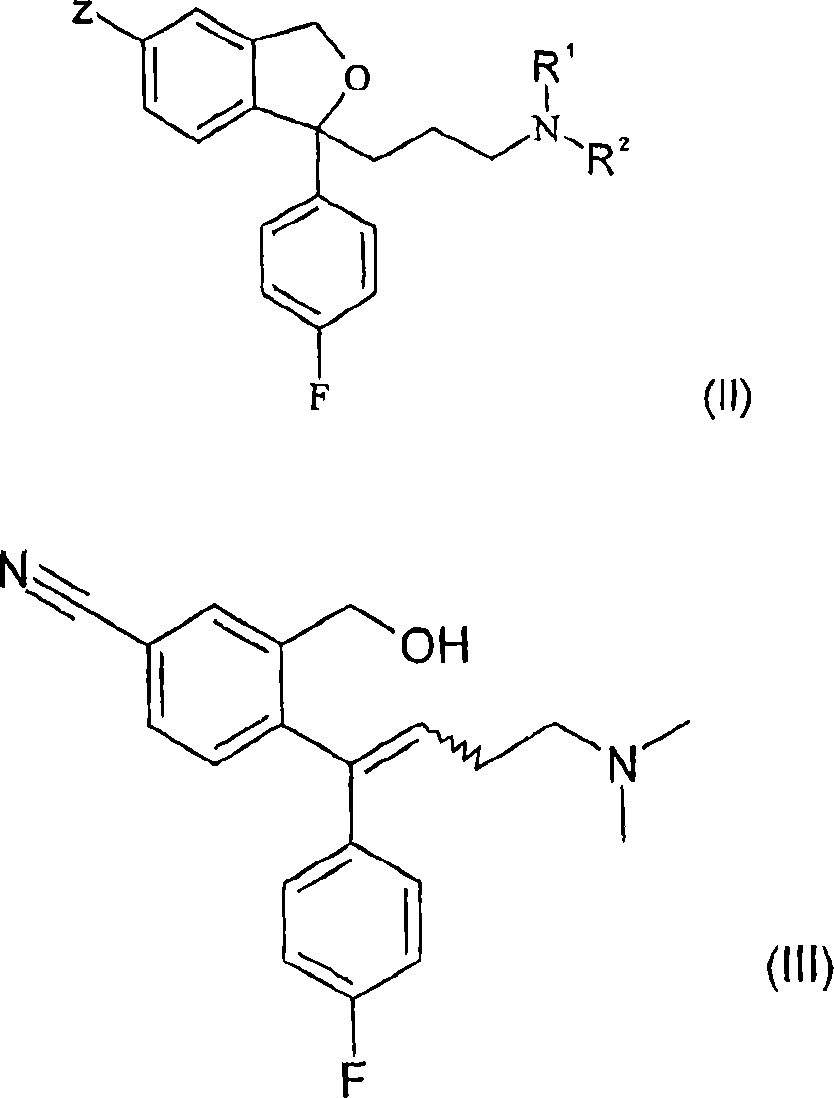
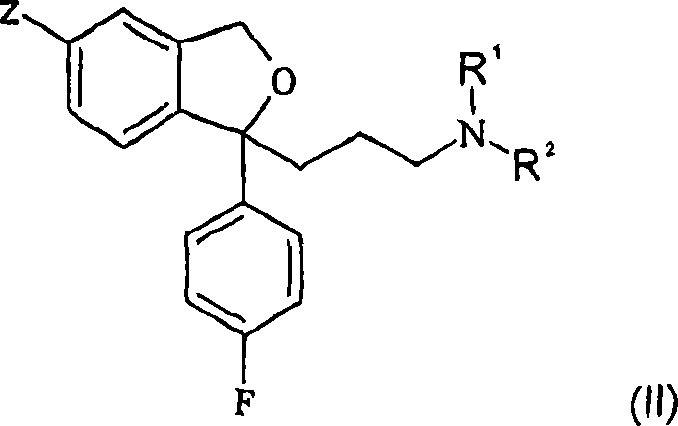
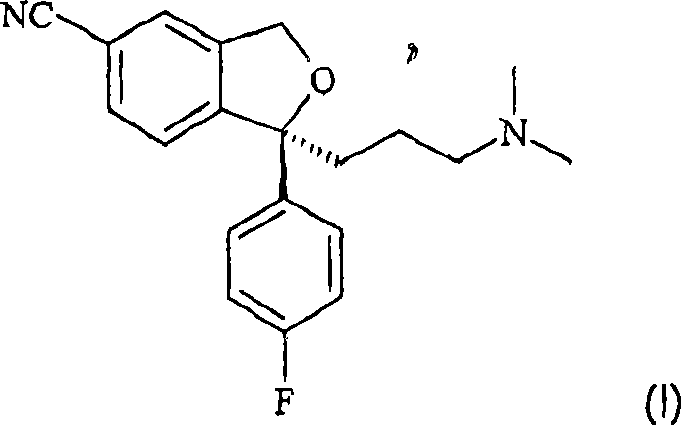
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
A technology of escitalopram and dispersible tablets, applied to the crystalline base of escitalopram: [0019] [, with melt agglomeration or melt coating filler, wherein the active pharmaceutical ingredient has 40-, It can solve the problems of fast disintegrating tablets, poor tablet strength, hindering the application of conventional packaging materials, and low friability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1, free release escitalopram free base from escitalopram oxalate
[0104] 704 g of escitalopram oxalate were placed in a 6 L three-necked flask equipped with a mechanical stirrer and a pressure compensating funnel. 3 L of water was added, followed by 600 mL of diethyl ether. The pH was adjusted to 9-9.5 by adding 27% w / w ammonia and the mixture was stirred for 1 / 2 hour. The phases were separated and the aqueous phase was re-extracted with 300 mL diethyl ether. The combined organic phases were washed twice with 300 mL of water and washed with MgSO 4 Drying, filtration and evaporation under vacuum at 40°C gave a light brown oil.
[0105] Yield: 542.5 g (98.4%)
Embodiment 2
[0106] Embodiment 2, the precipitation of escitalopram hydrobromide, the release of free base and the crystallization of base
[0107] Precipitation of escitalopram hydrobromide:
