Patents
Literature
38 results about "Escitalopram Oxalate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
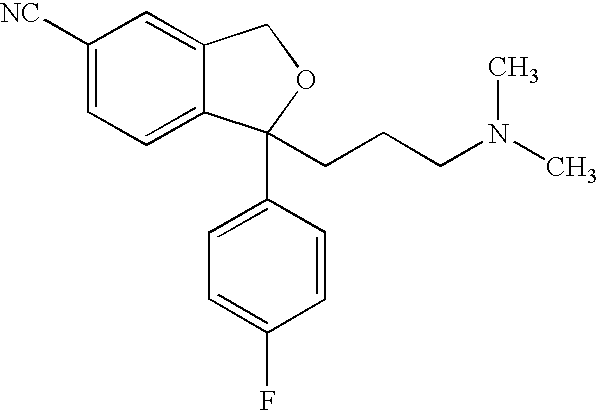
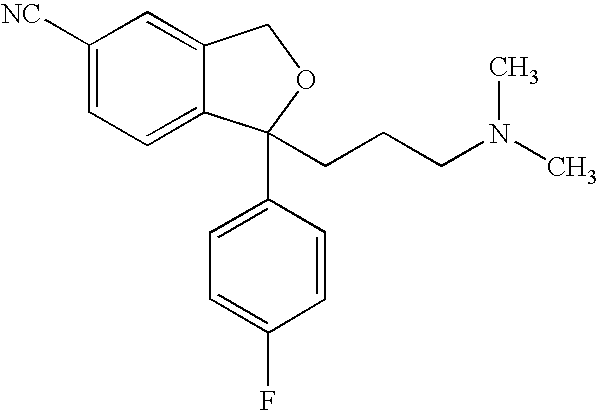
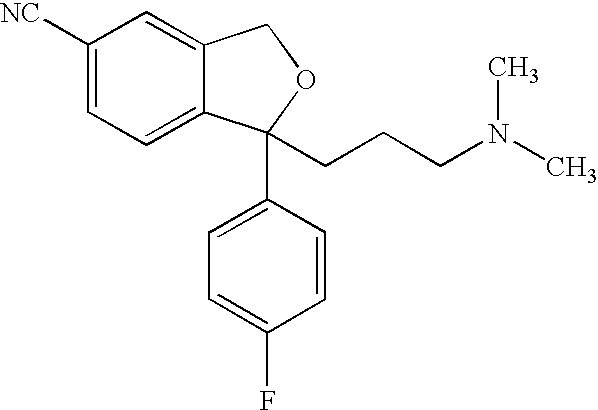
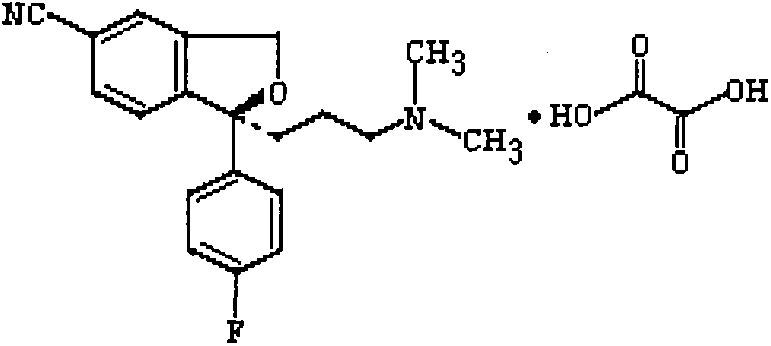
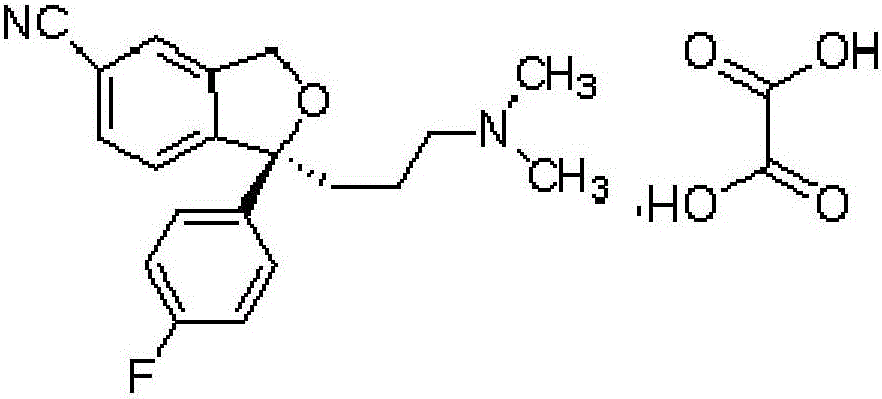
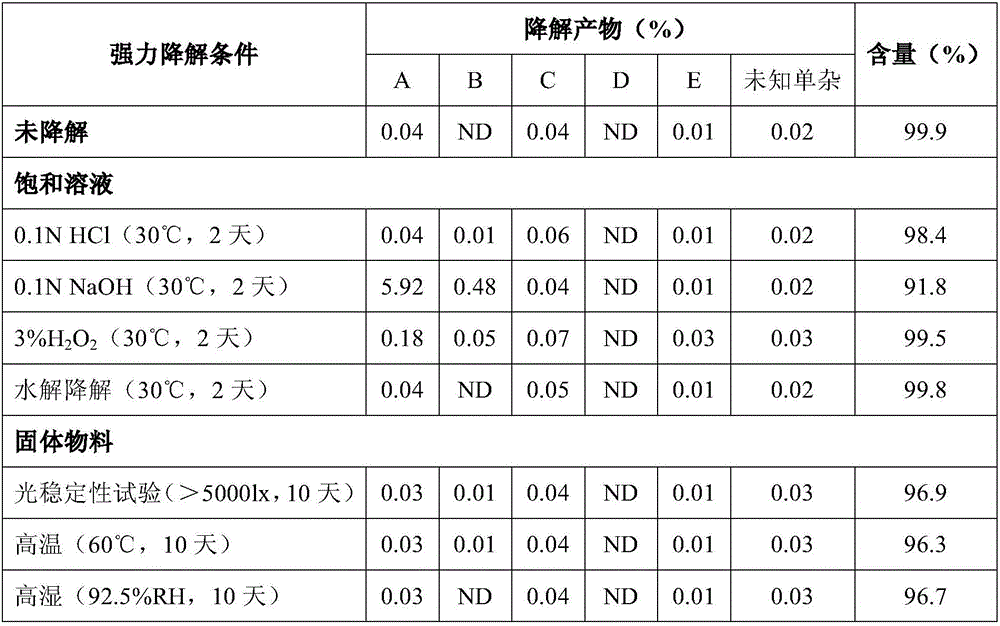
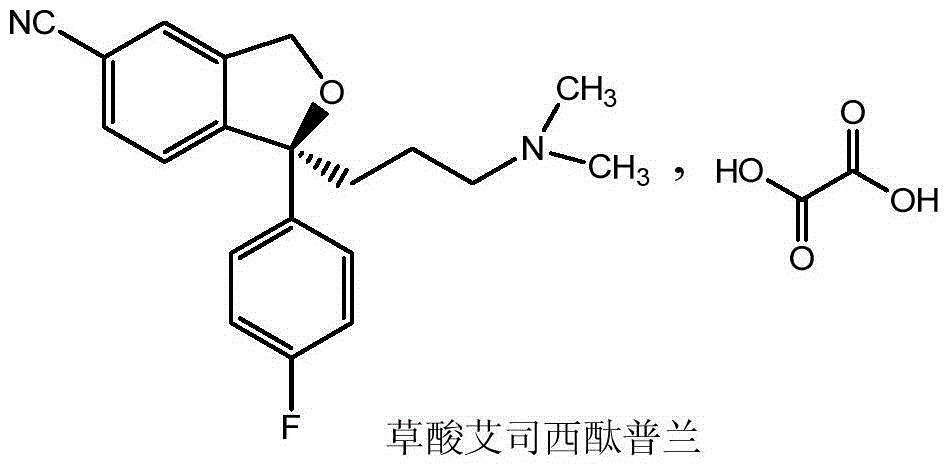
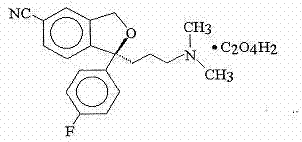
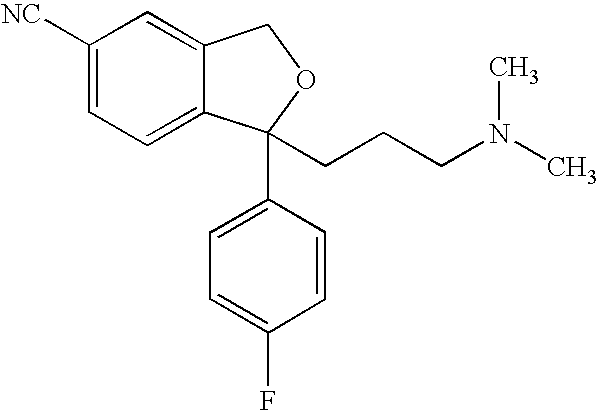
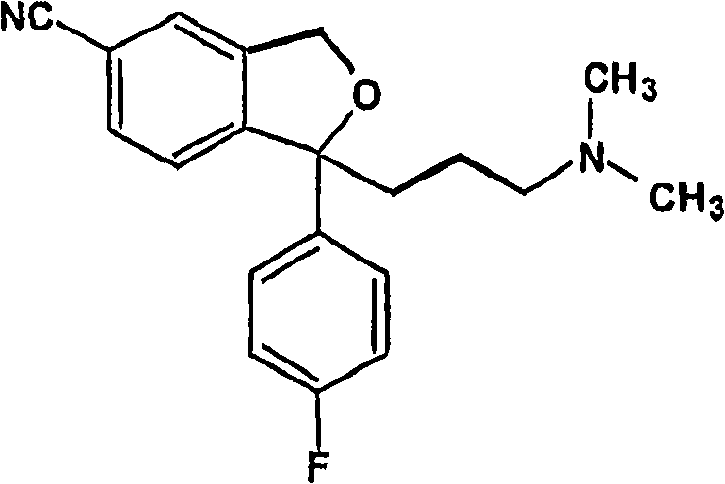
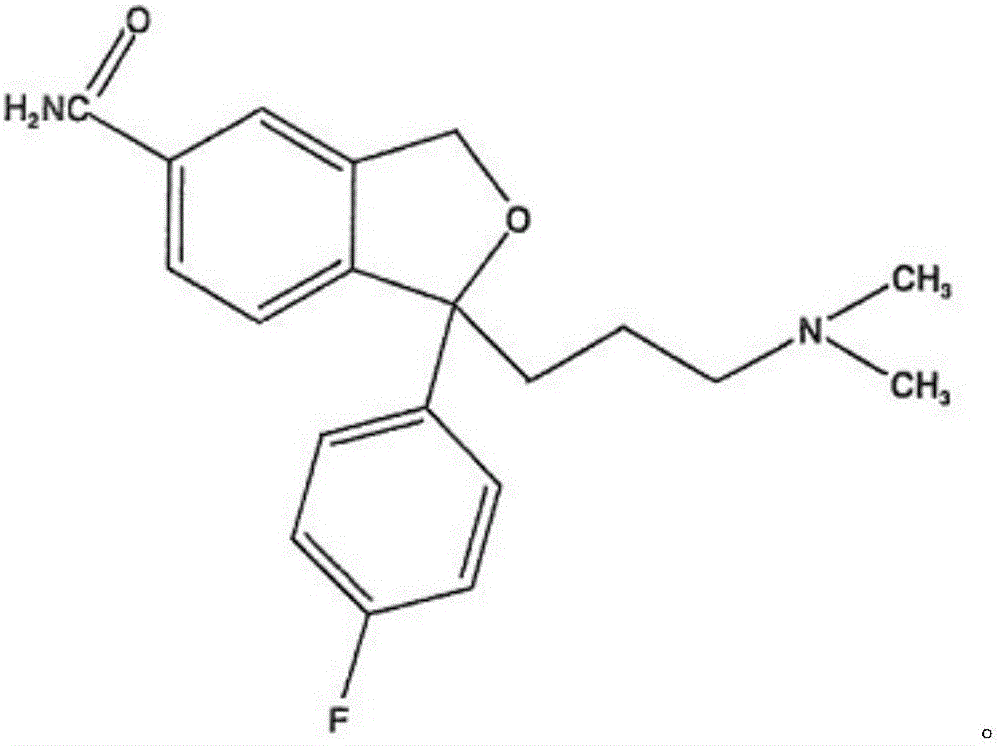
