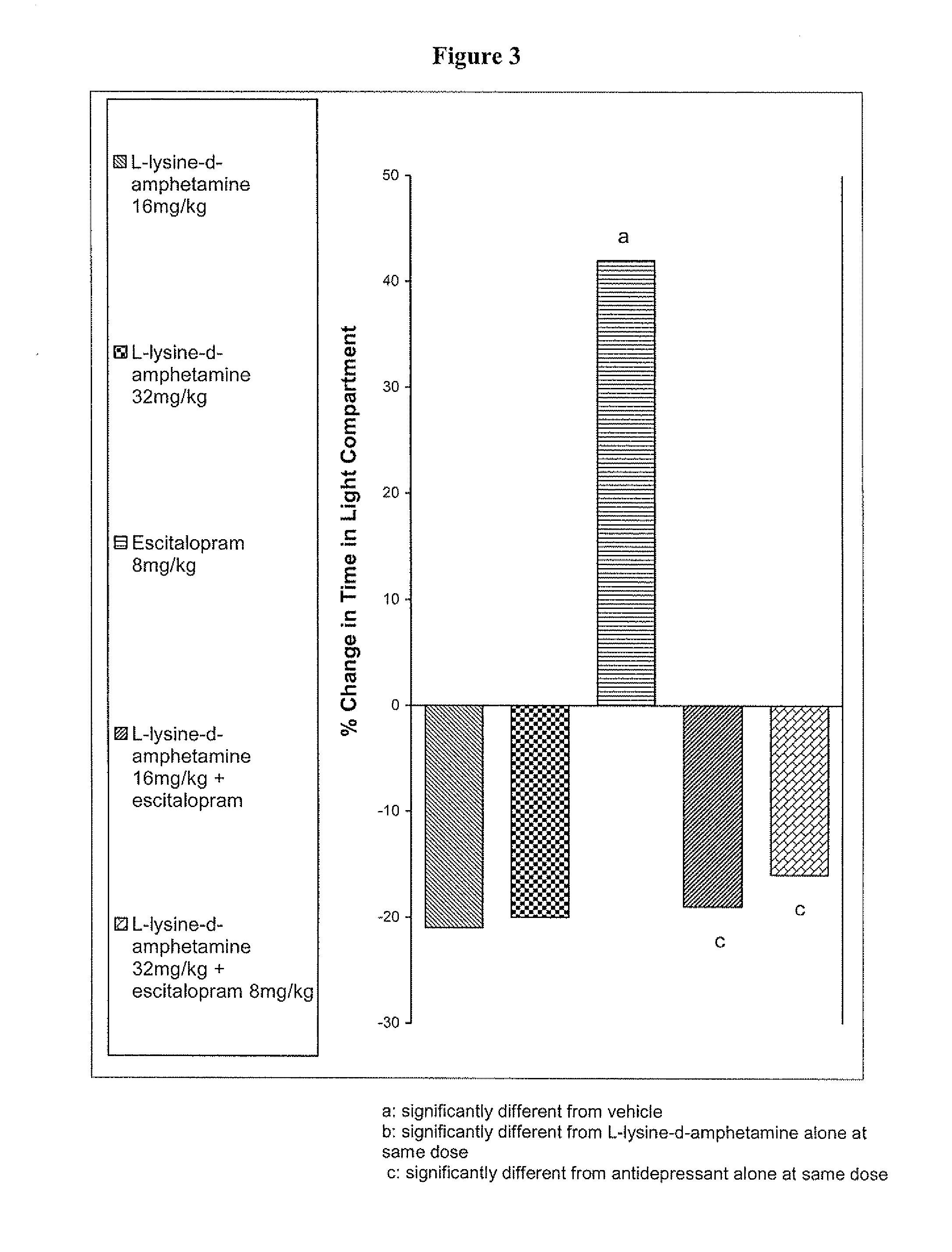
Methods of enhancing selective serotonin reuptake inhibitor effects in mammals
InactiveUS20100303903A1Convenient treatmentGood effectBiocideNervous disorderTreatment choicesEscitalopram
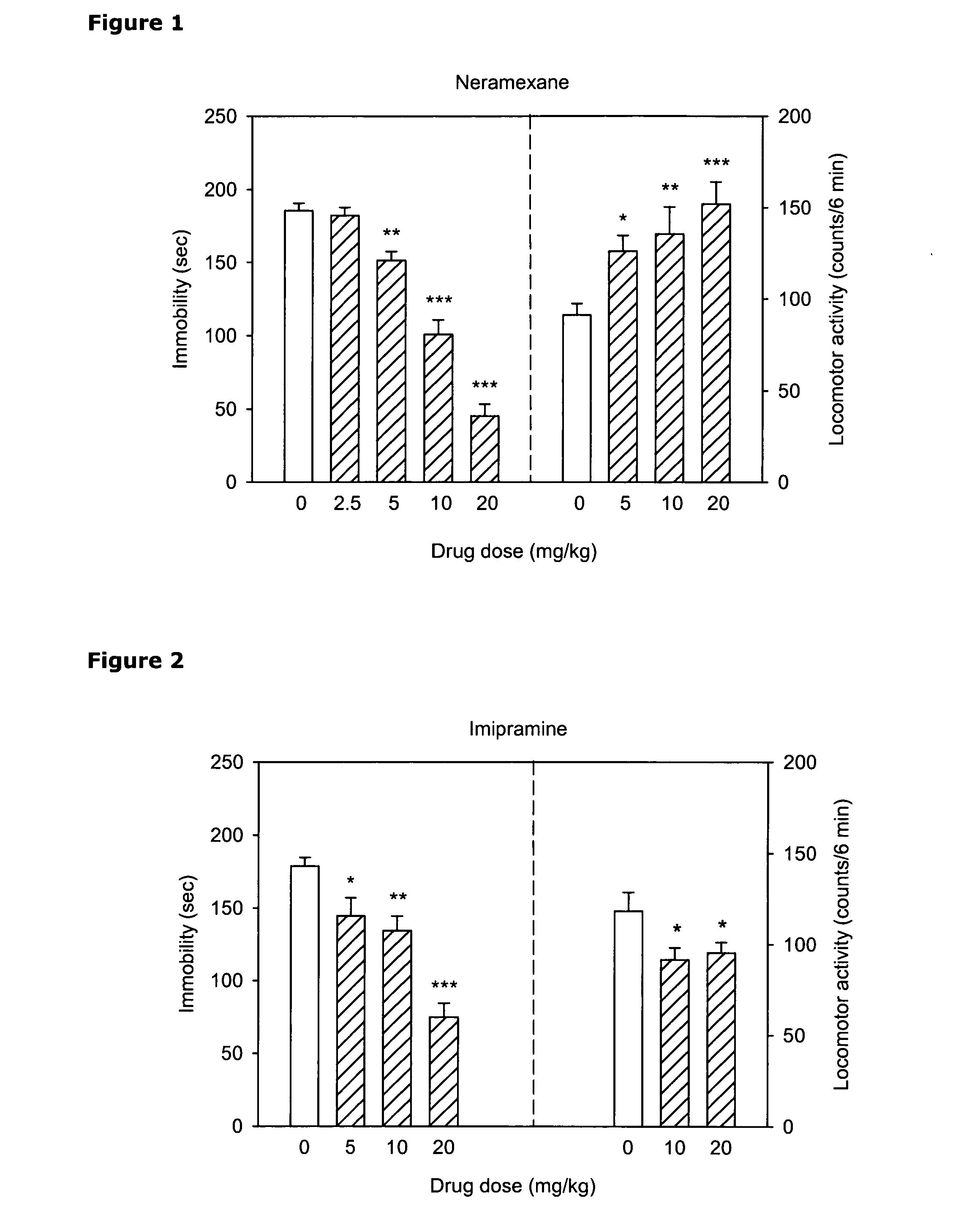
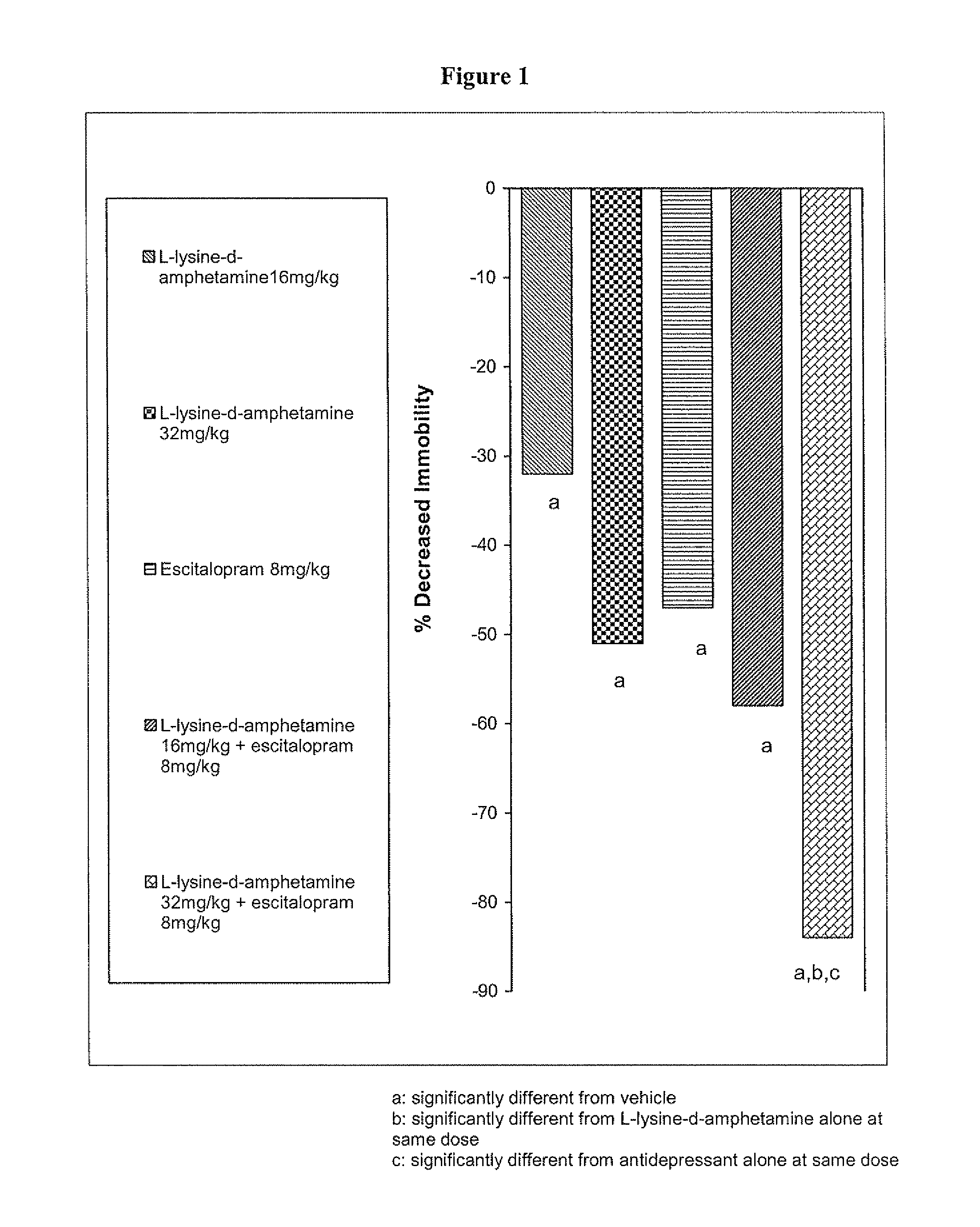
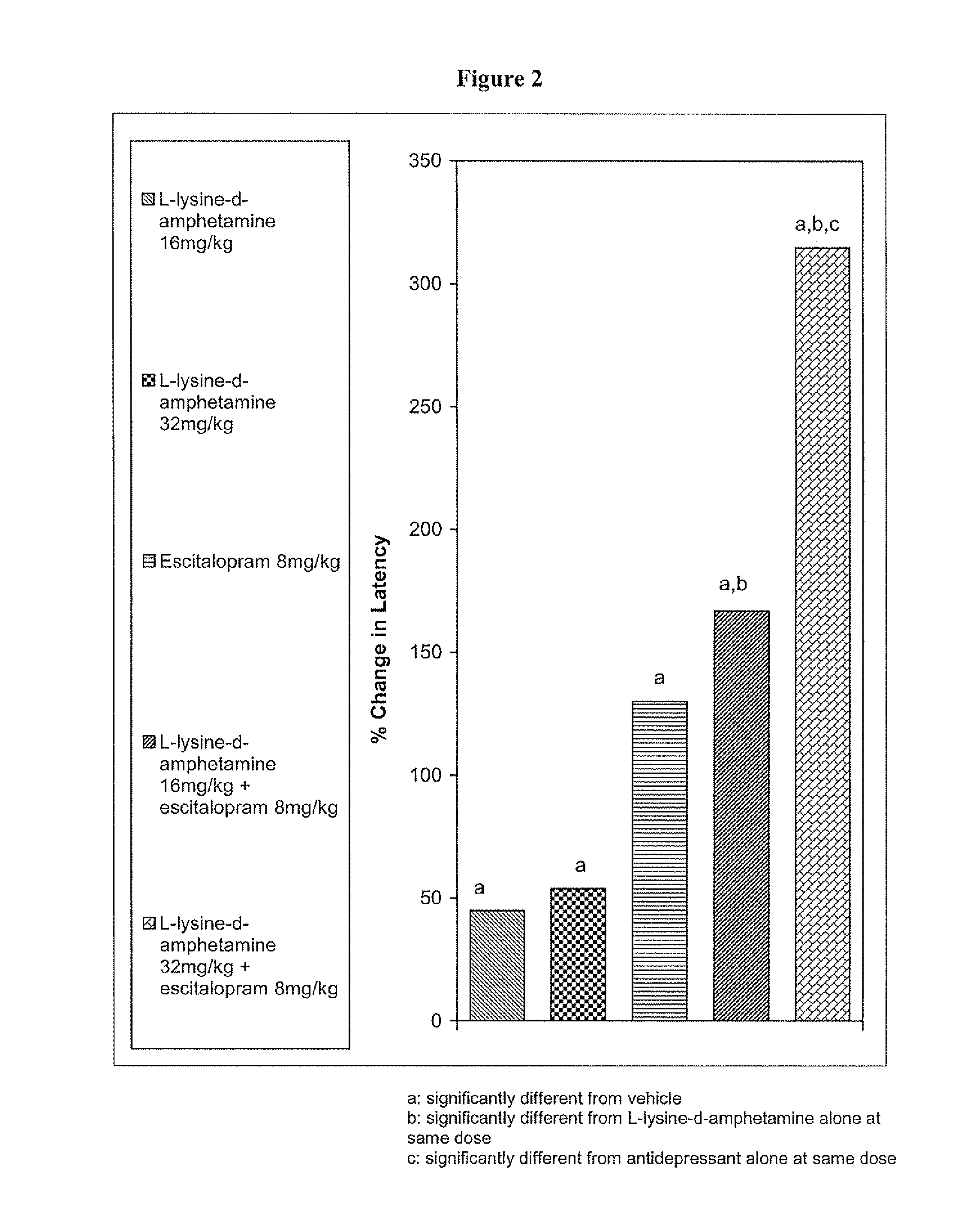
The invention relates to methods of enhancing selective serotonin reuptake inhibitor effects in mammals. In particular, the invention provides methods for treating selective serotonin reuptake inhibitor dependent conditions such as depression. More specifically, the present invention relates to a method of increasing the antidepressant activity of a selective serotonin reuptake inhibitor (“SSRI”) by administering L-lysine-d-amphetamine in combination with an SSRI and to formulates containing the same. In a preferred aspect, the combination is administered in connection with a method of treating depression. One preferred SSRI is escitalopram. The preferred amphetamine prodrug is L-lysine-d-amphetamine.