[0108] 3 kg of escitalopram free base (purified by HPLC: 99.16% (area %)) as a light brown oil dissolved in 12 kg of 2-propanol was added to 20 L with mechanical stirring, reflux condenser, scrubber , gas inlet and thermometer for thermostatic control of the reactor. The solution was heated to 40° C., and then HBr gas was injected into the solution until the pH was 3-4. The reaction is exothermic and the temperature in the reactor is maintained between 40-43°C. A small amount of seed crystals (escitalopram hydrobromide, 100-200 mg) was added and crystallization started within 10 minutes. The mixture was then cooled to 10° C. over 5 hours and kept at this temperature for a further 12 hours. The crystals were filtered off, rinsed on the filter with 3 x 1 L 2-propanol, and dried under vacuum at 60...
Embodiment 3
[0119] Embodiment 3, the crystallization of escitalopram free base
[0120] 520 escitalopram free base (purity: 99.25%; HPLC) of light brown oily state was placed in 2 L equipped with mechanical stirrer, reflux condenser, N 2 Inlet / outlet and thermometer for thermostatic control of the reactor. 50 mL of ethyl acetate was added, and the mixture was heated to 35° C., followed by the addition of 1.3 L of heptane. When the solution was homogeneous, slow cooling to -5°C was initiated over 12 hours. When the temperature was 20°C, a small amount of seed crystals (escitalopram base) (10-20 mg) was added. Crystallization begins at about 10°C. The mixture was stirred at -5°C for another 5-7 hours, then the crystals were filtered off by filtration. The crystals were rinsed with 2 x 150 mL heptane on the filter and dried to constant weight under vacuum at 25°C.
[0121] Yield: 485 g (93.3%)
[0122] HPLC purity of product: 99.58% (area %)
[0123] Melting point (DSC, onset): 45.8°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com