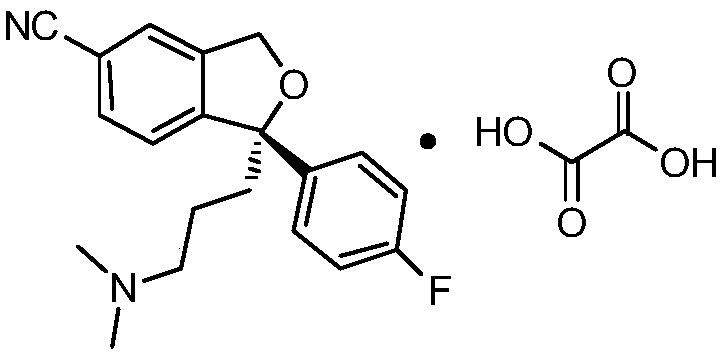
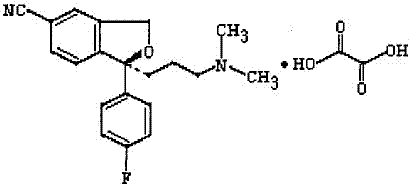
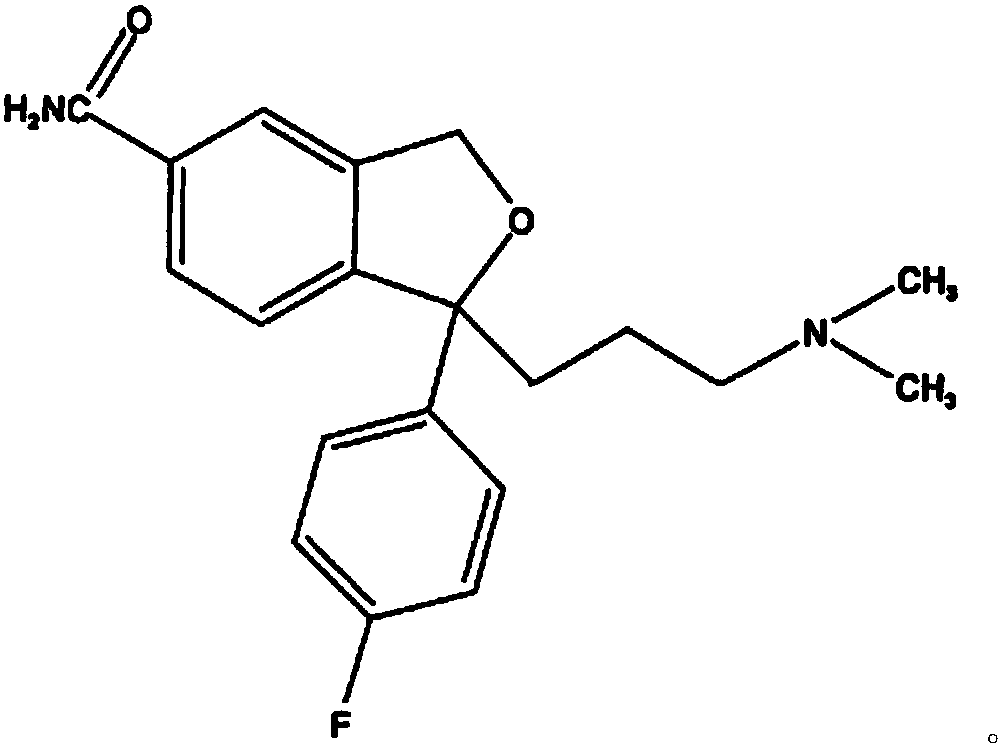
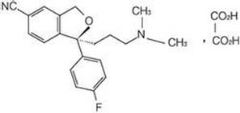
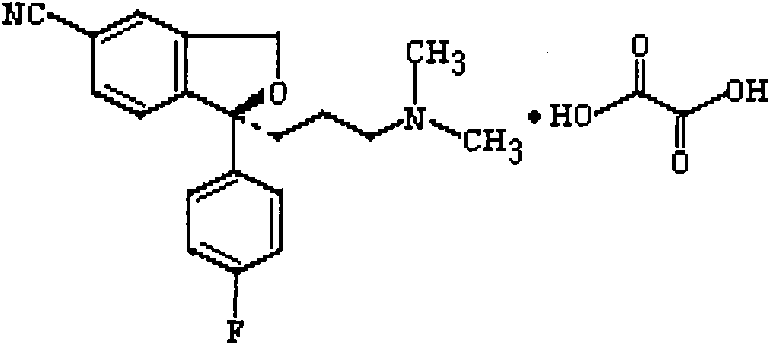
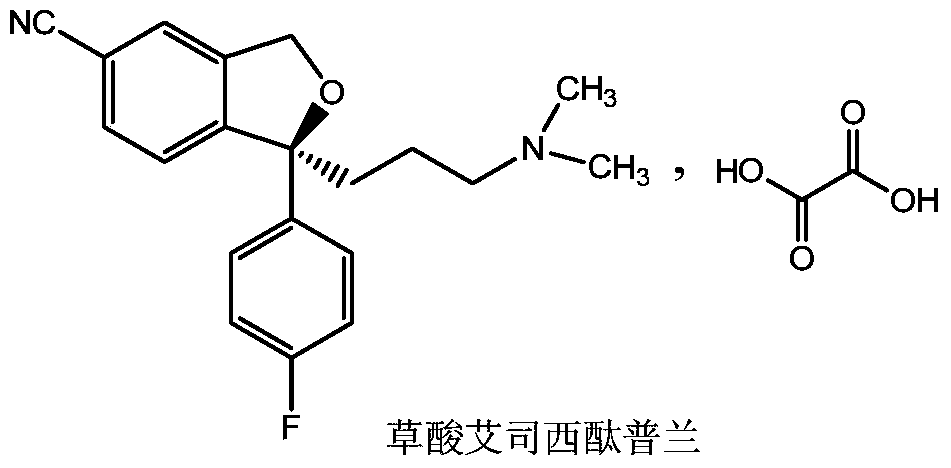
The oxalate salt of escitalopram, a pure S-enantiomer of the racemic bicyclic phthalane derivative citalopram, with antidepressant activity. As a selective serotonin reuptake inhibitor (SSRI), escitalopram blocks the reuptake of serotonin by neurons in the central nervous system (CNS), thereby potentiating CNS serotonergic activity.