Owner:SHIRE PLC
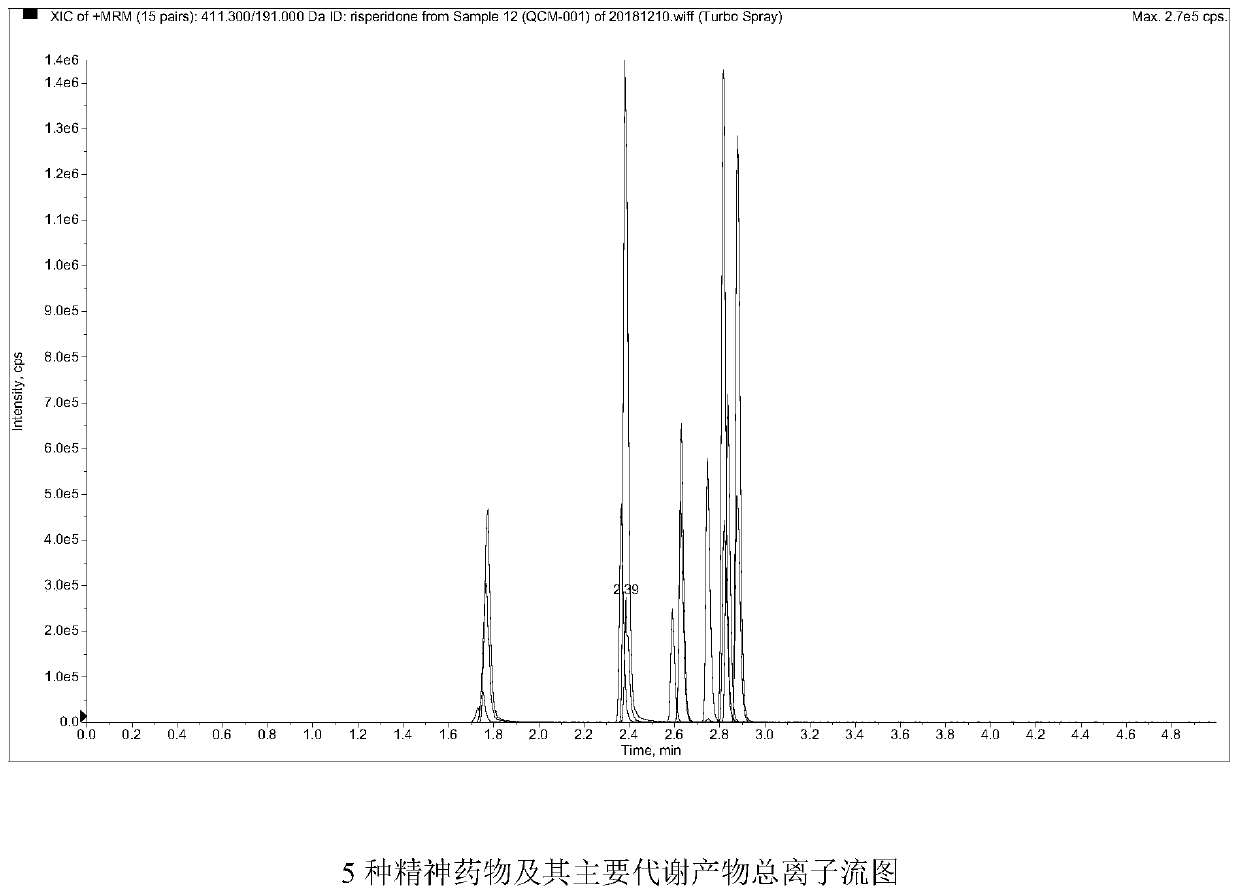
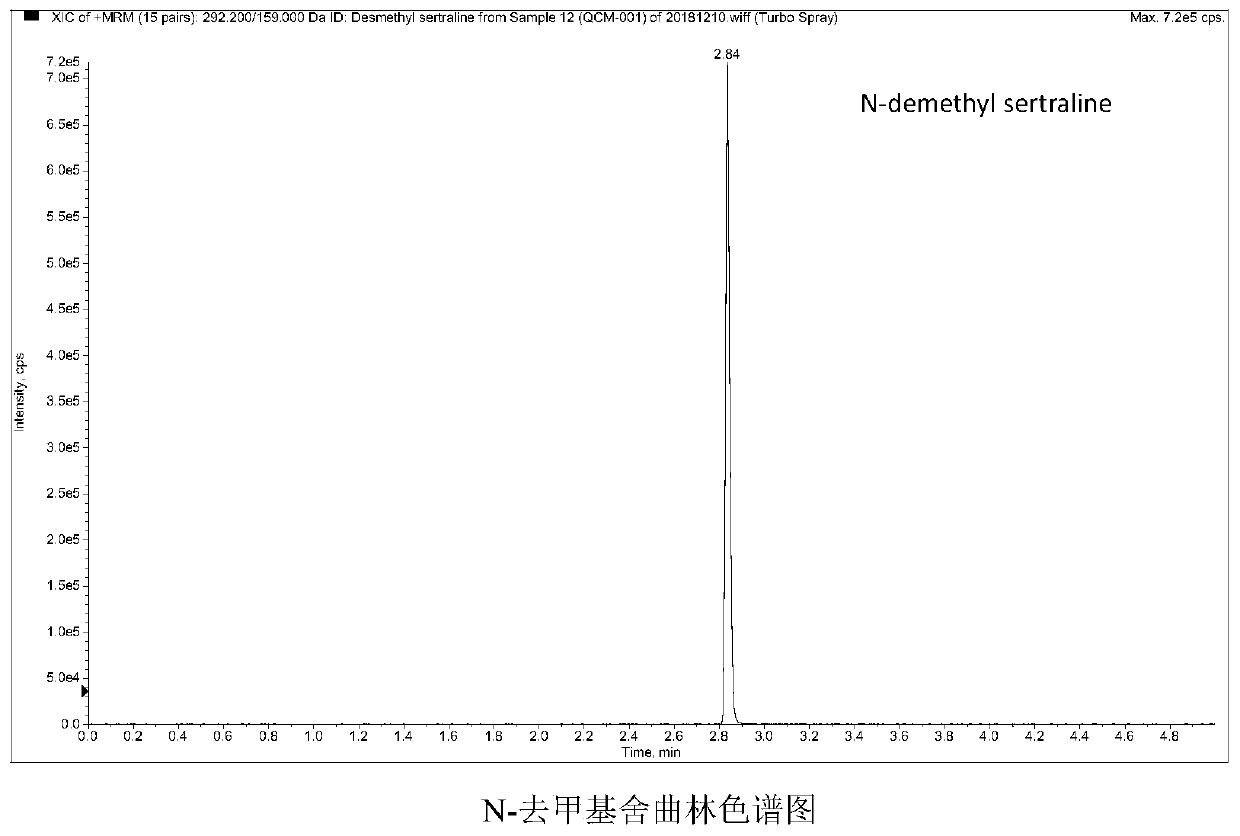
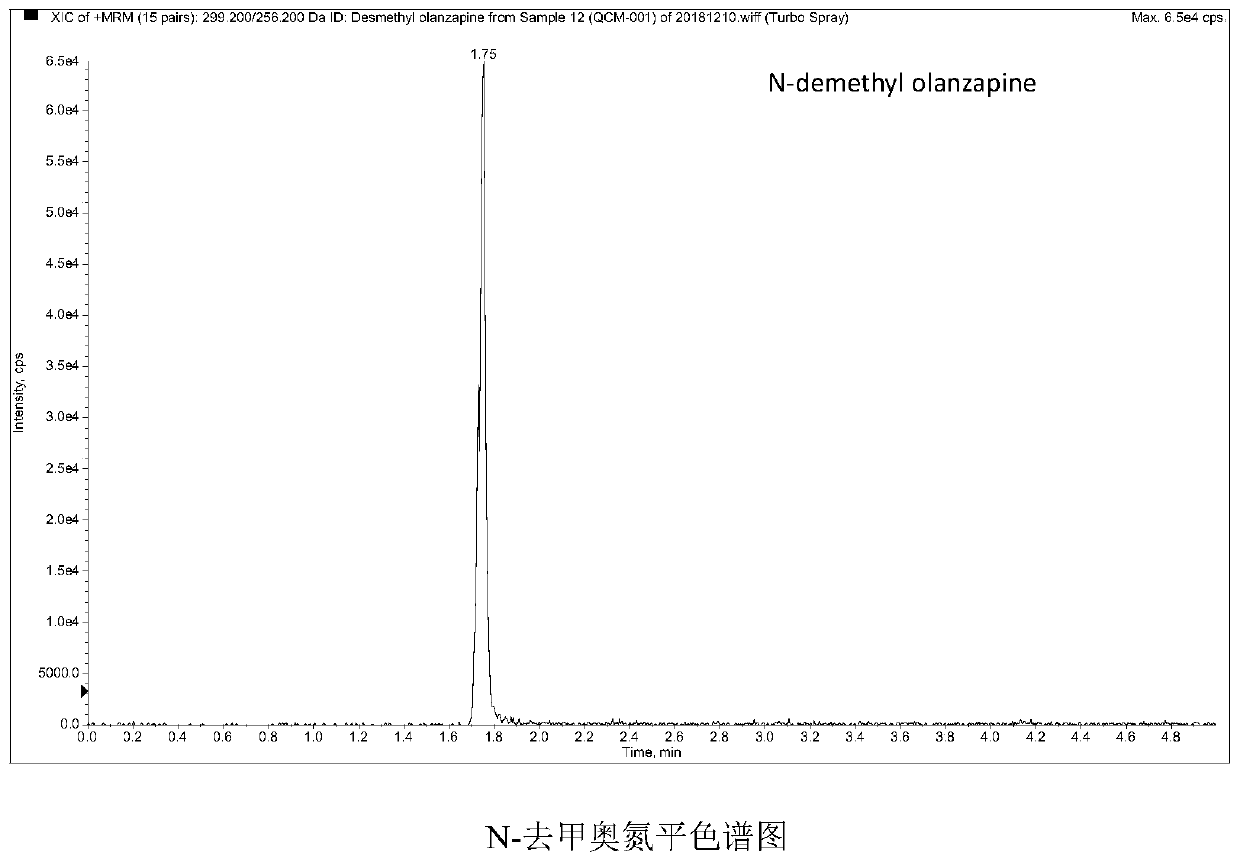
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
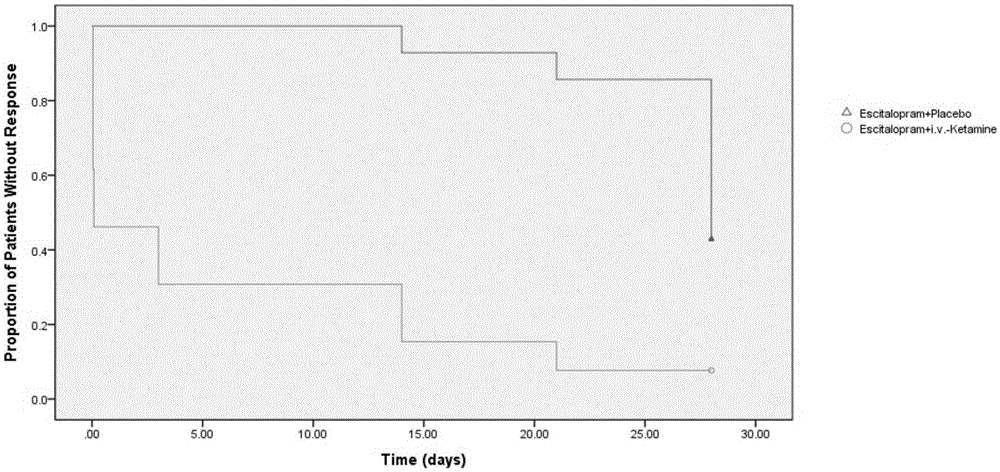
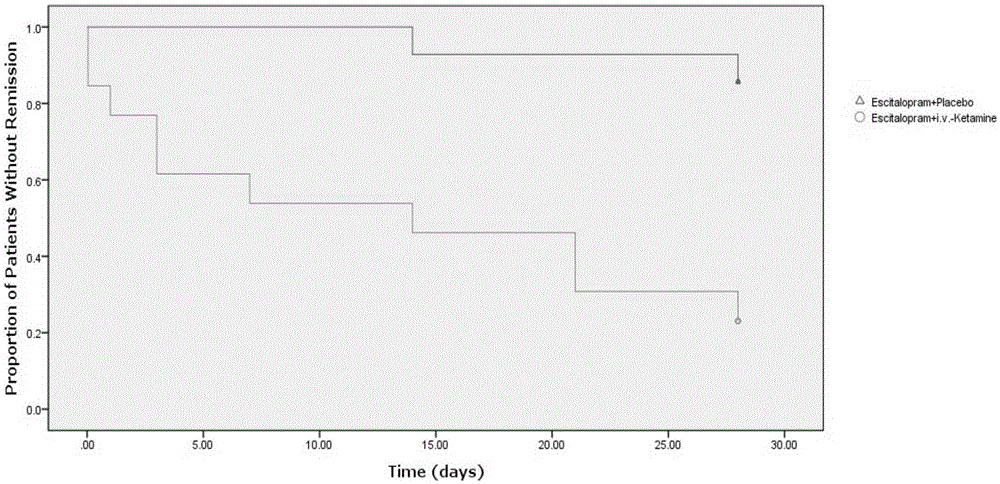
Application of ketamine to treatment of major depressive disorder
ActiveCN106562952AReduce suicideSignificant antidepressant effectOrganic active ingredientsNervous disorderDisplay lagEscitalopram
The invention provides an application of a combined drug composition of ketamine or S-(+)-ketamine or a pharmaceutically acceptable salt of ketamine or S-(+)-ketamine, and escitalopram or a pharmaceutically acceptable salt of escitalopram, to the preparation of drugs for treating major depressive disorder. The major depressive disorder can be major depressive disorder breaking out for the first time, major depressive disorder breaking out again or refractory depression. The invention furthermore provides an application of ketamine or S-(+)-ketamine or the pharmaceutically acceptable salt of ketamine or S-(+)-ketamine to the preparation of the drugs for treating the major depressive disorder, and a drug preparation containing ketamine or S-(+)-ketamine or the pharmaceutically acceptable salt of ketamine or S-(+)-ketamine, and escitalopram or the pharmaceutically acceptable salt of escitalopram. Ketamine and conventional antidepressants are used together; and a proper amount of ketamine is intravenously injected once at first day of start of treatment, so that the anti-depression effect of oral antidepressants can be effectively improved, the effect display lag period is remarkably shortened, and suicidal actions of patients can be remarkably reduced.