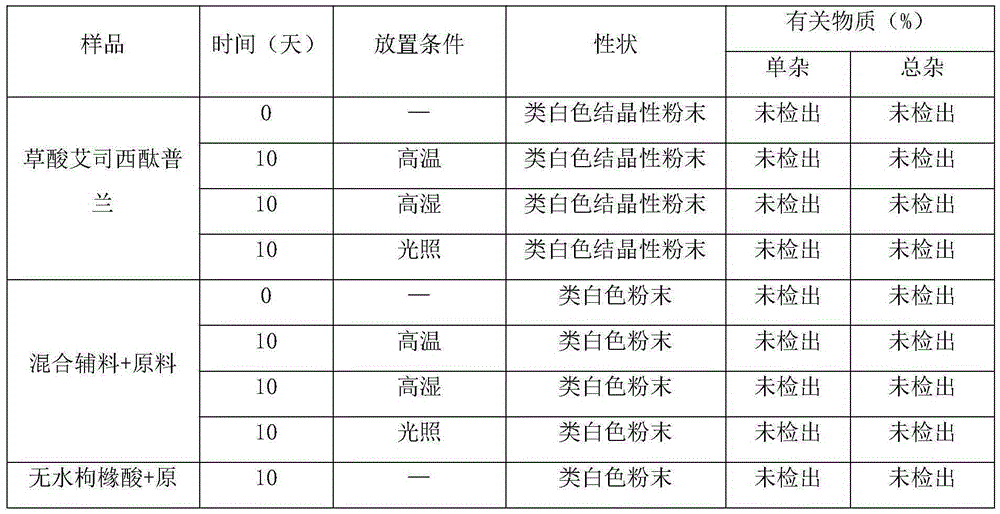
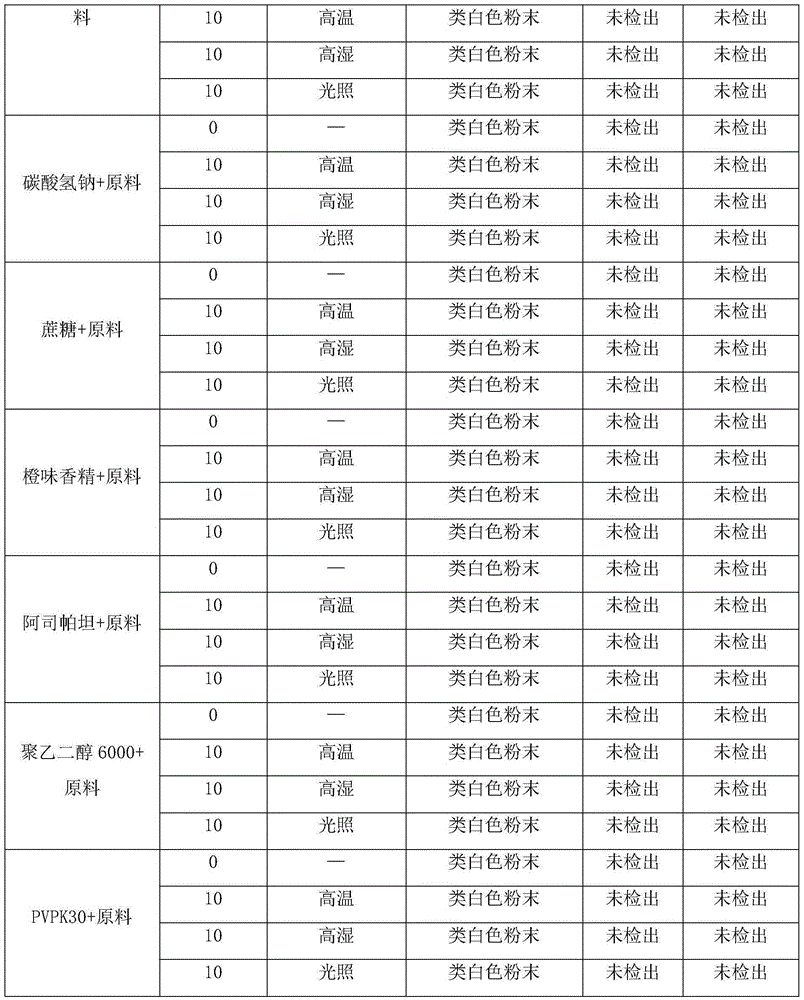
Crystalline composition containing escitalopram
The present invention discloses crystalline particles of escitalopram oxalate which either have a broad particle size distribution or comprise at least 0.01% (w / w) of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, said particles being suitable for use in direct compression. Furthermore, the invention discloses a novel pharmaceutical unit dosage form containing such crystalline particles of escitalopram oxalate as well as methods for manufacture of such crystalline particles of escitalopram oxalate Finally, the invention provides a method for reduction of the amount of hydroxyl containing impurities in a solution of citalopram or escitalopram.
Owner:H LUNDBECK AS
Crystalline composition containing escitalopram
InactiveUS6916941B2Granulation avoidedReduce stepsBiocidePowder deliveryCrystallographyCrystalline particle
Crystalline particles of escitalopram oxalate with a particle size of at least 40 μm is disclosed. Method for the manufacture of said crystalline particles and pharmaceutical compositions comprising said crystalline particles are also disclosed.
Owner:H LUNDBECK AS
Crystalline composition containing escitalopram
The present invention discloses crystalline particles of escitalopram oxalate which either have a broad particle size distribution or comprise at least 0.01% (w / w) of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, said particles being suitable for use in direct compression. Furthermore, the invention discloses a novel pharmaceutical unit dosage form containing such crystalline particles of escitalopram oxalate as well as methods for manufacture of such crystalline particles of escitalopram oxalate Finally, the invention provides a method for reduction of the amount of hydroxyl containing impurities in a solution of citalopram or escitalopram.