Owner:北京安博睿达医药科技有限公司 +1
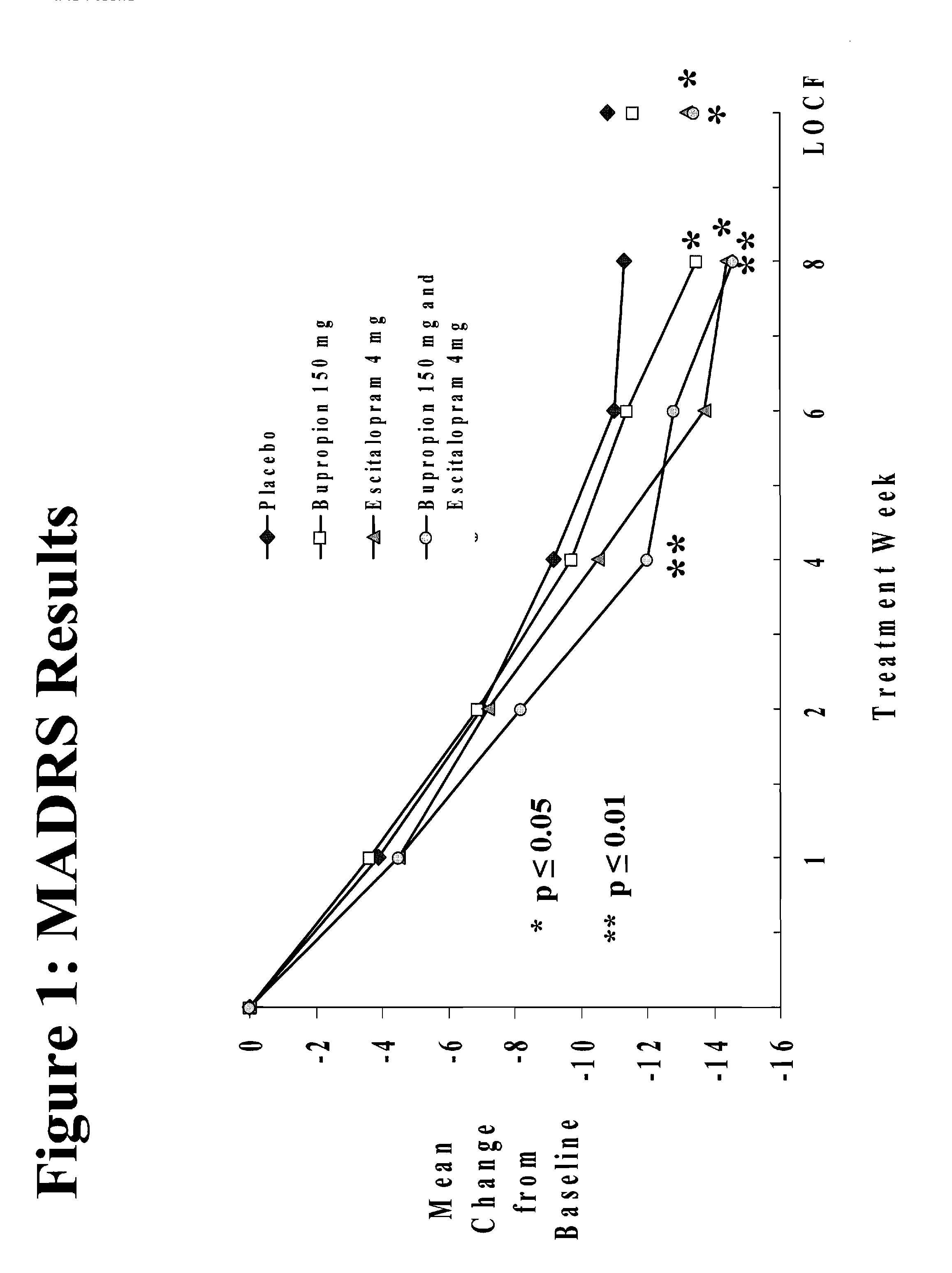
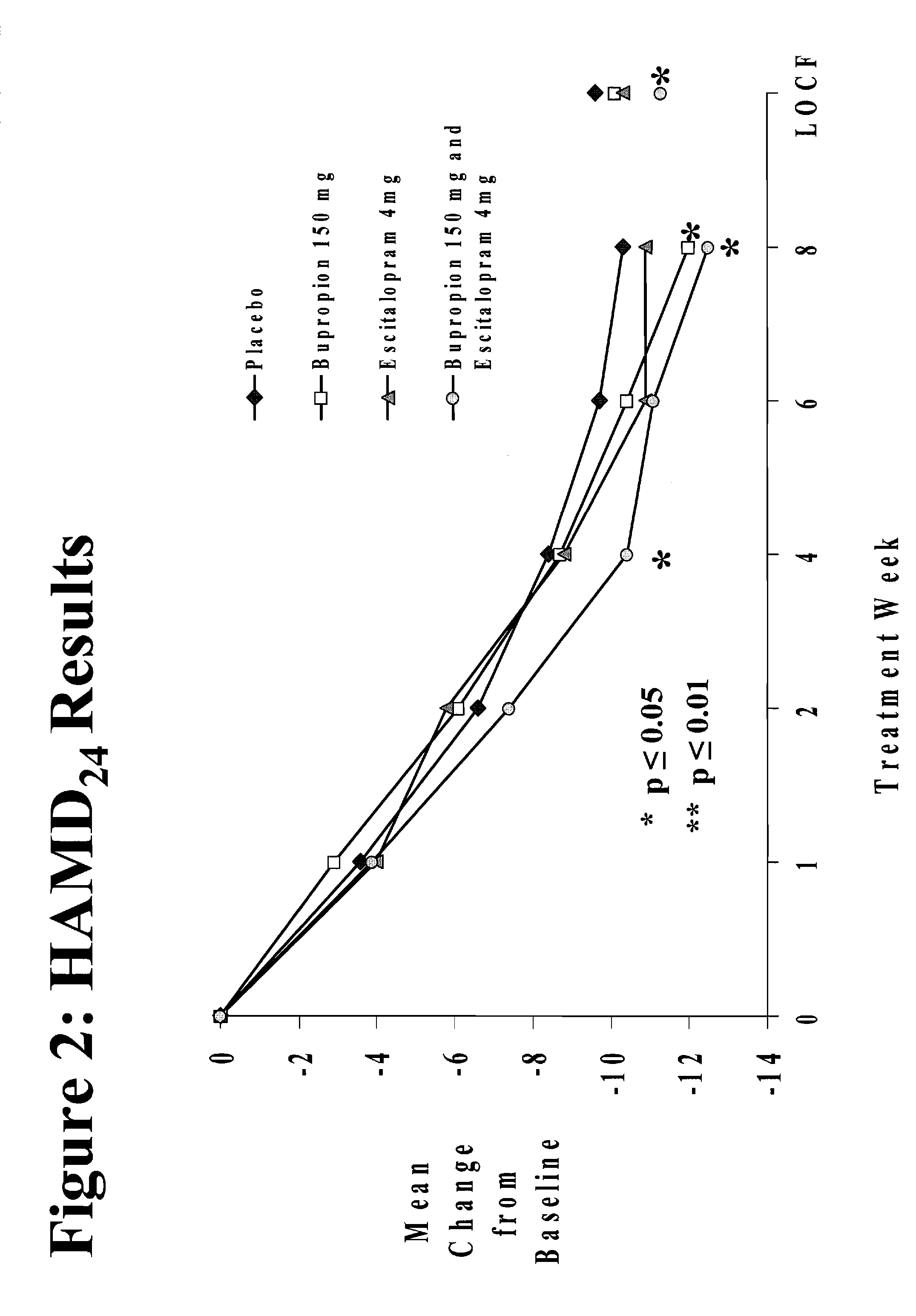
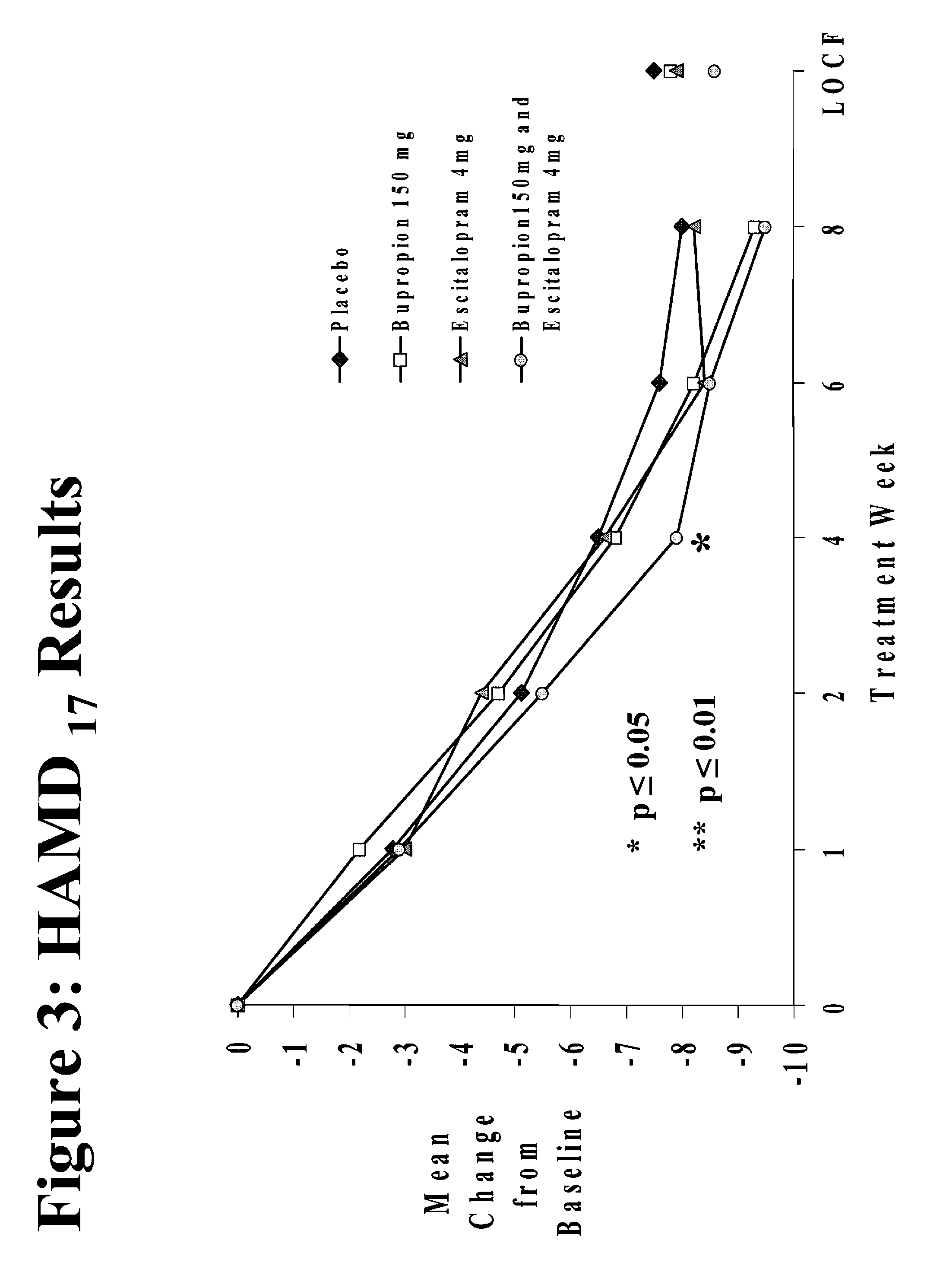
Methods of treating central nervous system disorders with a low dose combination of escitalopram and bupropion
The present invention relates to a method of treating a central nervous system disorder, such as a mood disorder (e.g., major depressive disorder) or an anxiety disorder (e.g., general anxiety disorder, social anxiety disorder, post traumatic stress disorder, and panic disorder) with a low dose combination of escitalopram and bupropion.
Owner:H LUNDBECK AS +1
Uses of escitalopram
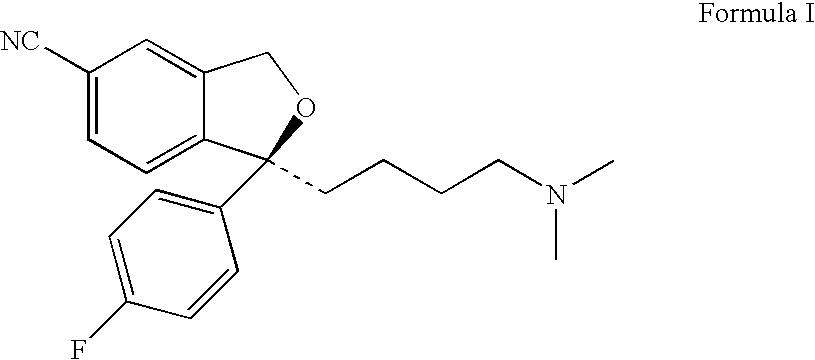
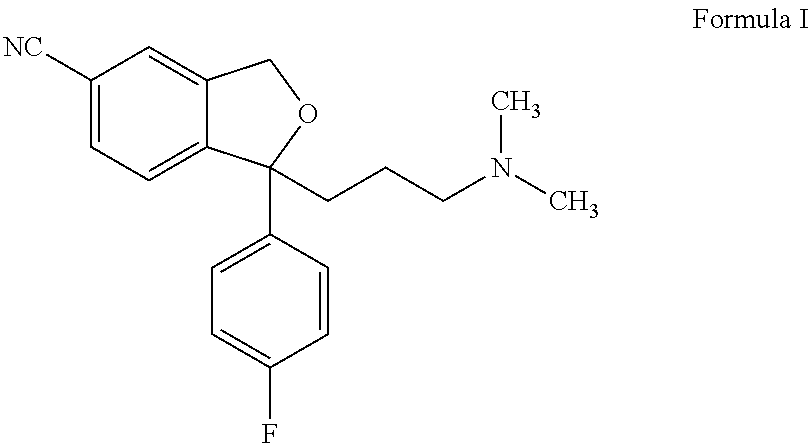
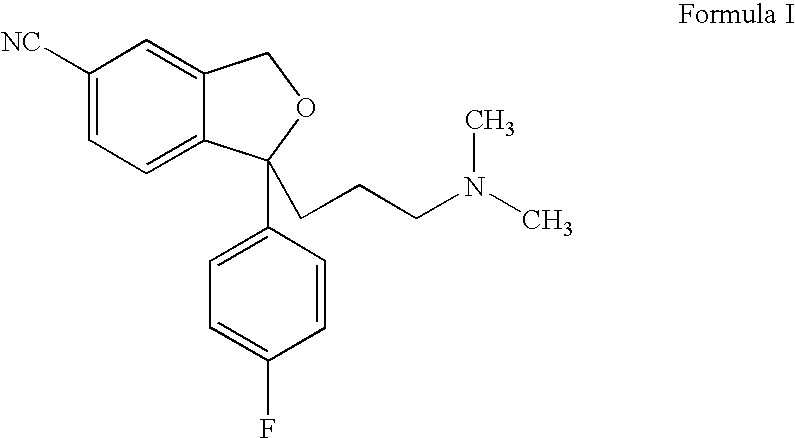
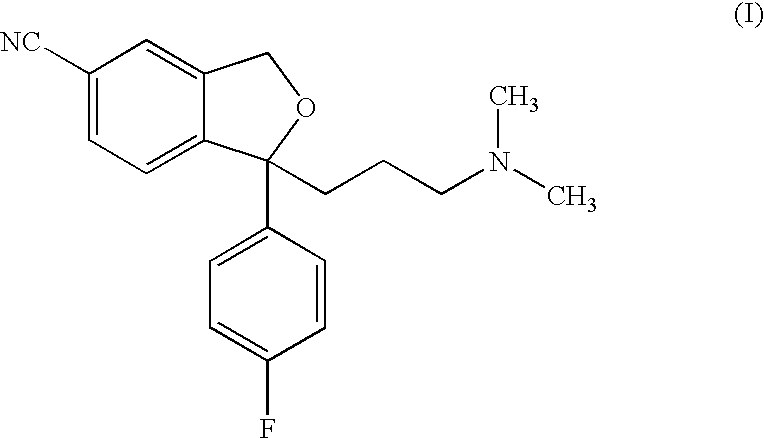
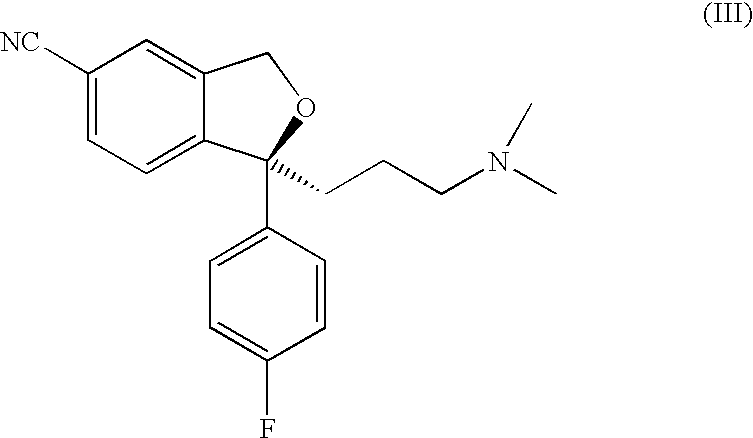
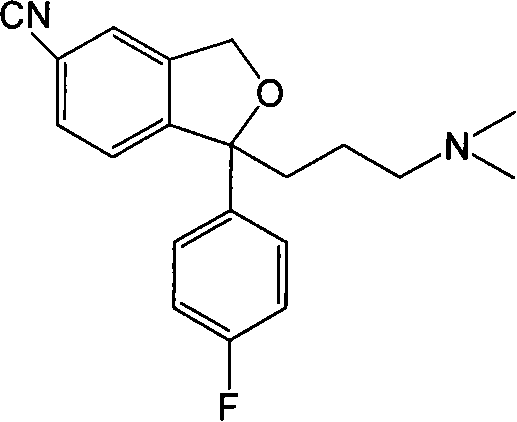
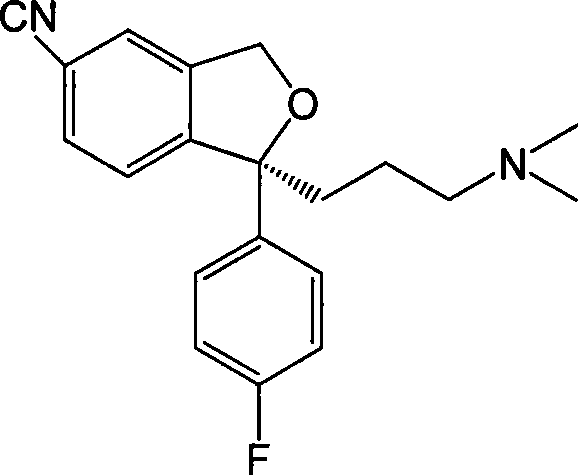
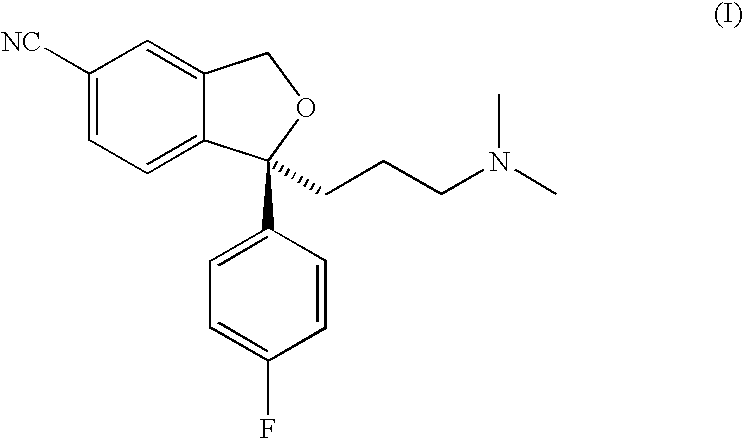
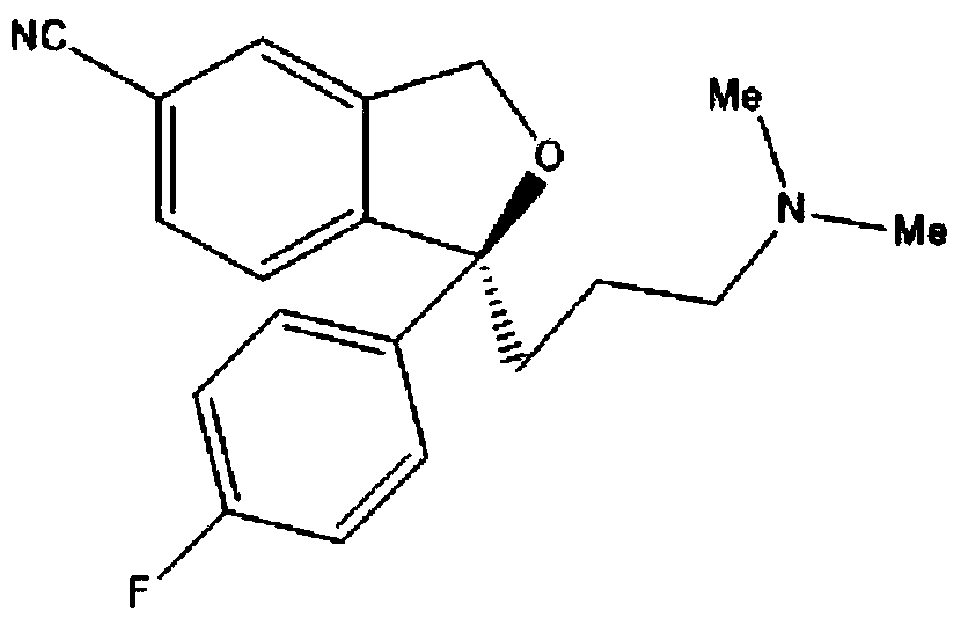
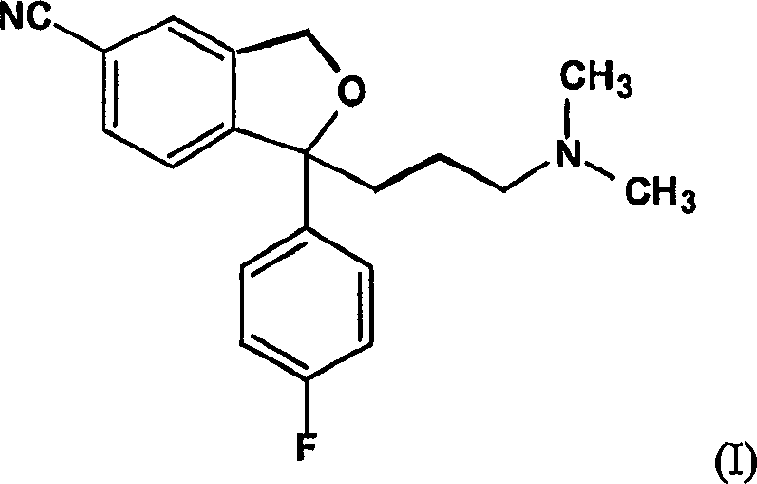
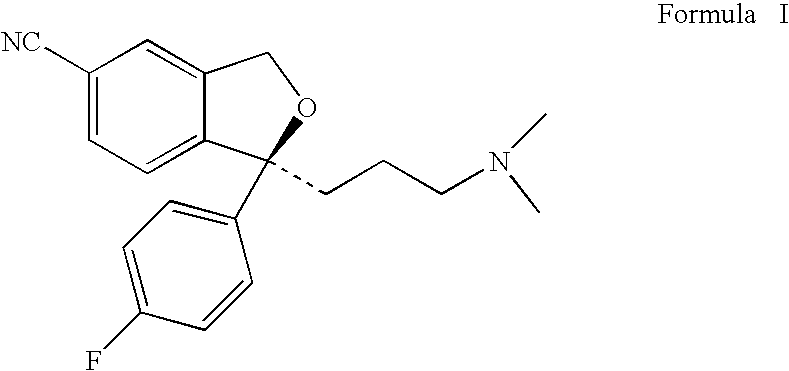
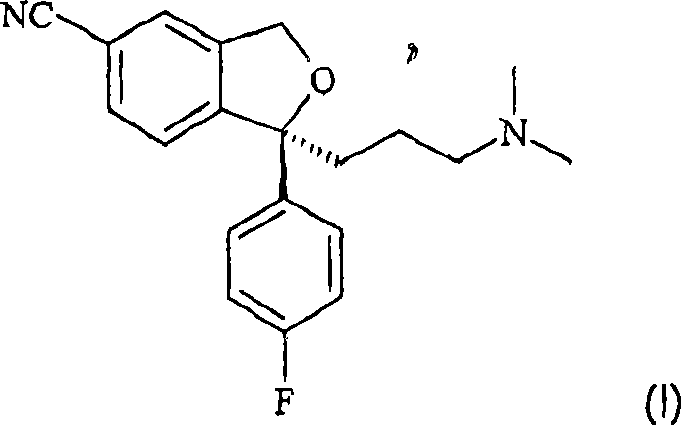
The present invention relates to the use of the compound escitalopram (INN-name), i.e. (S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, or a pharmaceutically acceptable salt thereof for the preparation of a medicament for improving cognition in a condition where the cognitive processes are diminished.