Owner:H LUNDBECK AS
Tablet containing escitalopram oxalate and preparation method thereof
ActiveCN104523638ASolve Content Uniformity ProblemsSolve the sticking problemOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveChemistry
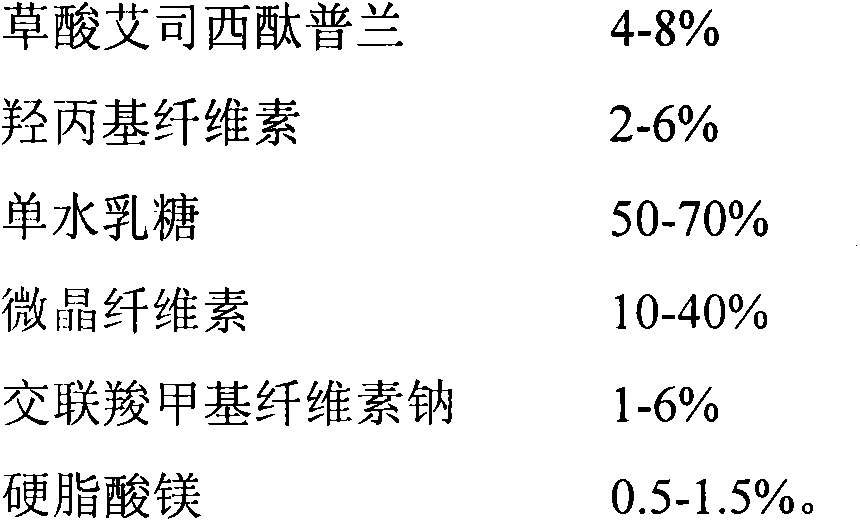
The invention discloses a tablet containing escitalopram oxalate and a preparation method thereof. According to the invention, the tablet containing escitalopram oxalate uses hydroxy propyl cellulose as an adhesive and uses escitalopram oxalate with median particle size less than or equal to 20mum, and the prepared tablet containing escitalopram oxalate has the advantages of beautiful appearance and stable quality. The invention also provides the preparation method of oral tablet, and has the advantages of simple process and adaptability of commercialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
HPLC (high-performance liquid chromatography) detection method for escitalopram oxalate related substances
ActiveCN106324141AGood linear relationshipStrong specificityComponent separationAcetonitrileGradient elution
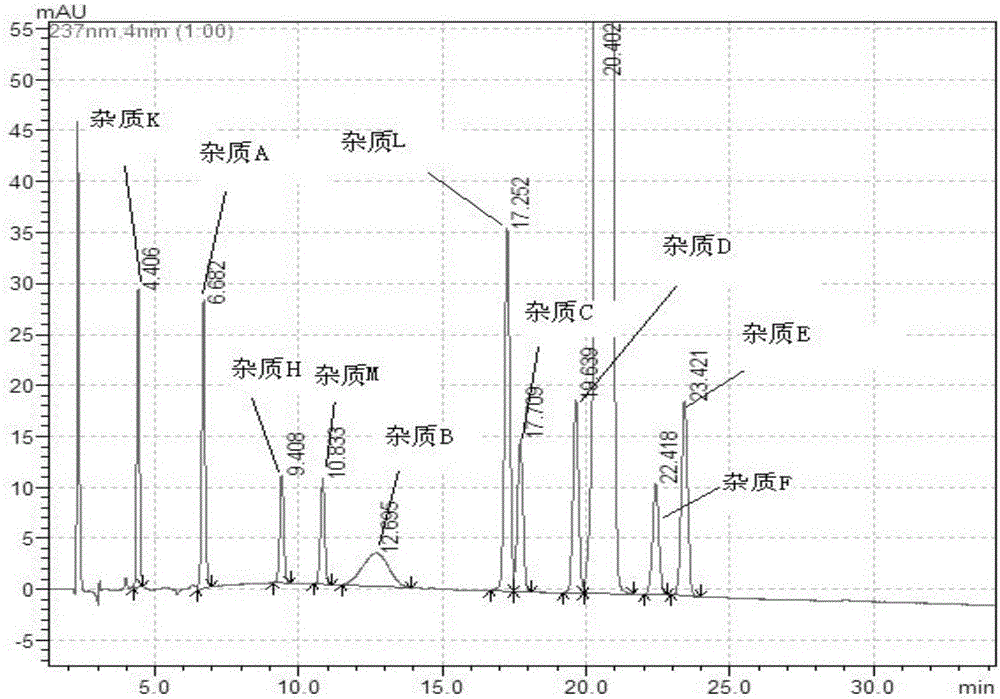
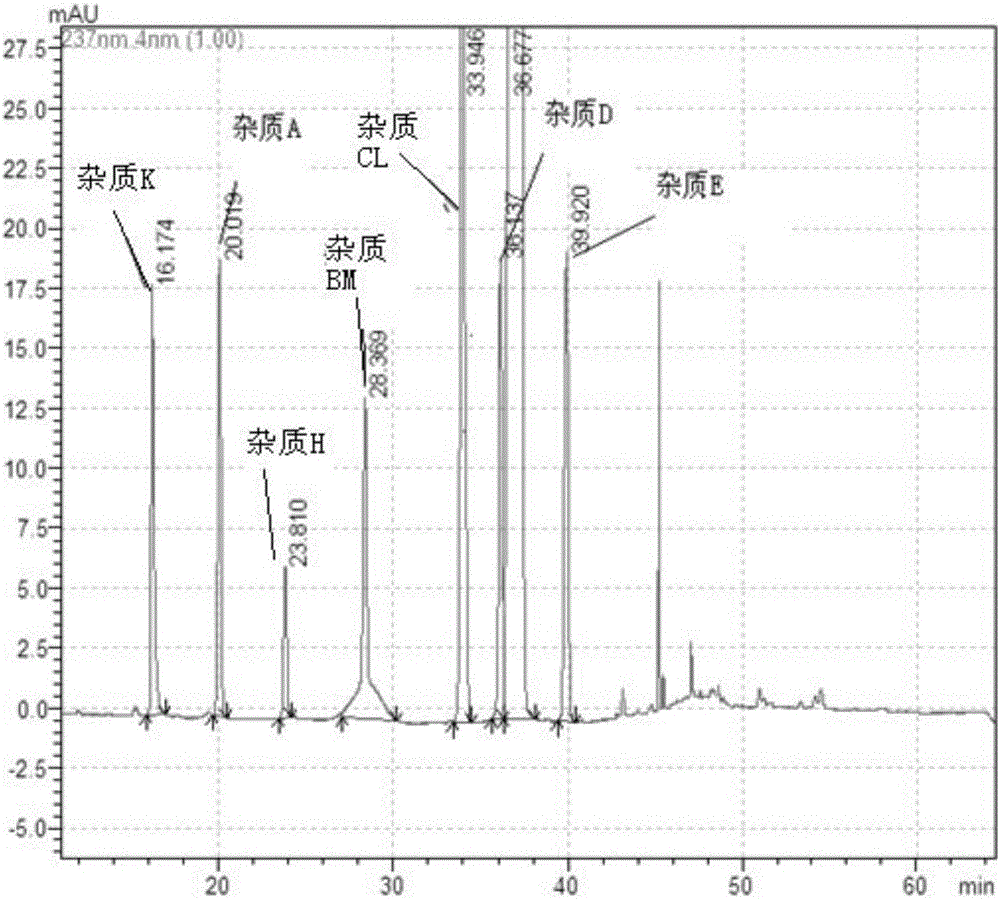
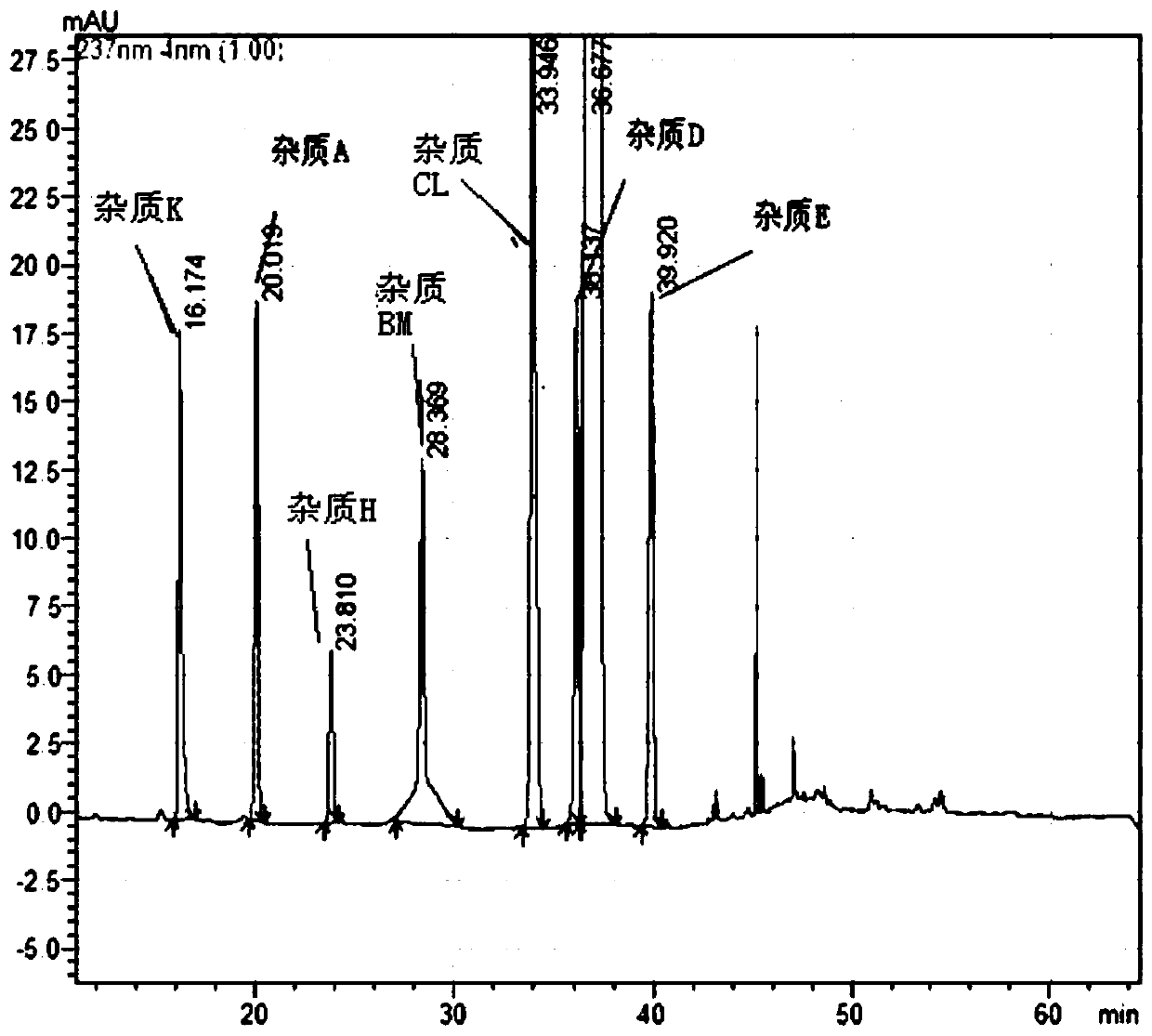