Owner:H LUNDBECK AS
Taste masked dosage form of pharmaceutically acceptable salt of escitalopram
A taste masked dosage form of pharmaceutical acceptable salt of escitalopram comprising (a) resin complex of pharmaceutical acceptable salt of escitalopram and cationic exchange resin or adsorbing or coating non-pareil seeds or inert particles with a mixture of pharmaceutically acceptable salt of escitalopram, cationic polymer and optionally other polymer(s) or loading non-pareil seeds or inert particles with pharmaceutically salt of escitalopram followed by polymer coating with cationic polymer and optionally other polymer(s); and (b) at least one pharmaceutical excipient.
Owner:GENEPHARM A E
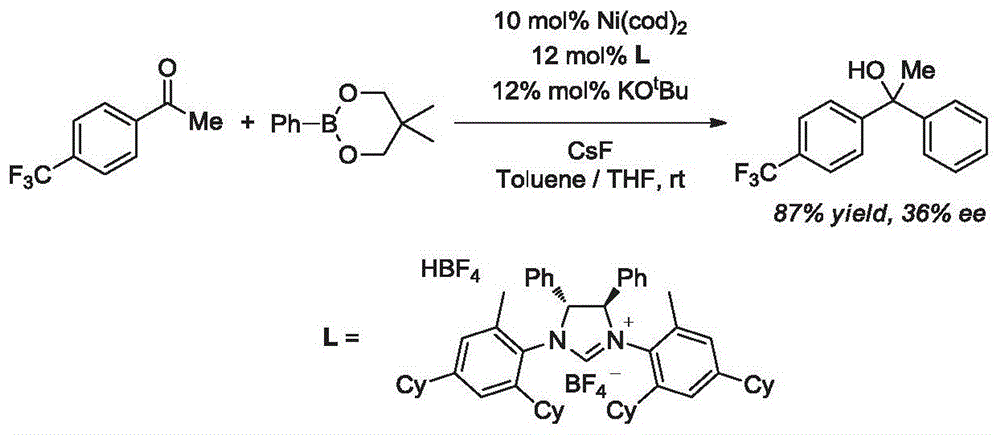
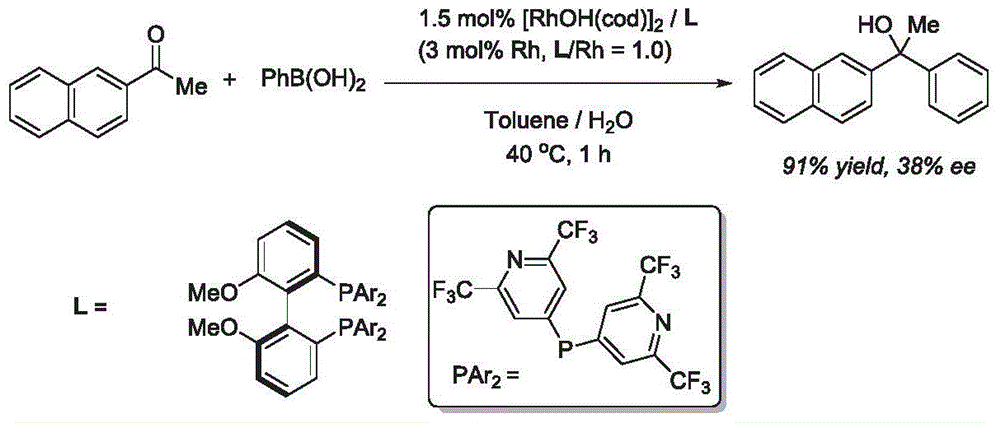
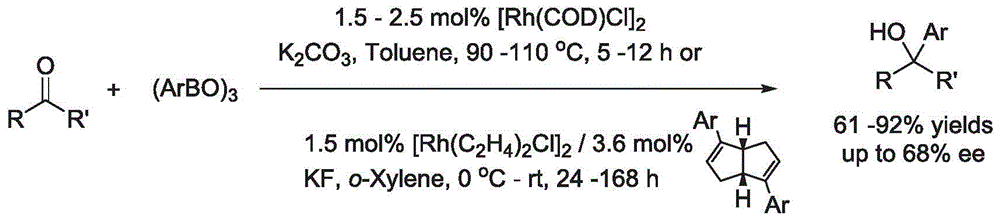
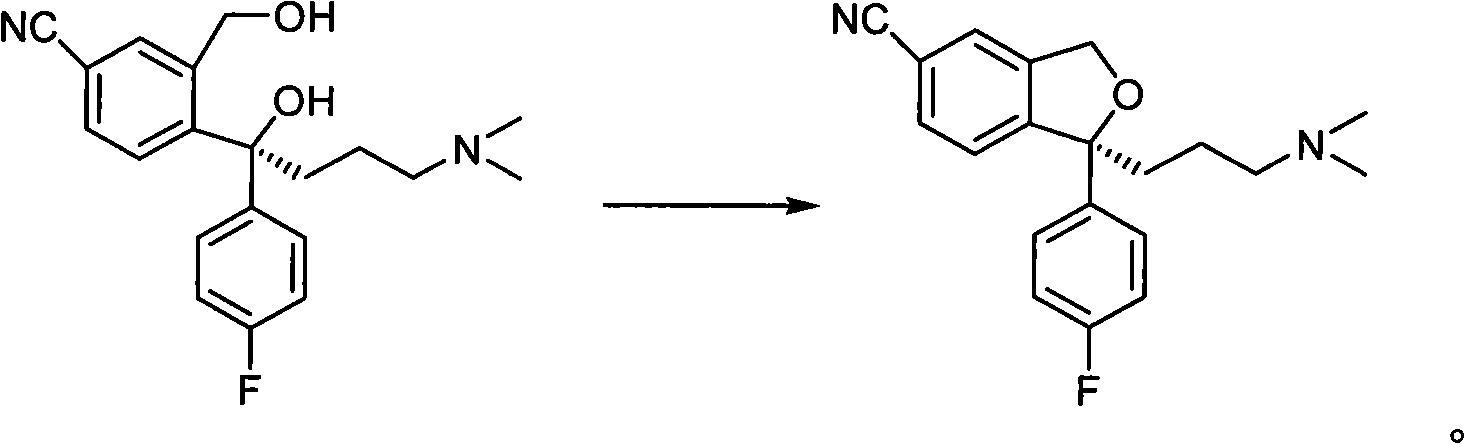
Synthesis method of aryl alcohol compound and Escitalopram
ActiveCN105732249AHigh yieldHigh enantioselectivityOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsEscitalopram
The invention discloses a synthesis method of an aryl alcohol compound and Escitalopram. The synthesis method of the aryl alcohol compound comprises the following steps: under gas protection and in an organic solvent and in the presence of transition metal, diphosphine ligand and alkali, the compound of formula 1 reacts with an aryl boron reagent 2. The synthesis method of Escitalopram comprises the following steps: (1) under gas protection and in an organic solvent and in the presence of transition metal, diphosphine ligand and alkali, the compound of formula 4 reacts with the aryl boron reagent 2; (2) under gas protection and in an organic solvent and in the presence of alkali, the compound of formula 5 reacts with dimethylamine or hydrochloride thereof; (3) under gas protection and in an organic solvent and in the presence of organic phosphine ligand and a palladium catalyst, the compound of formula 7 reacts with metal cyanide; and (4) under gas protection and in an organic solvent and in the presence of a reducing agent, the compound of formula 6 reacts. The synthesis method disclosed by the invention has high yield and enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
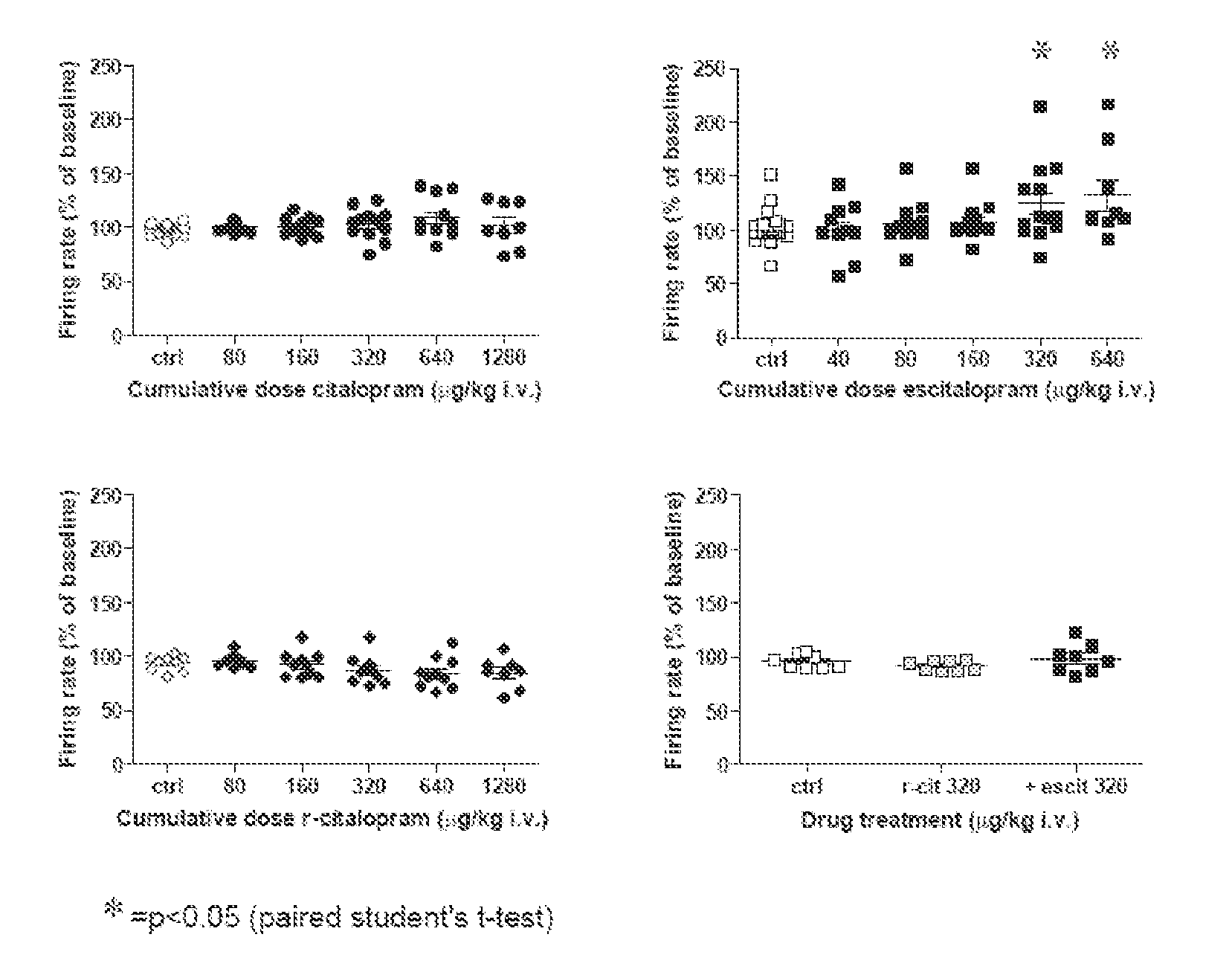
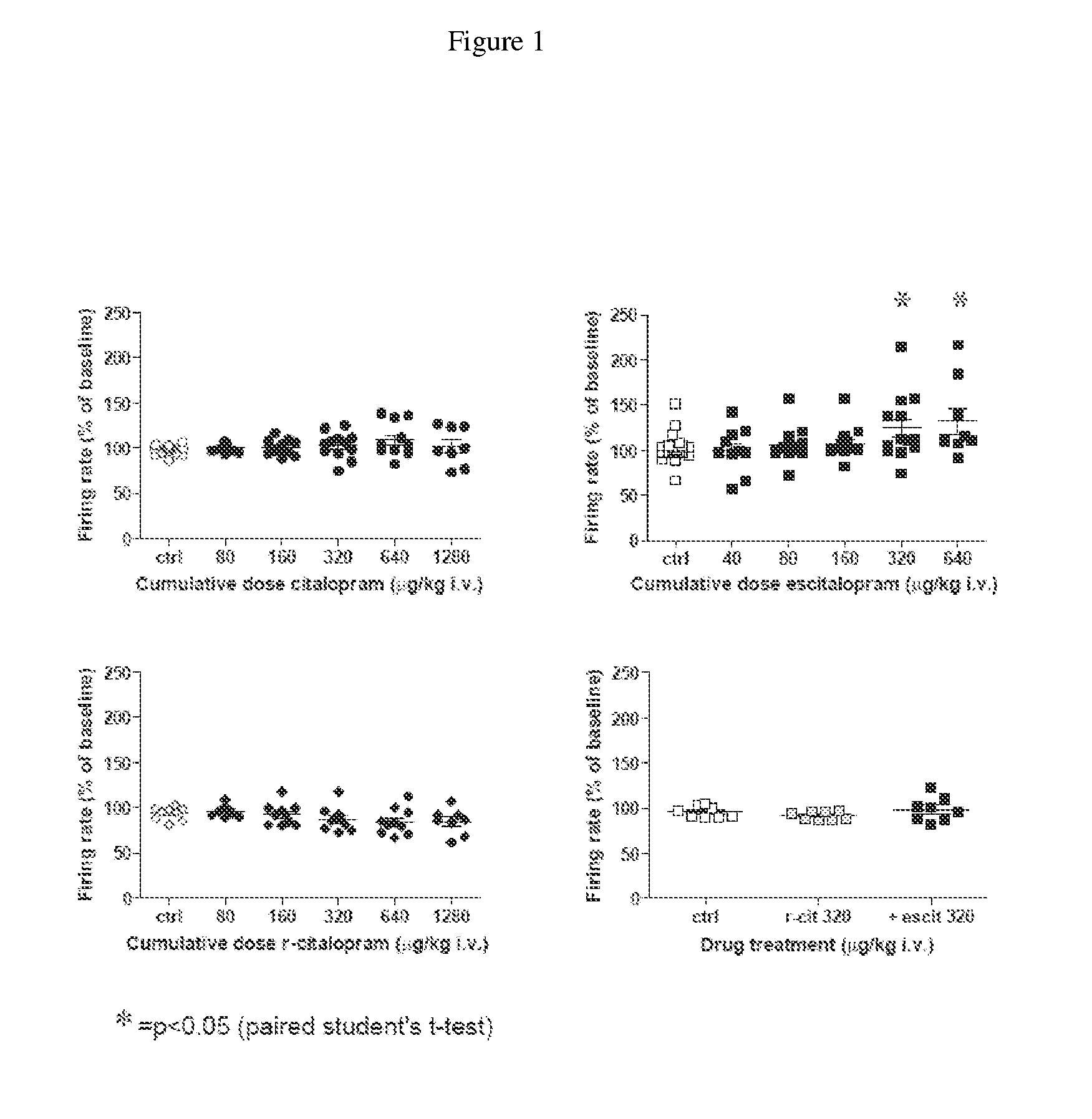
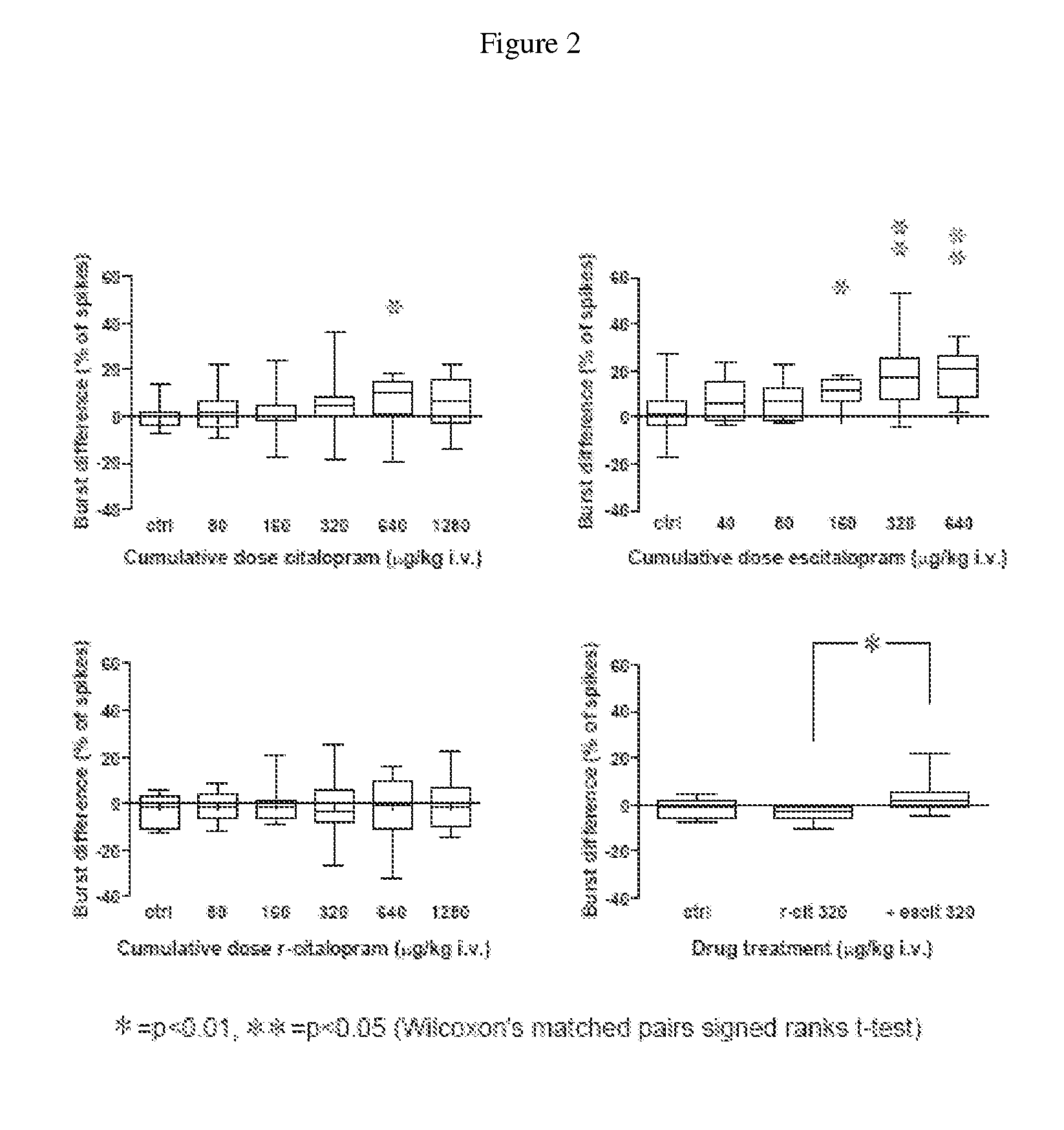
Use of enantiomeric pure escitalopram
The present invention relates to the use of anatiomeric pure escitalopram and / or of low dose medicaments thereof for the improved treatment of depression, in particular major depression disorder, neurotic disorders, acute stress disorder, eating disorders such as bulimia, anorexia and obesity, phobias, dysthymia, pre-mentrual syndrome, cognitive disorders, impulse control disorders, attention deficit hyperactivity disorder or drug abuse. The medicaments may also be used in the treatment of major depression disorder in "treatment resistant" patients.
Owner:H LUNDBECK AS
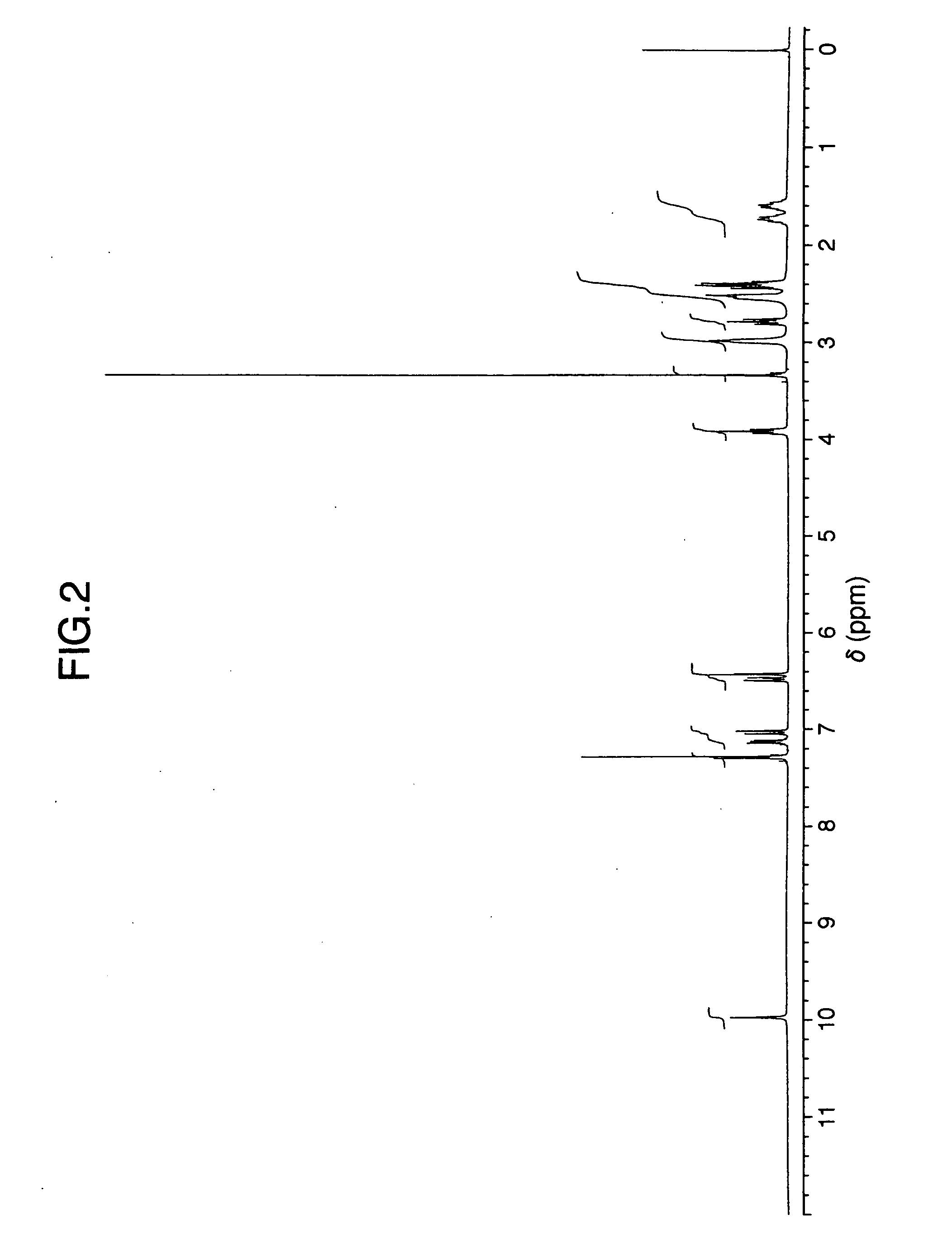
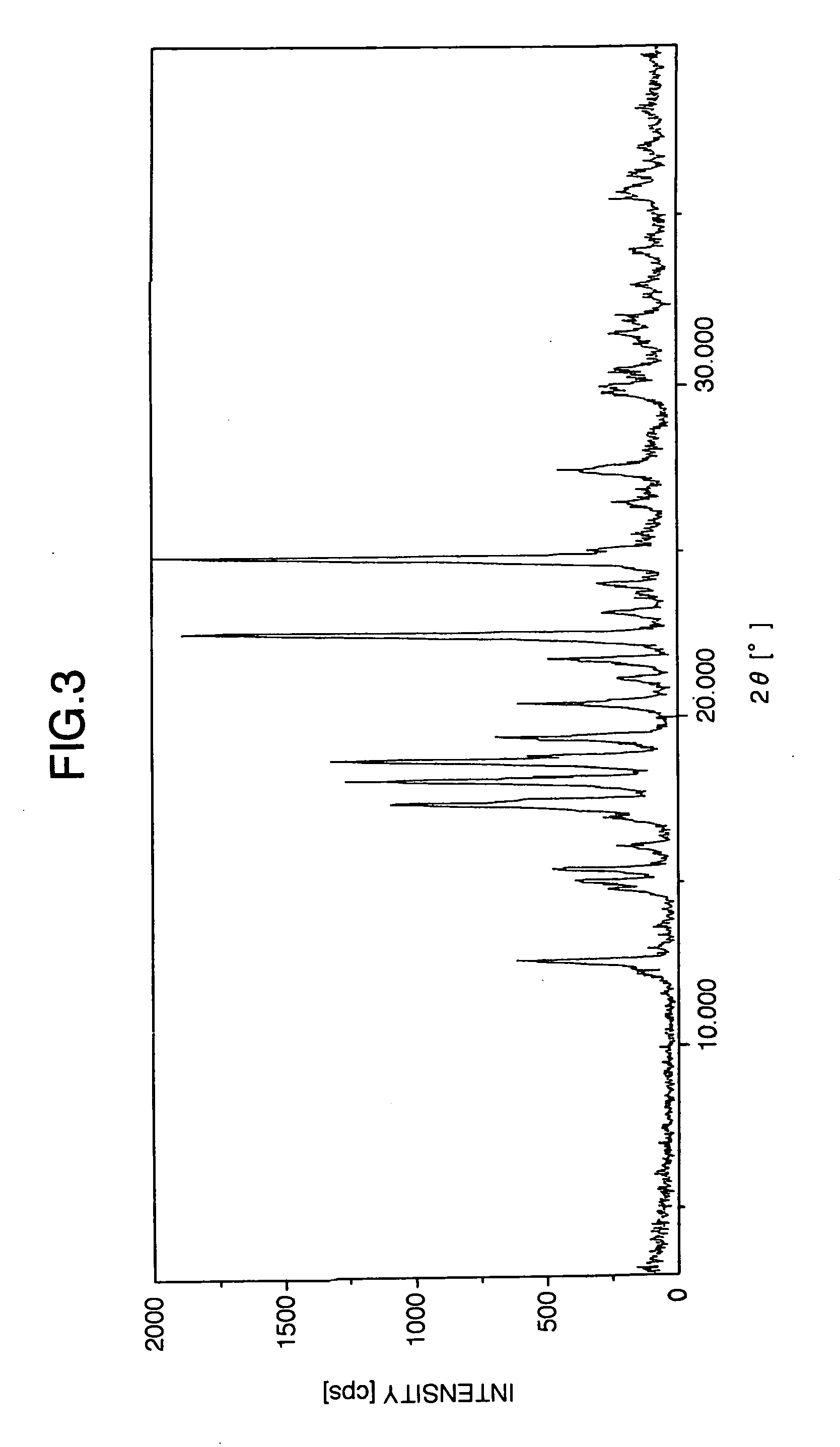
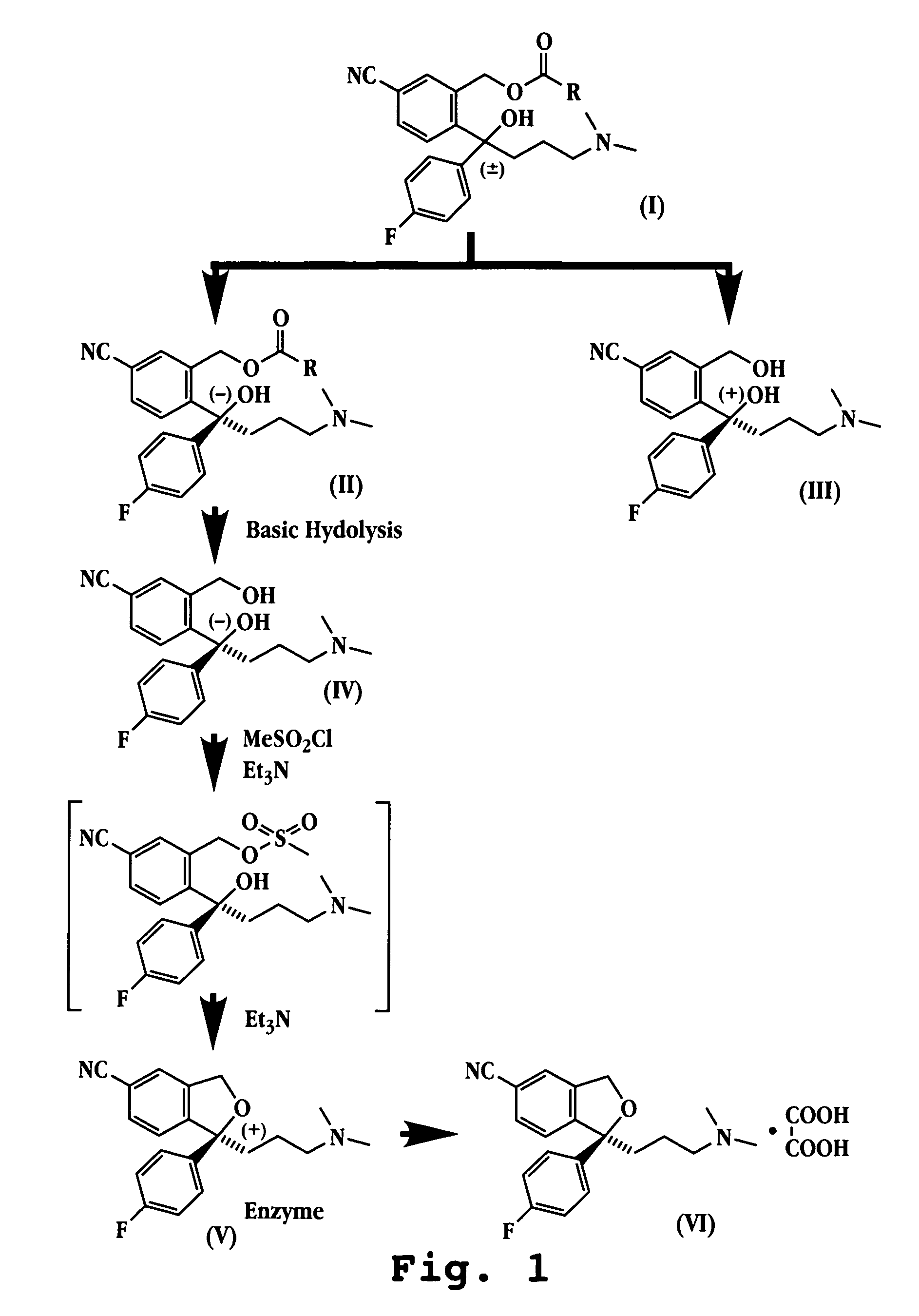
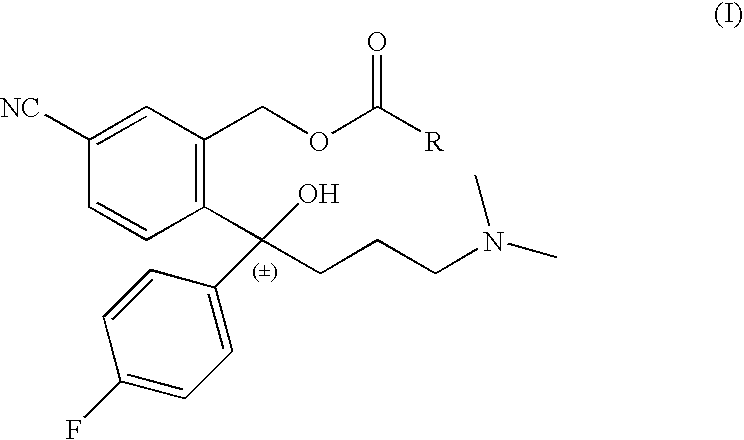
Preparation of Escitalopram, Its Salts and Intermediates
The present patent application relates to an improved process for the preparation of escitalopram, its salts and intermediates. It also relates to a novel crystalline form S of citalopram diol intermediate, process for preparation and its use in the preparation of citalopram, escitalopram and their salts.
Owner:SHODHANA LAB LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Treatment of neurotic disorders
Use of escitalopram (the S-(+)-enantiomer of citalopram) or a pharmaceutically acceptable salt thereof for the preparation of a medicament useful in the treatment of neurotic disorders is provided, including anxiety states, in particular generalised anxiety disorder and social anxiety disorder, post traumatic stress disorder, obsessive compulsive disorder and panic attacks.
Owner:H LUNDBECK AS
Method for the preparation of escitalopram
A novel method is provided for the manufacture of escitalopram. The method comprises chromatographic separation of the enantiomers of citalopram or an intermediate in the production of citalopram using a chiral stationary phase such as Chiralpak™ or Chiralcel™ OD. Novel chiral intermediates for the synthesis of Escitalopram made by said method are also provided.