The invention belongs to the field of pharmaceutical analysis and particularly relates to a HPLC (high-performance liquid chromatography) detection method for escitalopram oxalate related substances. The escitalopram oxalate related substances are determined with HPLC, a C18 column is taken as a chromatographic column, a phosphate buffer solution is taken as a mobile phase A, the mobile phase A and an acetonitrile solution are mixed in a certain ratio to form a mobile phase B, the mobile phase A and the acetonitrile solution are mixed in a certain ratio to form a mobile phase C, and the escitalopram oxalate related substances are detected with a gradient elution method. The method can separate impurities, is high in specificity and sensitivity and good in repeatability and durability, can well control known impurities and unknown impurities of escitalopram oxalate and guarantees safety of escitalopram oxalate.
Owner:山东锐顺药业有限公司
Preparation methods for impurities of escitalopram oxalate
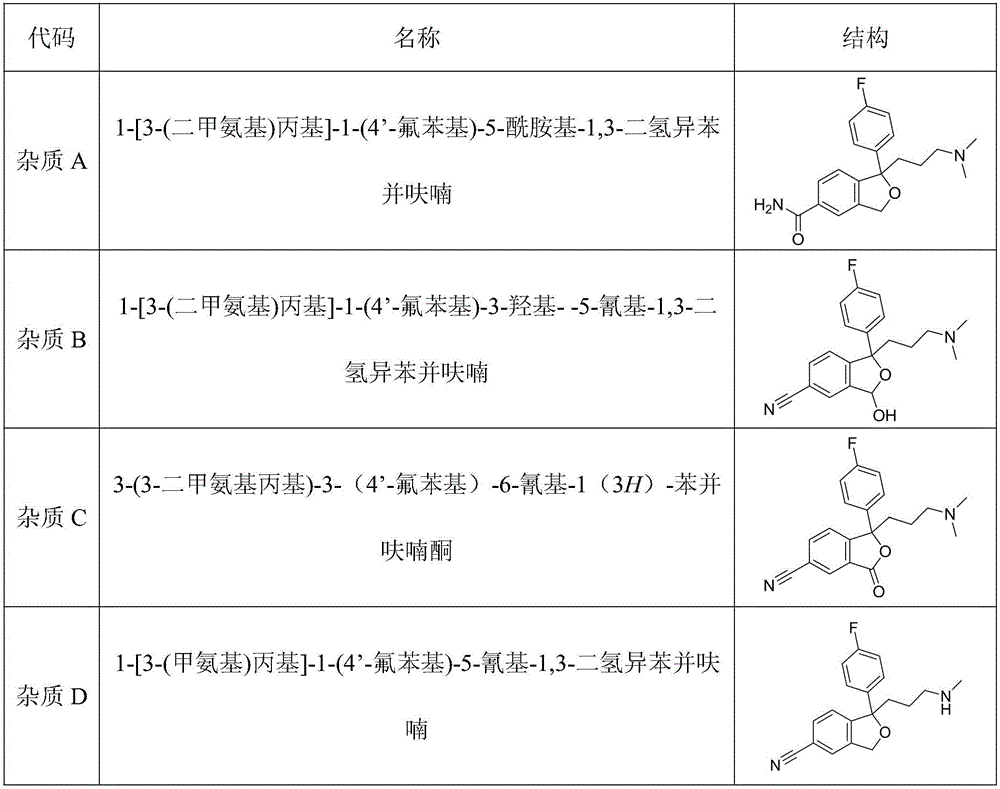
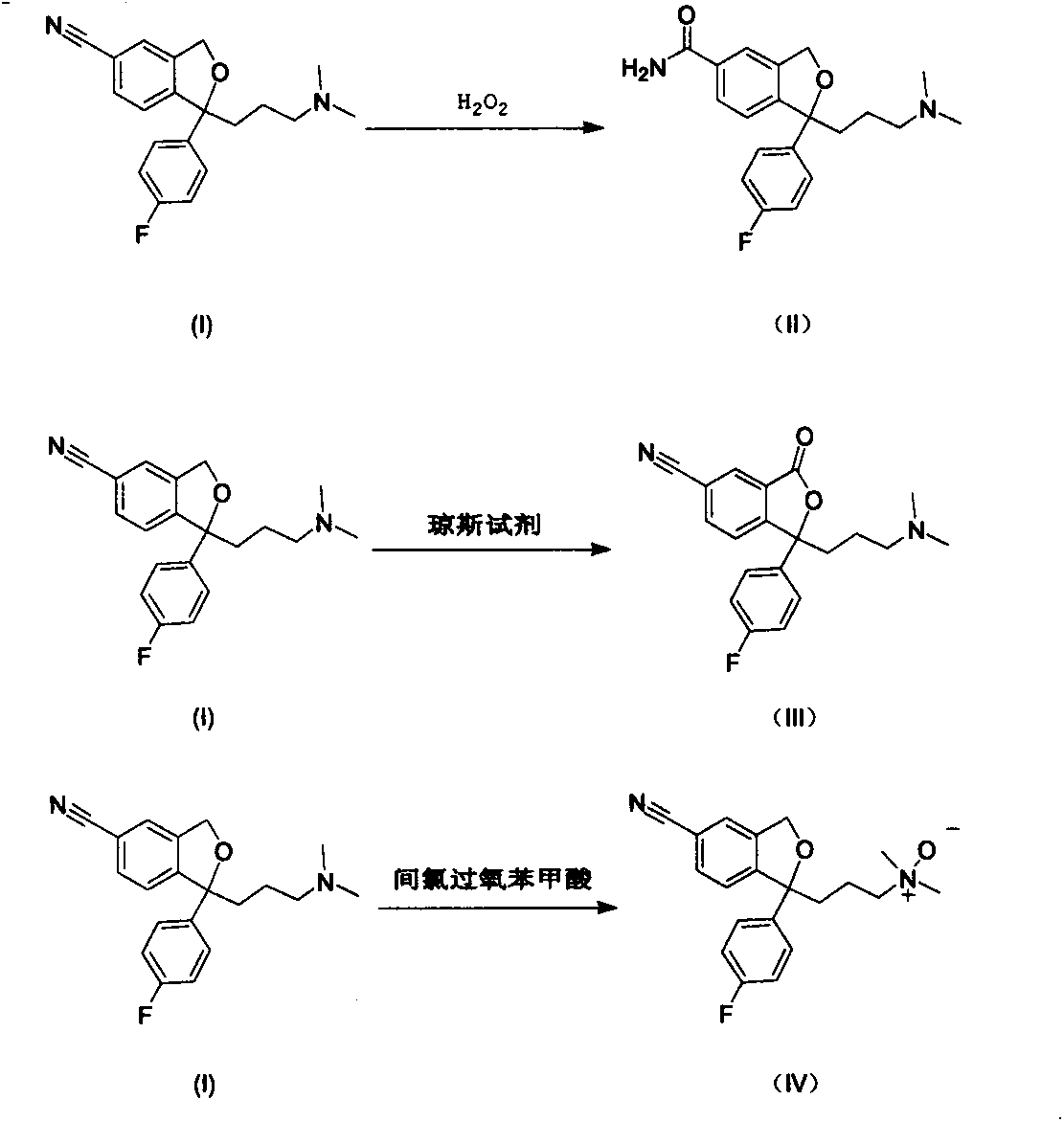
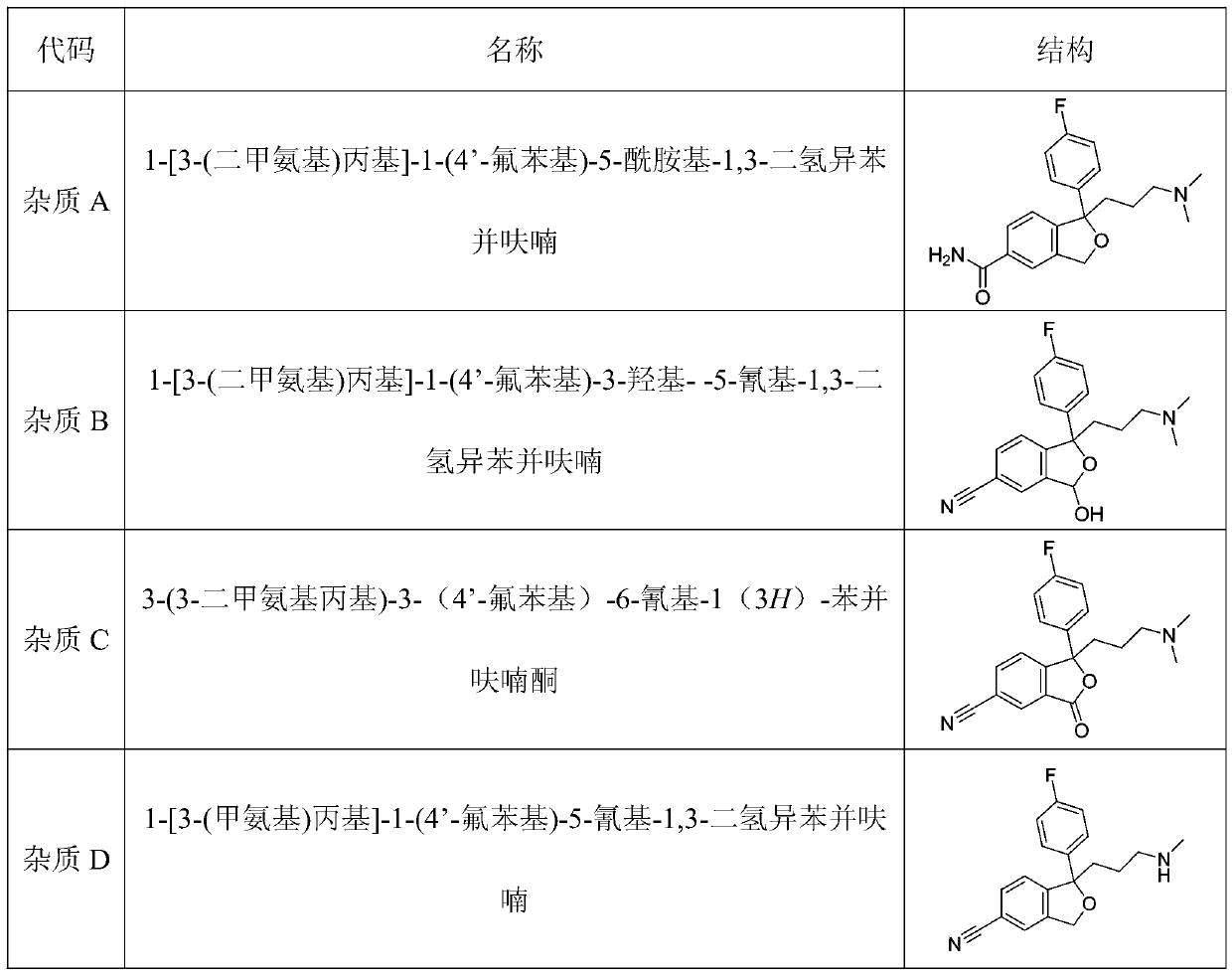
The invention relates to novel synthetic methods for three impurities of escitalopram oxalate. The methods have great significance for synthesis of the escitalopram oxalate with high purity. The invention mainly study syntheses of a citalopram amide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-formamide (II), a citalopram lactone impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3-oxo-1,3-dihydro-isobenzofuran-5-carbonitrile (III) and a citalopram-N-oxide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-cyano-N-oxide (IV). Specific synthetic routes of the impurities are showed as follows.
Owner:CHINA PHARM UNIV
Escitalopram oxalate oral dissolution film agent and preparation method thereof
InactiveCN106176691ADisperse fastDissolve fastOrganic active ingredientsNervous disorderMedicineEconomic benefits
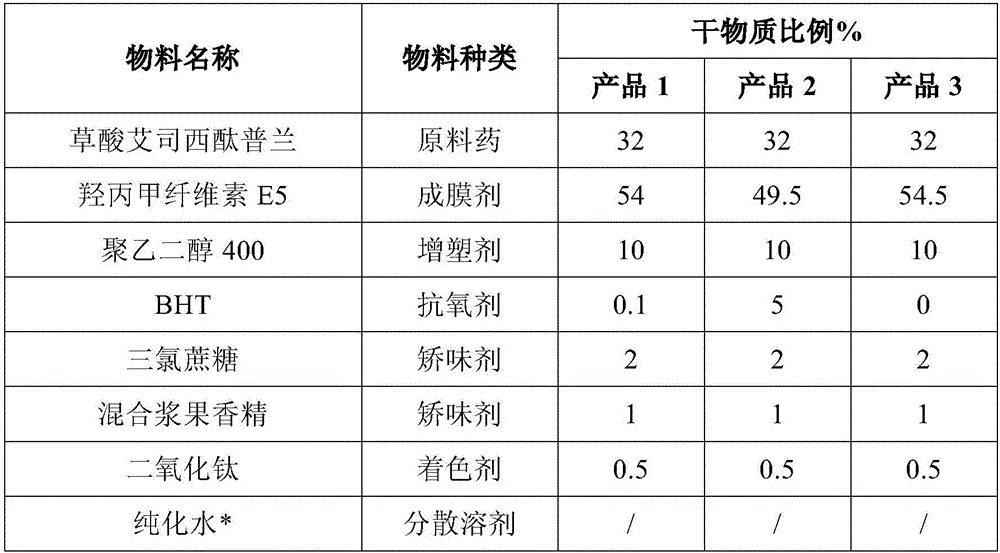
The invention belongs to the technical field of medicinal preparation and particularly relates to an escitalopram oxalate oral dissolution film agent and a preparation method thereof. The escitalopram oxalate oral dissolution film agent comprises an antioxygen, a high polymer film-forming material and the like. The film agent provided by the invention is novel in appearance and excellent in taste, can be dissolved in an oral cavity quickly, can be taken without water, and can be accepted and adopted by patients easily; in addition, compared with common drugs, the film agent provided by the invention is different in appearance and taste, not only can relieve mental stress of patients, but also can protect patients' privacy. The product adopts the process of low temperature drying, is used together with the antioxygen, and ensures excellent product stability. The preparation process is environmentally friendly, low in cost, and has excellent environmental benefits and economic benefits.