Owner:H LUNDBECK AS
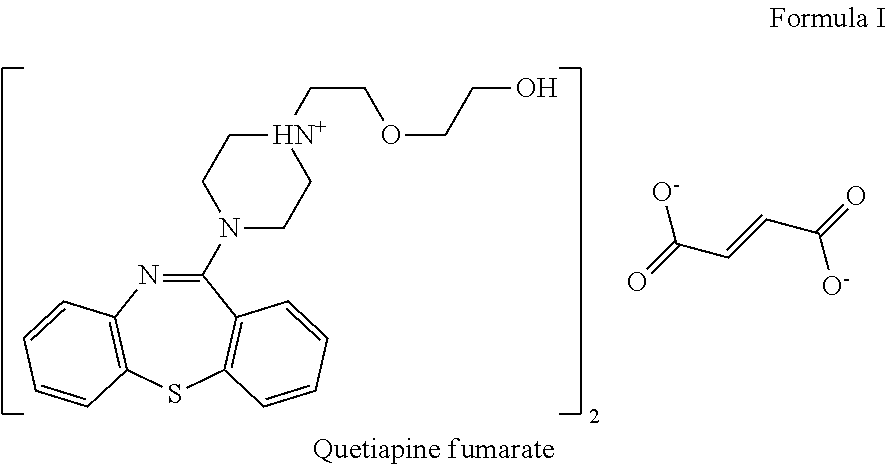
Pharmaceutical formulations comprising quetiapine and escitalopram
The present invention relates to a multilayer tablet formulation comprising a combination of quetiapine or a pharmaceutically acceptable salt thereof and escitalopram or a pharmaceutically acceptable salt thereof.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Chemo-enzymatic process for the preparation of escitalopram
Owner:ADORKEN TECH
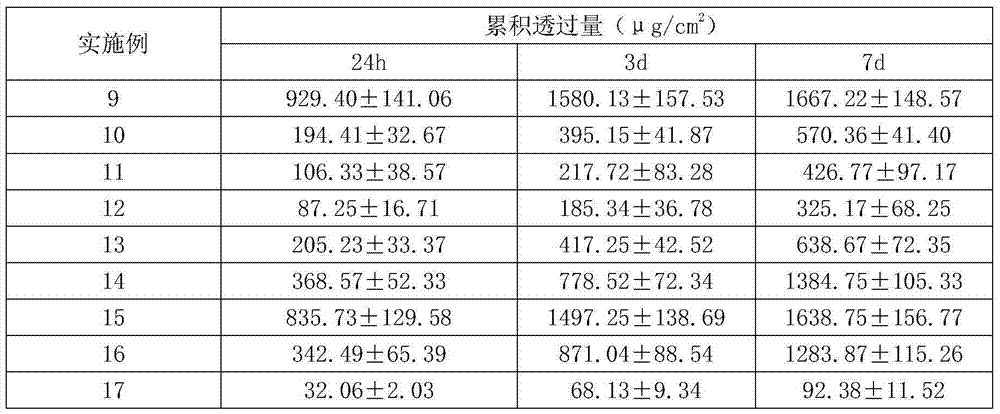
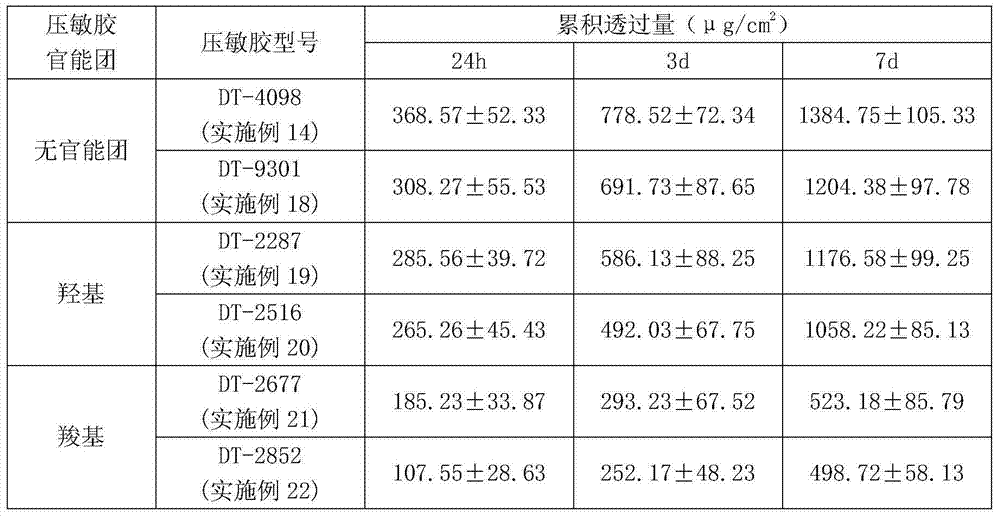
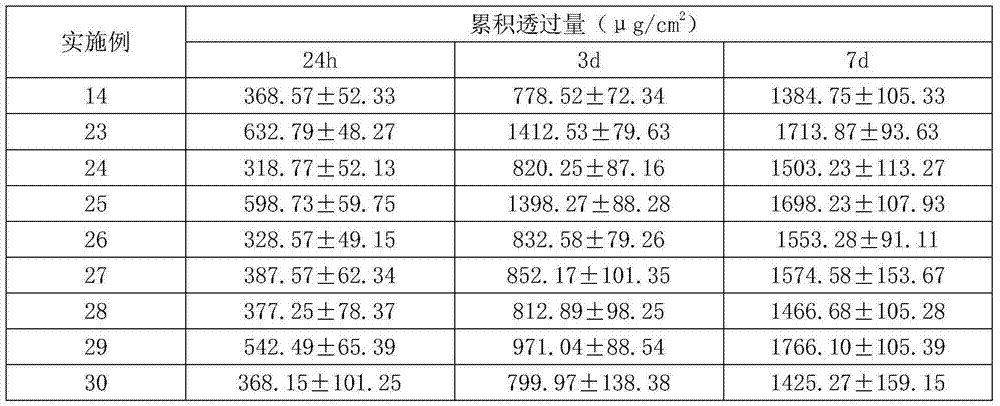
Escitalopram percutaneous patch and preparation method thereof
ActiveCN104840973AEffective regulation of transdermal penetration rateSimple technologyOrganic active ingredientsNervous disorderOrganic acidEscitalopram
The invention belongs to the technical field of medicine and relates to an escitalopram percutaneous patch and a preparation method thereof. The escitalopram percutaneous patch is composed of a backing layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-sticking layer, the medicine-carrying pressure-sensitive adhesive layer comprises escitalopram free alkali or organic acid ion pair compound thereof, pressure-sensitive adhesive and percutaneous absorption promoter, the escitalopram free alkali or organic acid ion pair compound thereof accounts for 2.0-20wt% of total weight of the medicine-carrying pressure-sensitive adhesive layer, pressure-sensitive adhesive accounts for 77-97wt% of the total weight, the percutaneous absorption promoter accounts for 0-6.8wt% of the total weight, and in the escitalopram organic acid ion pair compound, a ratio of escitalopram free alkali and different organic acids is 0.5:1-2:1. Compared with an escitalopram organic acid ion pair compound percutaneous absorption patch and an escitalopram free alkali percutaneous absorption patch, the escitalopram percutaneous patch has the advantages that medicine release up to seven days and similar to constant rate can be provided, so that medication compliance of a patient is improved greatly.
Owner:SHENYANG PHARMA UNIVERSITY
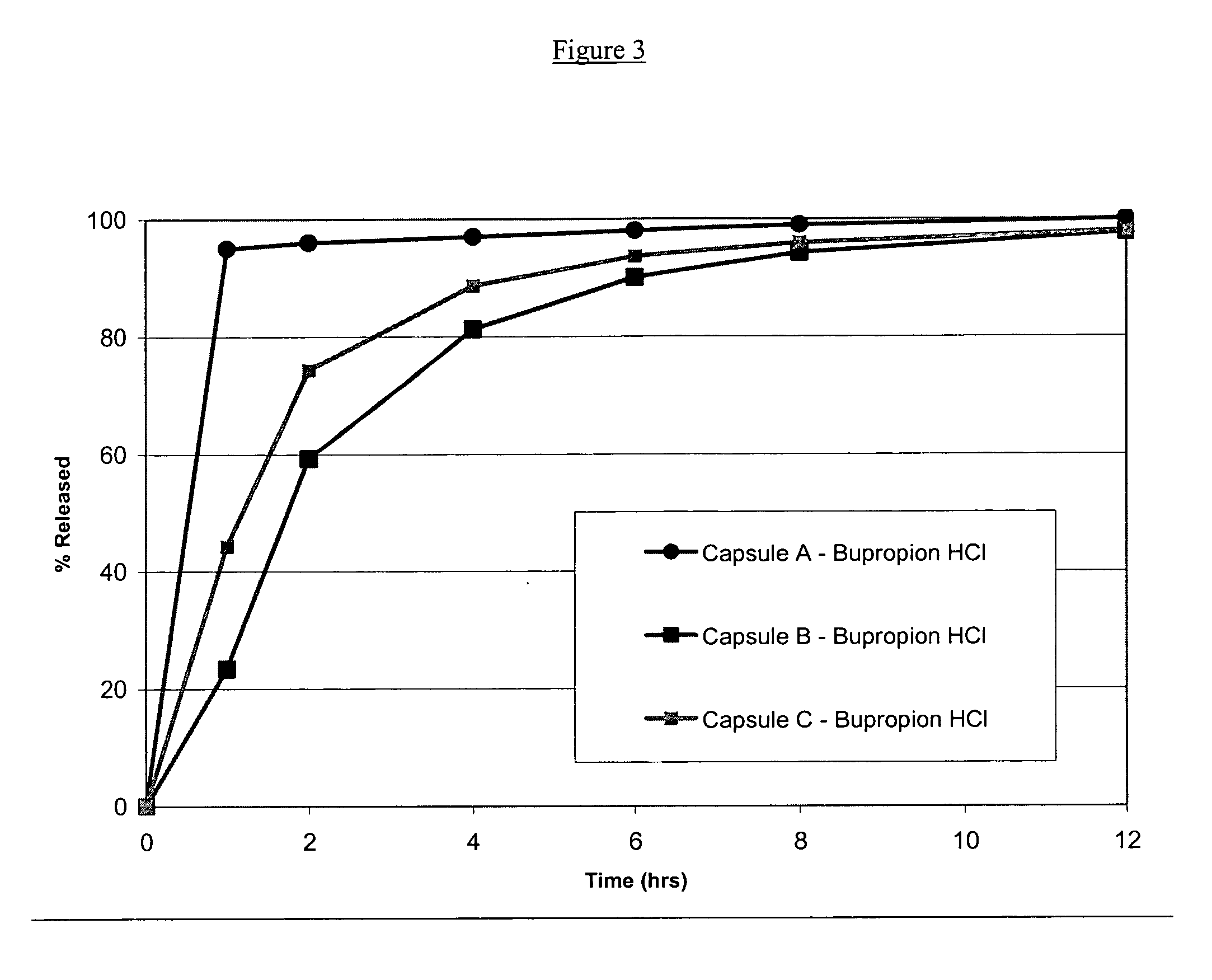
Stable pharmaceutical formulations containing escitalopram and bupropion
InactiveUS20070112075A1Treat and reduce suicidal thoughtImprove survivalBiocideOrganic active ingredientsDiseaseEscitalopram
The present invention relates to stable pharmaceutical formulations of escitalopram and bupropion and their use for the treatment a central nervous system disorder, such as a mood disorder (e.g., major depressive disorder) or an anxiety disorder (e.g., general anxiety disorder, social anxiety disorder, post traumatic stress disorder, or panic disorder).
Owner:H LUNDBECK AS
Method for preparing escitalopram
InactiveCN101607951AGood shape retentionWill not racemizeNervous disorderOrganic chemistryOrganic solventEscitalopram
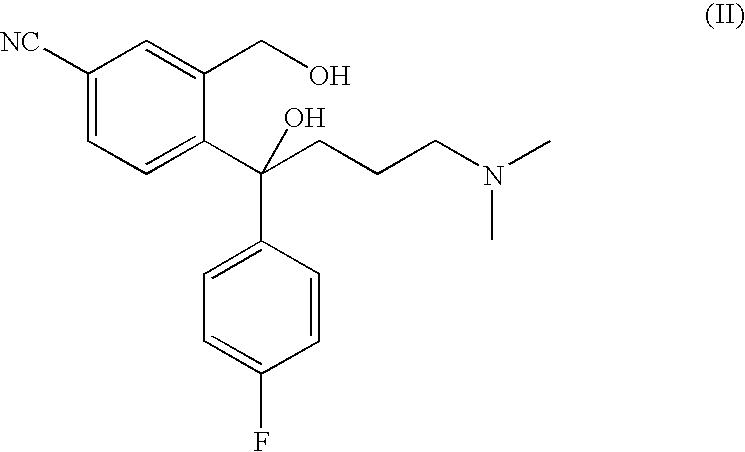
The invention discloses a method for preparing escitalopram represented by a formula (I), which comprises the following step: reacting S-diol represented by a formula (II) in an aprotic organic solvent under the action of a phosphine complex compound, an azo reagent and a proton supplying agent in an atmosphere of an inert gas to obtain the escitalopram represented by the formula (I). The method uses a Mitsunobu reaction in the cyclization preparation of escitalopram represented by the structural formula (I) for the first time and has the unexpected advantages of excellent product configuration retention, prevention of racemization in a cyclization process and high optical purity and yield. In addition, the method of the invention is simple in operation, mild in condition, simple and convenient in post processing and suitable for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
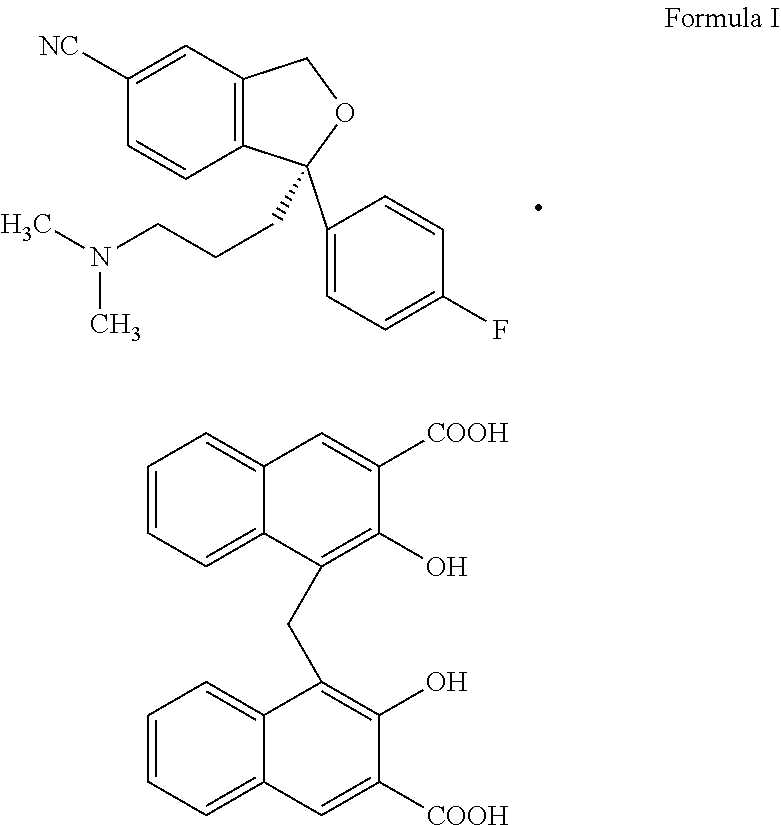
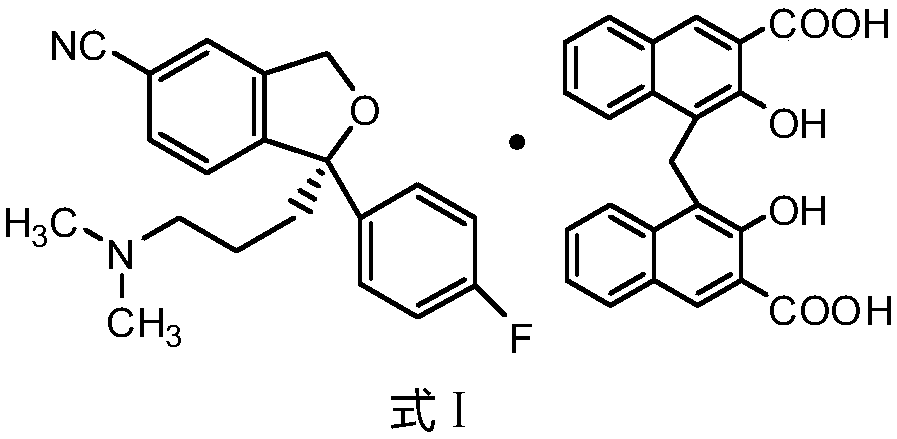
New preparation method for escitalopram pamoate
The present invention relates to a new preparation method for escitalopram pamoate ((S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-cyanoisob enzofuran pamoate), wherein the method is environmentally friendly and pollution-free, and the escitalopram pamoate prepared by means of the method has a high purity and a good repeatability.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
The present invention relates to the crystalline base of the well known antidepressant drug escitalopram, S-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, formulations of said base, a process for the preparation of purified salts of escitalopram, such as the oxalate, using the base, the salts obtained by said process and formulations containing such salts, and a process for the preparation of purified escitalopram free base or salts of escitalopram, such as the oxalate, using the hydrobromide, the salts obtained by said process and formulations containing such salts. Finally the present invention relates to an orodispersible tablet having a hardness of at least 22 N and an oral-disintegration time of less than 120 s and comprising an active pharmaceutical ingredient adsorbed onto a water soluble filler wherein the active pharmaceutical ingredient has a melting point in the range of 40-100° C., as well as a method for making such an orodispersible tablet.
Owner:H LUNDBECK AS
Combination therapy for male sexual dysfunction
InactiveUS20170326139A1Reliable and effective and convenient on demand treatmentImproves overall sexual well-beingPill deliveryAmide active ingredientsTadalafilEscitalopram
Pharmaceutical formulations containing a serotonin reuptake inhibitor and a smooth muscle relaxant are provided for the treatment of premature ejaculation and the increase in intravaginal ejaculatory latency time. Specific formulations contain tadalafil (1-30 mg per dose) or tamsulosin (0.05-2 mg) and escitalopram (1-30 mg) in daily dose and on-demand formulations.
Owner:ATP THERAPEUTICS
Method for detecting escitalopram in blood
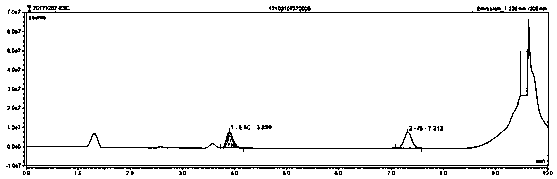
InactiveCN110646558AShorten detection timeAccurate identificationComponent separationLiquid chromatography mass spectroscopyEscitalopram
The invention provides a method for detecting escitalopram in blood. The method comprises the following steps of preparing a standard stock solution of the escitalopram, preparing a standard intermediate solution of the escitalopram, preparing an internal standard working solution, preparing a standard solution and detecting the standard solution by using a liquid chromatography-gas chromatograph-mass spectrometer, and fitting to obtain a standard curve equation corresponding to the escitalopram, wherein the standard curve equation is y1=a*x1+b; and processing the blood to be tested, detectingthe blood to be tested by using the liquid chromatography-gas chromatograph-mass spectrometer, and calculating the concentration of the escitalopram in the blood to be tested. According to the methodfor detecting the escitalopram in the blood provided by the invention, a processing method of a blood sample to be tested and the selection of an internal standard substance enable the recognition ofthe escitalopram to be more accurate, the analysis time is short, the interference is small, the internal standard is quantitative and suitable, the specificity is strong, and the sensitivity is high; and meanwhile, all technical indexes such as the recovery rate, the detection limit and the precision meet the requirements, so that the accuracy of a detection result is improved, and system errorsare eliminated.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
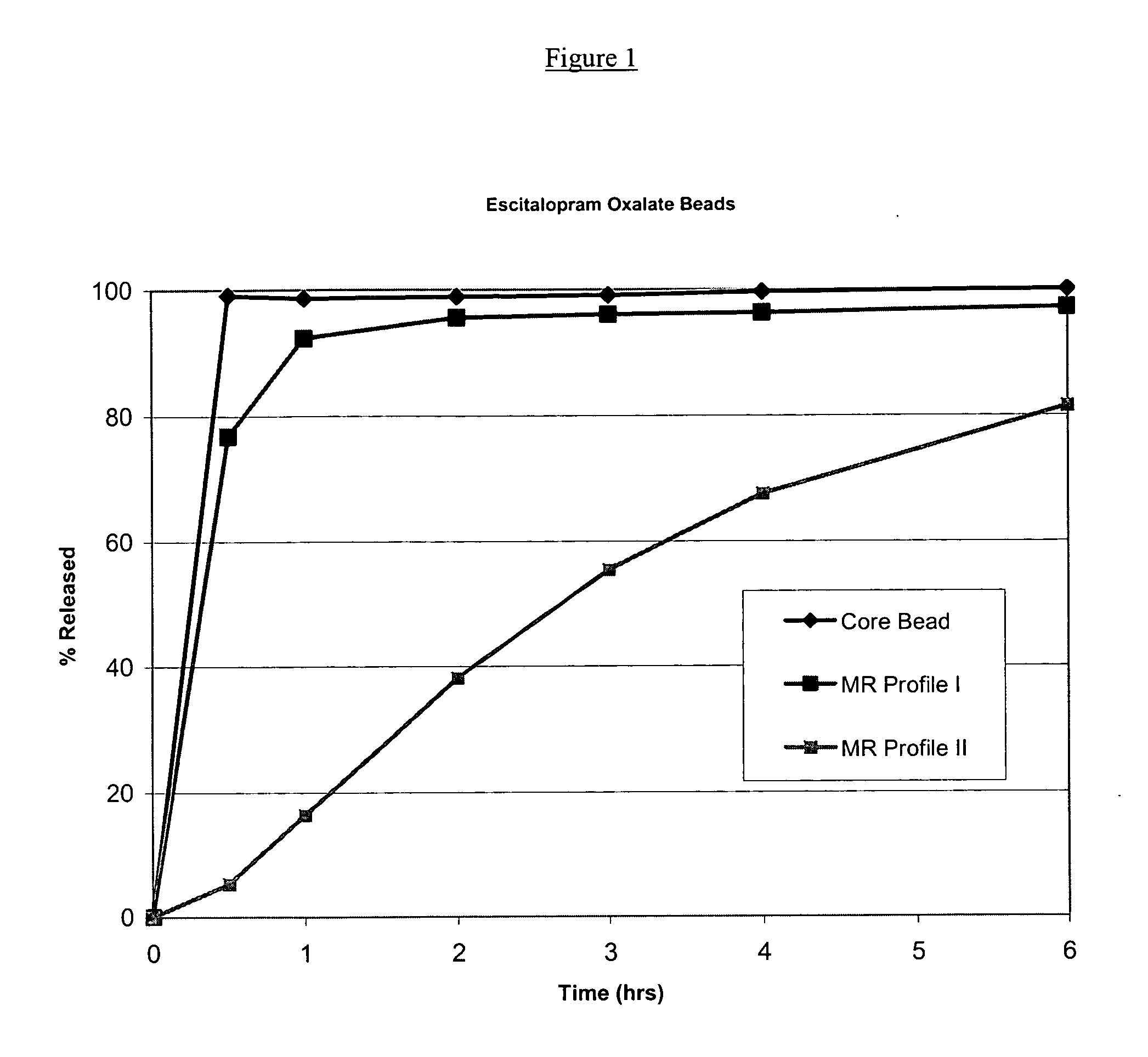
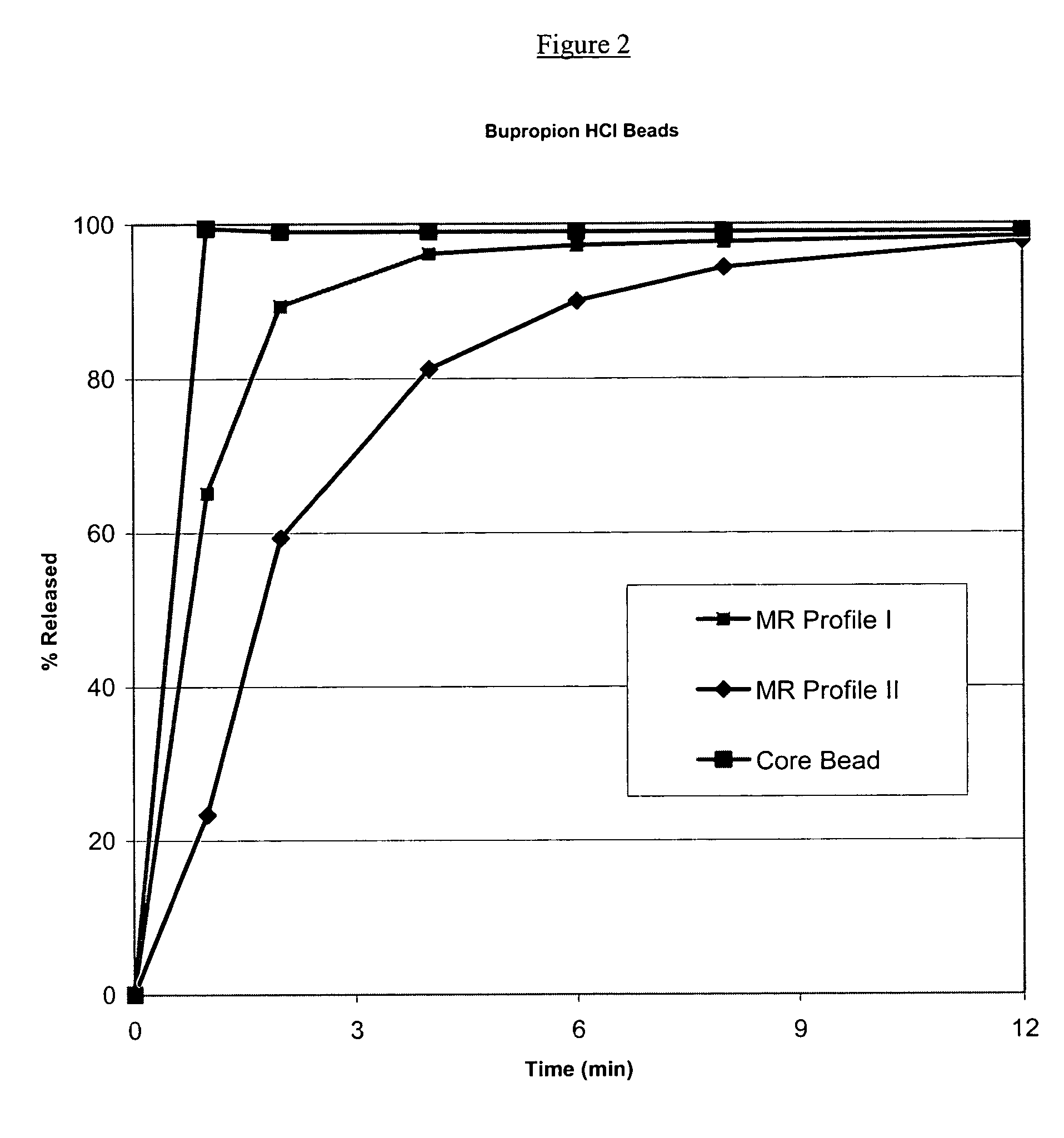
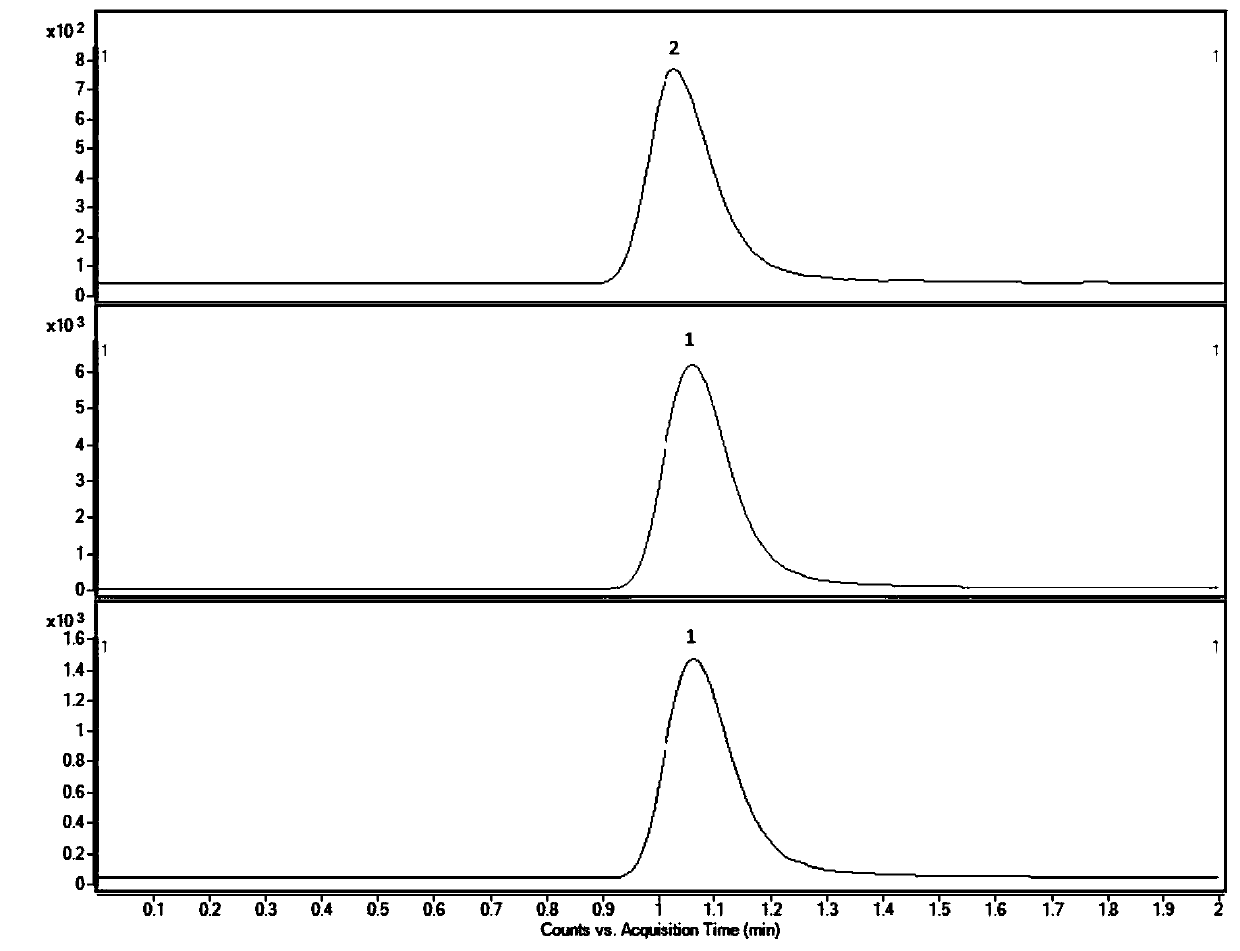
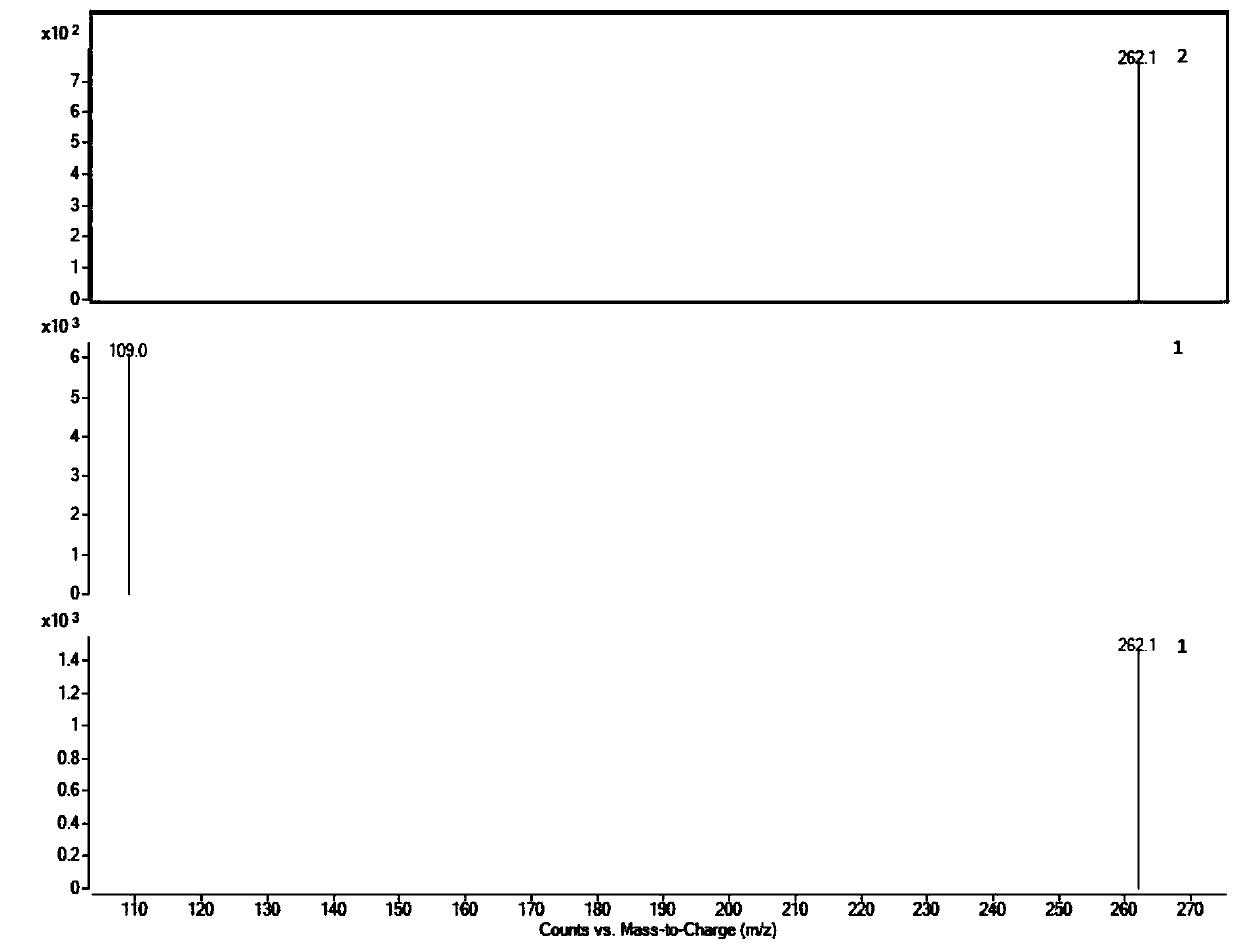
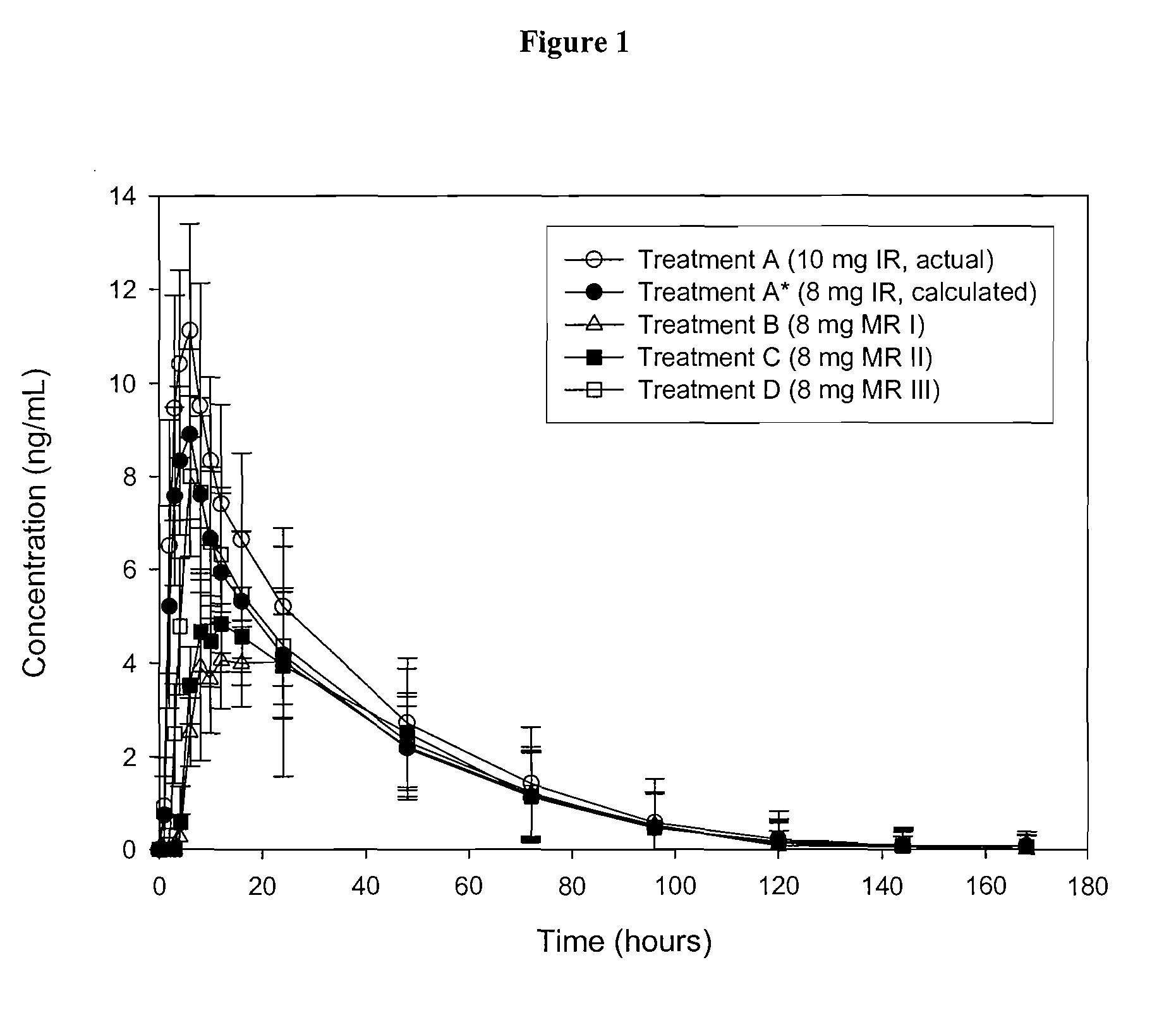
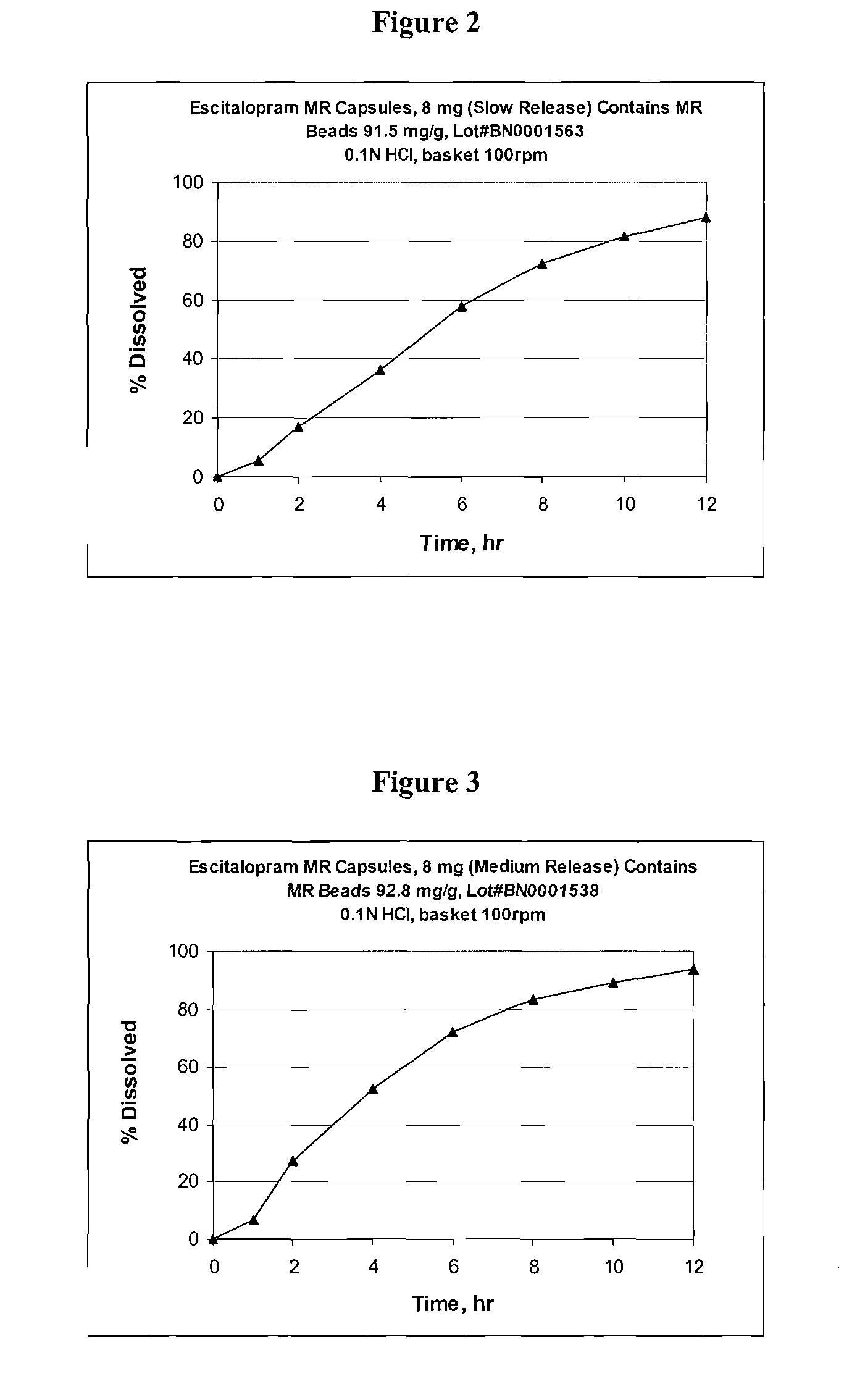
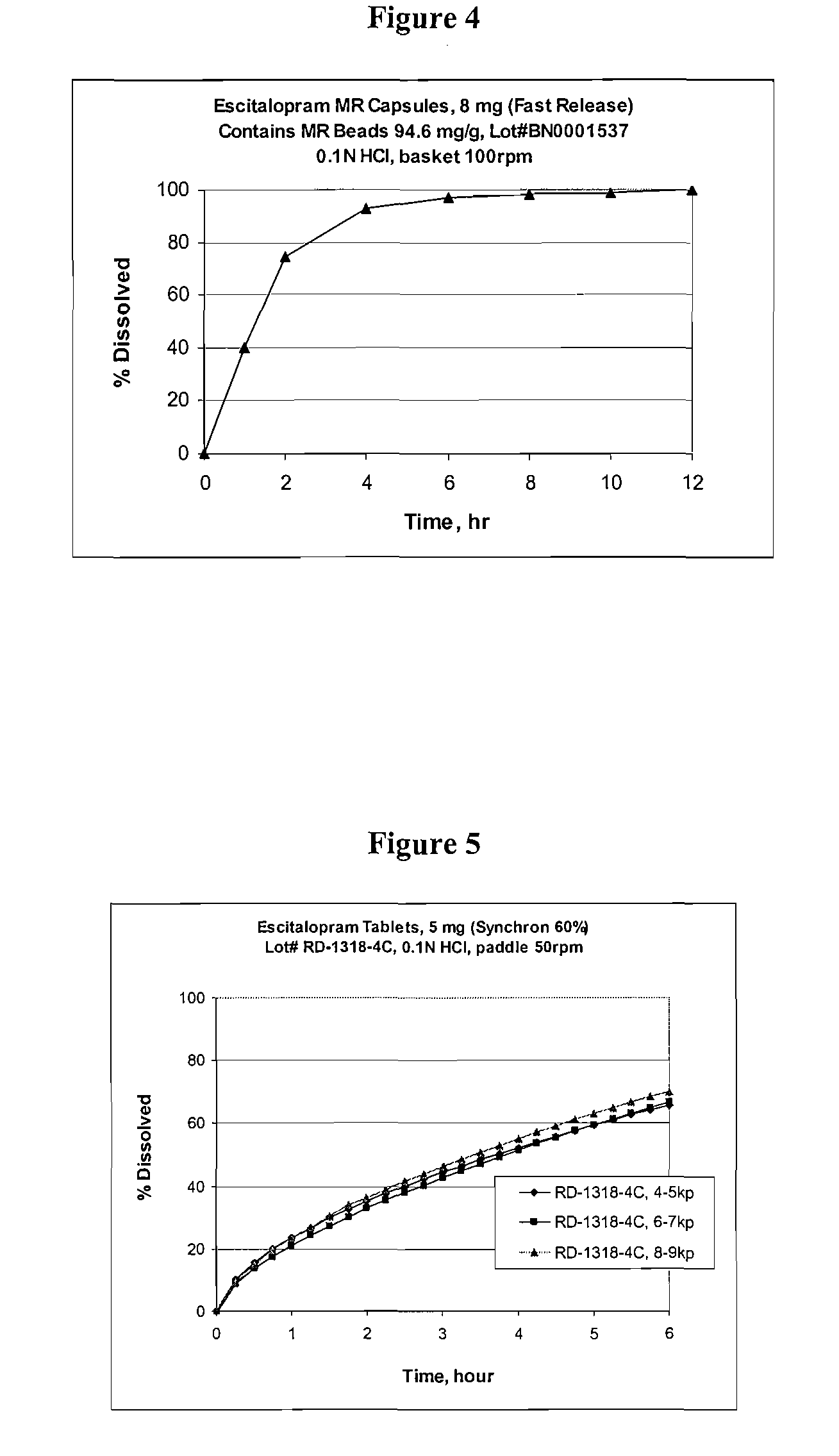
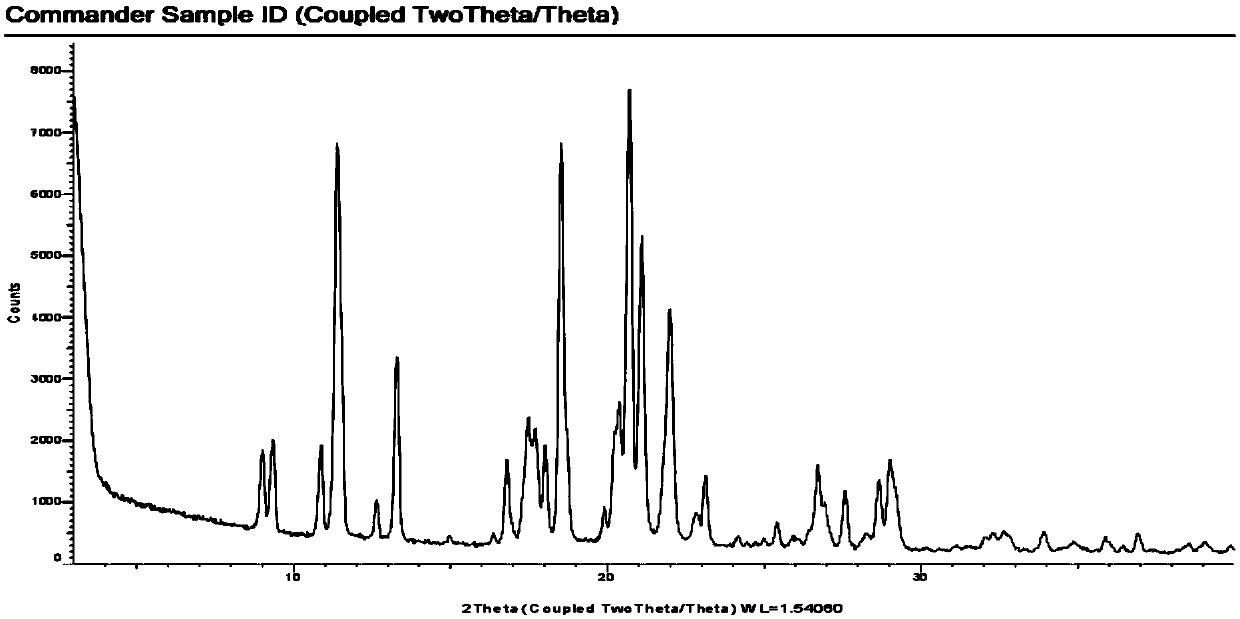
Modified and pulsatile release pharmaceutical formulations of escitalopram
The present invention relates to modified and pulsatile release pharmaceutical formulations of escitalopram and their use for the treatment of central nervous system disorders, including mood disorders (e.g., major depressive disorder) and anxiety disorders (e.g., generalized anxiety disorder, social anxiety disorder, post traumatic stress disorder, and panic disorder, including panic attacks).
Owner:H LUNDBECK AS +1
Novel preparation process of high-purity escitalopram pamoate
The invention relates to a novel preparation method of escitalopram pamoate ((S)-(+)-1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydro-5-cyano isobenzofuran pamoate), wherein the method is environment-friendly and pollution-free, and escitalopram pamoate prepared by the method is high in purity and good in repeatability.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
Crystalline escitalopram hydrobromide and methods for preparing the same