Owner:QILU PHARMA CO LTD
Oral solid preparation of escitalopram oxalate and preparation method thereof
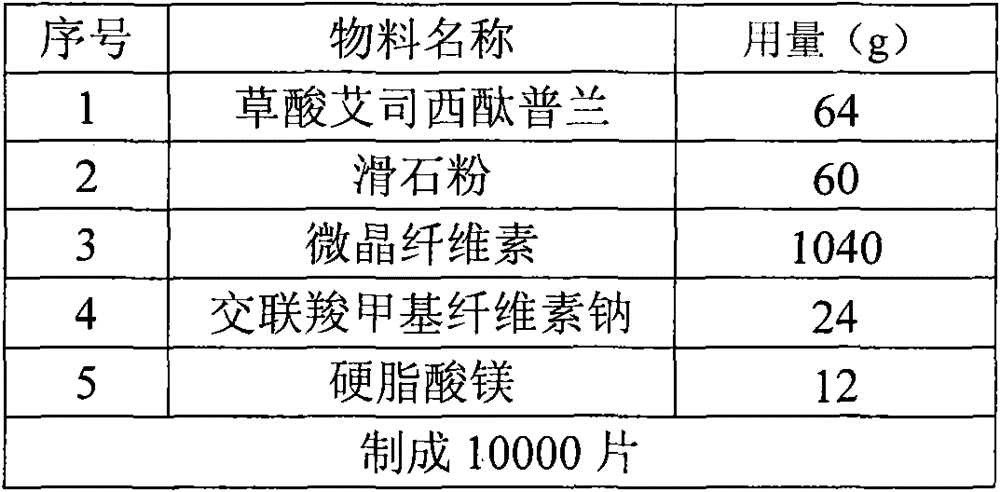
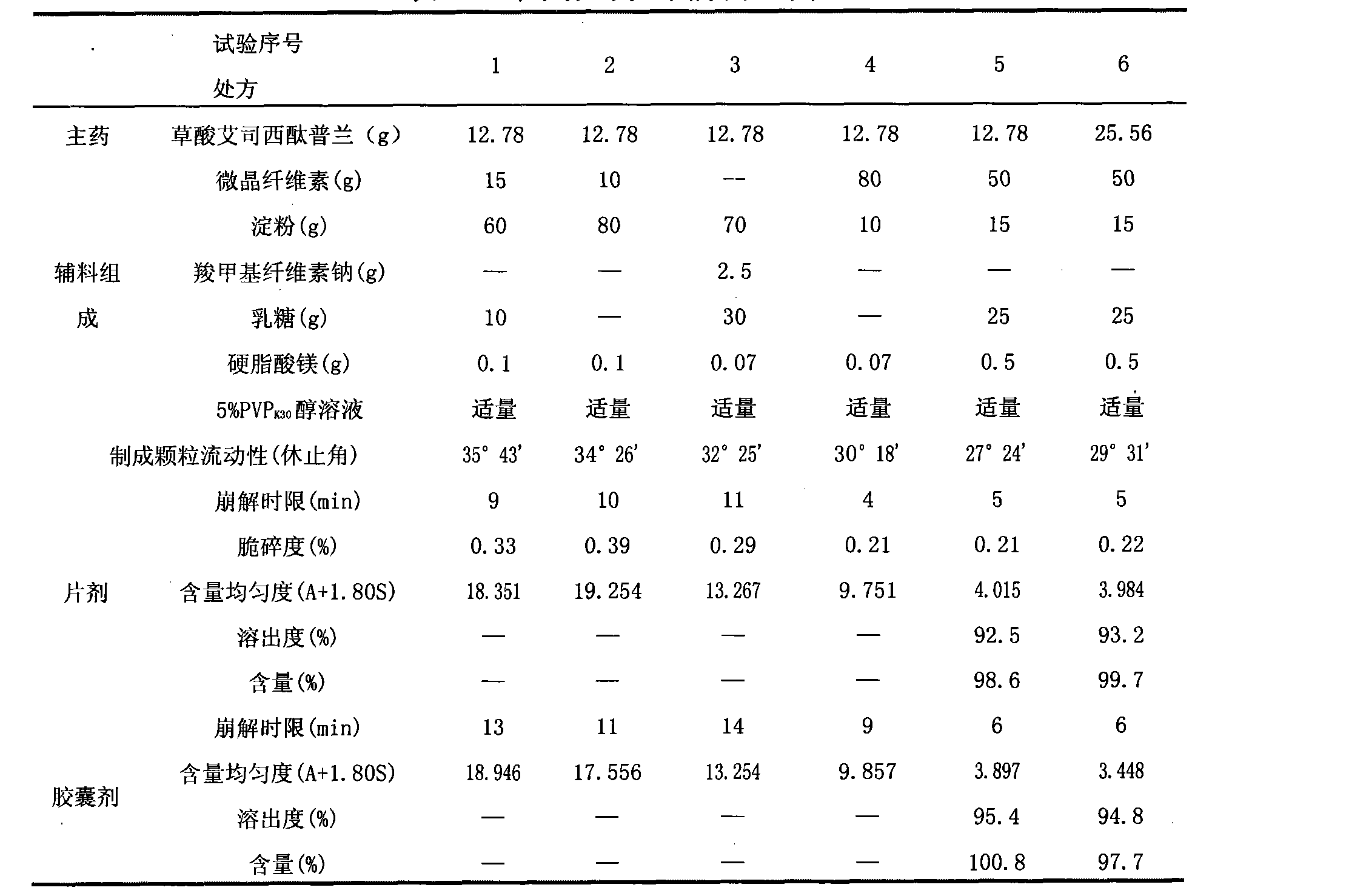
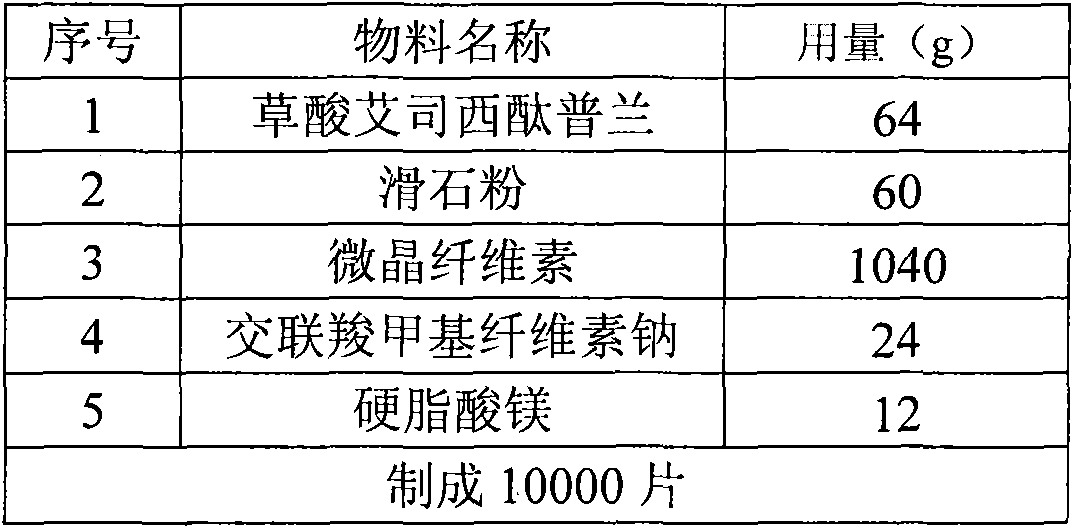
The invention discloses an oxalic acid esmolol citalopram oral solid preparation and a preparation method. The oral solid preparation comprises raw materials of: 2-40 parts by weight of oxalic acid esmolol citalopram, 5-60 parts by weight of starch, 5-200 parts by weight of microcrystalline cellulose, 5-60 parts by weight of lactose, 0.05-5 parts by weight of binding agent and 0.05-3 parts by weight of lubricant; the binding agent is one or a plurality of mixed aqueous solutions or alcoholic solutions from the group consisting of polyvinyl pyrrolidone, hydroxyethyl methylcellulose, methylcellulose, hydroxypropyl cellulose and ethyl cellulose, the concentration of the binding agent is 0.1-10.0%; and the lubricant is stearic acid, magnesium stearate, calcium stearate, talcum or superfine silica gel powder.