The present invention provides an escitalopram (S-citalopram) in the form of its hydrobromide, methods for the preparation thereof and pharmaceutical compositions thereof.
Owner:H LUNDBECK AS
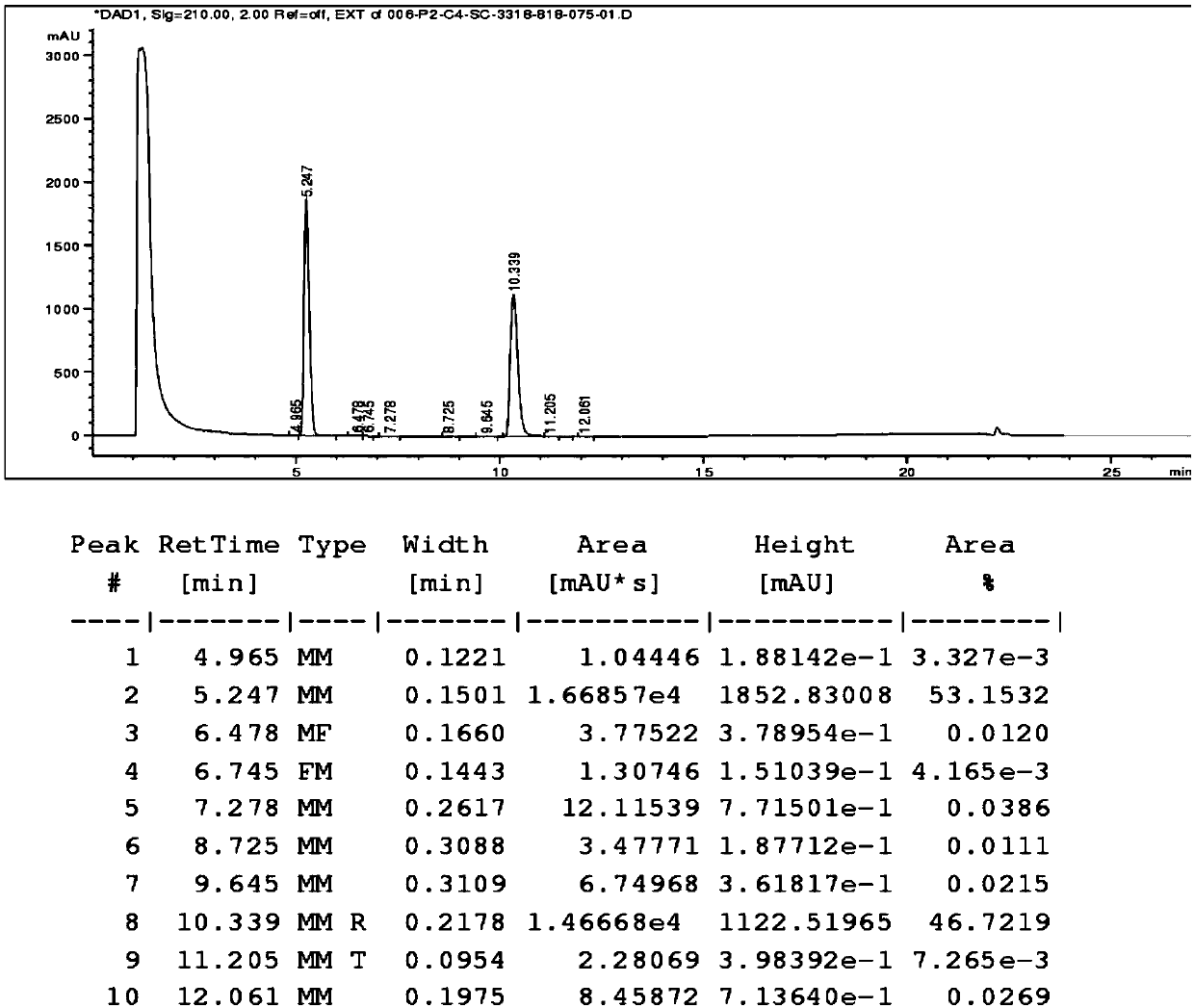
Liquid chromatographic analysis method for detecting escitalopram content in blood
The invention provides a liquid chromatographic analysis method for detecting escitalopram content in blood. The method is characterized in that a standard solution is calibrated through a liquid chromatographic analysis device and a fluorescence detector, and a standard curve equation can be obtained as y=a*x+b by fitting; a blood sample to be detected is prepared and processed and then is detected to obtain y value of the blood to be detected through the liquid chromatographic analysis device and the fluorescence detector; the y value of the blood to be detected is substituted into the standard curve equation, and the relative content x of a target substance in the blood sample to be detected can be calculated; the content of working liquid of an internal standard substance is known, andon that basis, the concentration of escitalopram in the blood to be detected in the sample is calculated. With the adoption of the method, the accuracy of the quantitation result is improved; the system error is avoided; the analyzing time is extremely shortened; the detection process is simple, convenient and quick; experimental foundation is provided for personalized dosing of escitalopram andreducing toxic and side effects of escitalopram.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Method of treating premenstrual dysphoric disorder with escitalopram
The present invention relates to a method of treating the symptoms of premenstrual dysphoric disorder (PMDD) in a patient in need thereof by administering an effective amount of escitalopram or a pharmaceutically acceptable salt thereof.
Owner:H LUNDBECK AS
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
InactiveCN101189220AOrganic active ingredientsOrganic compounds purification/separation/stabilisationOxalateHydrobromide
The present invention relates to the crystalline base of the well known antidepressant drug escitalopram, S-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, formulations of said base, a process for the preparation of purified salts of escitalopram, such as the oxalate, using the base, the salts obtained by said process and formulations containing such salts, and a process for the preparation of purified escitalopram free base or salts of escitalopram, such as the oxalate, using the hydrobromide, the salts obtained by said process and formulations containing such salts. Finally the present invention relates to an orodispersible tablet having a hardness of at least 22 N and an oral-disintegration time of less than 120 s and comprising an active pharmaceutical ingredient adsorbed onto a water soluble filler wherein the active pharmaceutical ingredient has a melting point in the range of 40-100 DEG C, as well as a method for making such an orodispersible tablet.
Owner:H LUNDBECK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com