Owner:SICHUAN KELUN PHARMA CO LTD
Escitalopram oxalate related substance and preparation method thereof
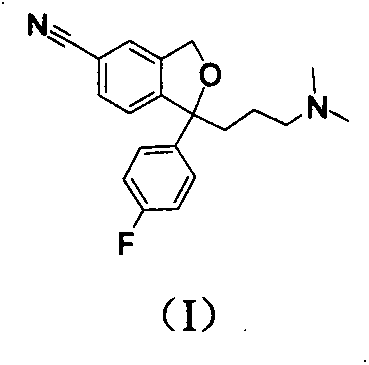
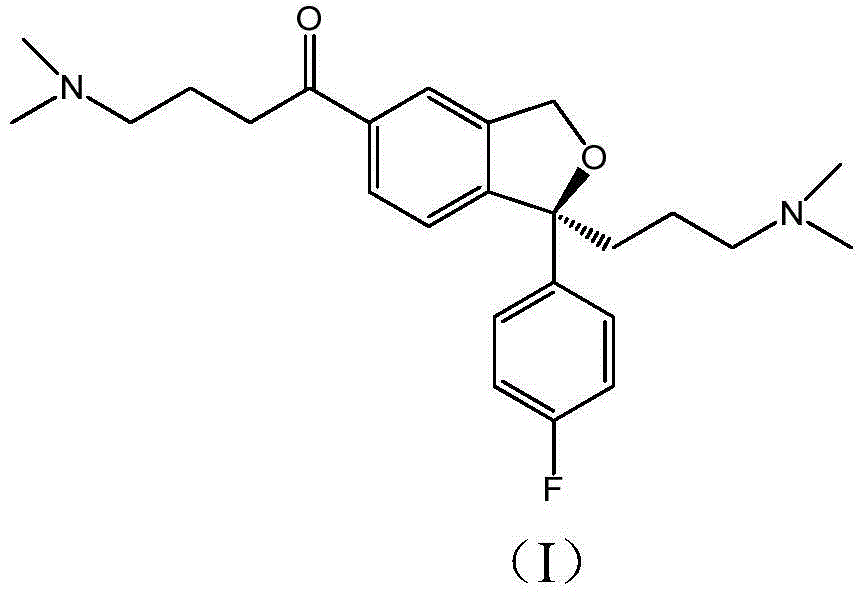
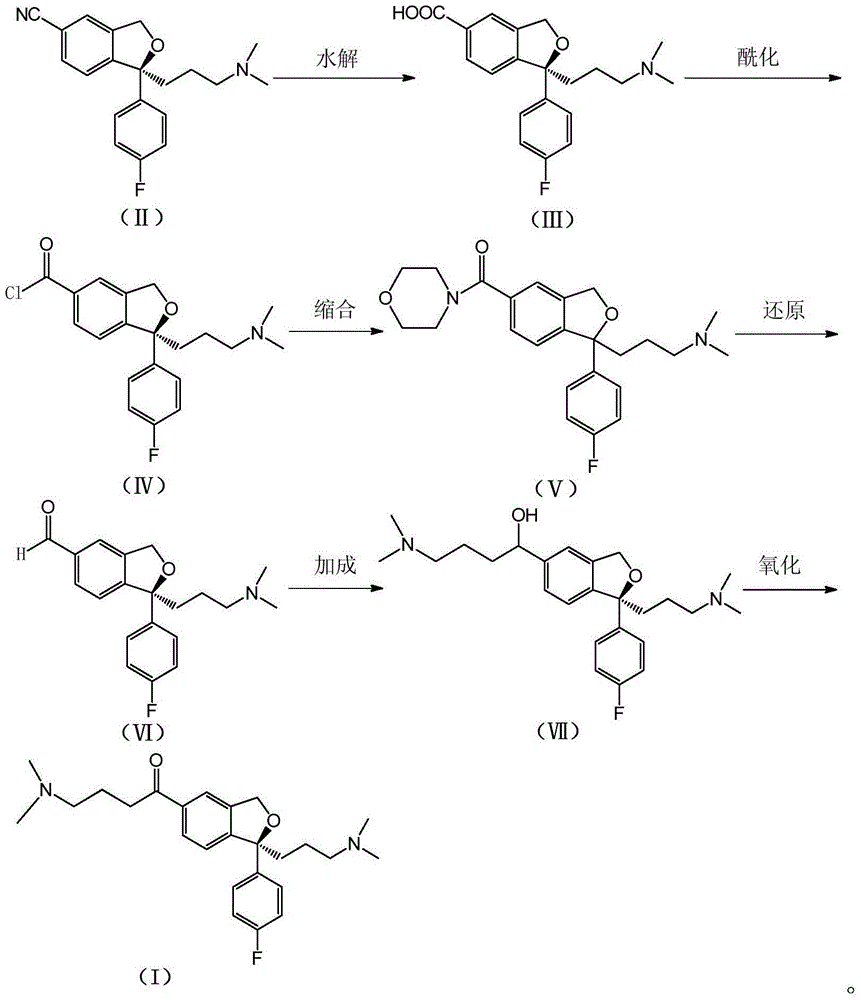
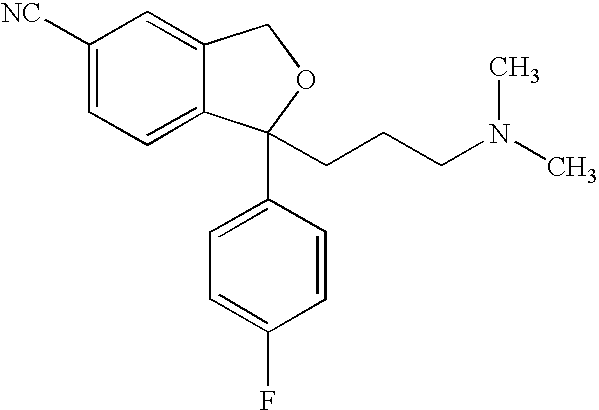
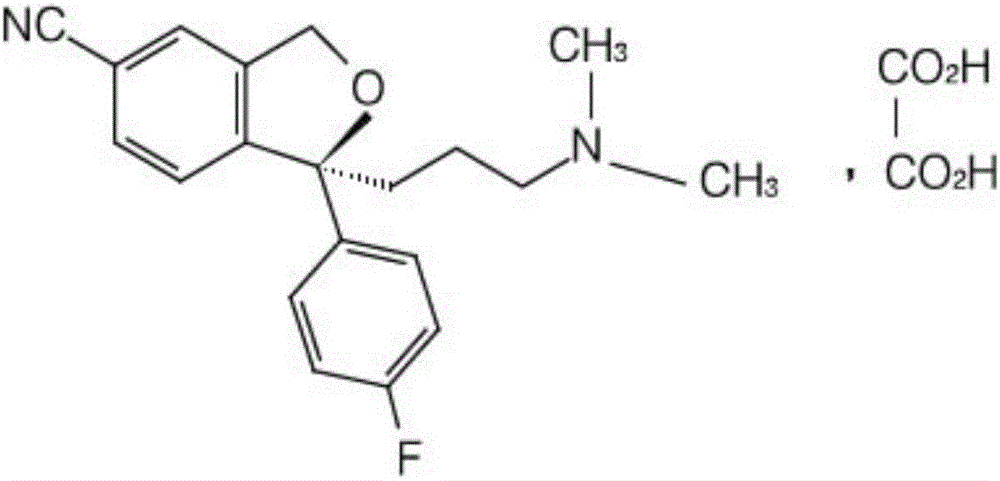
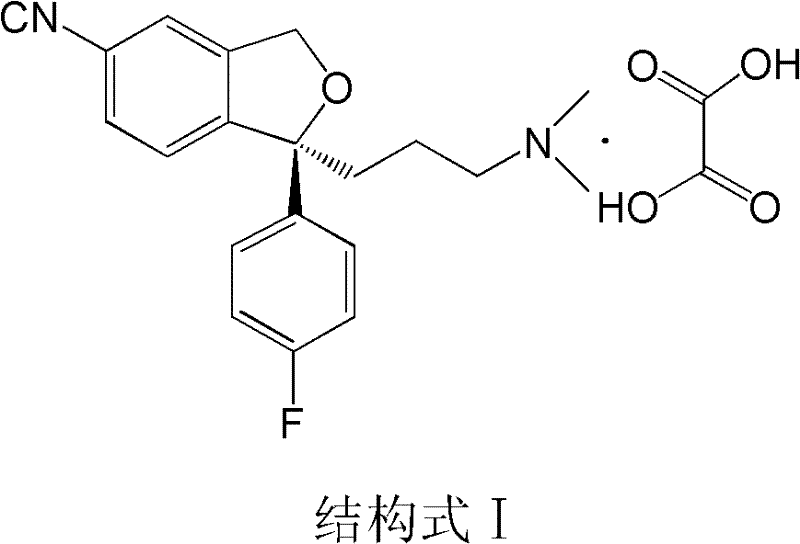
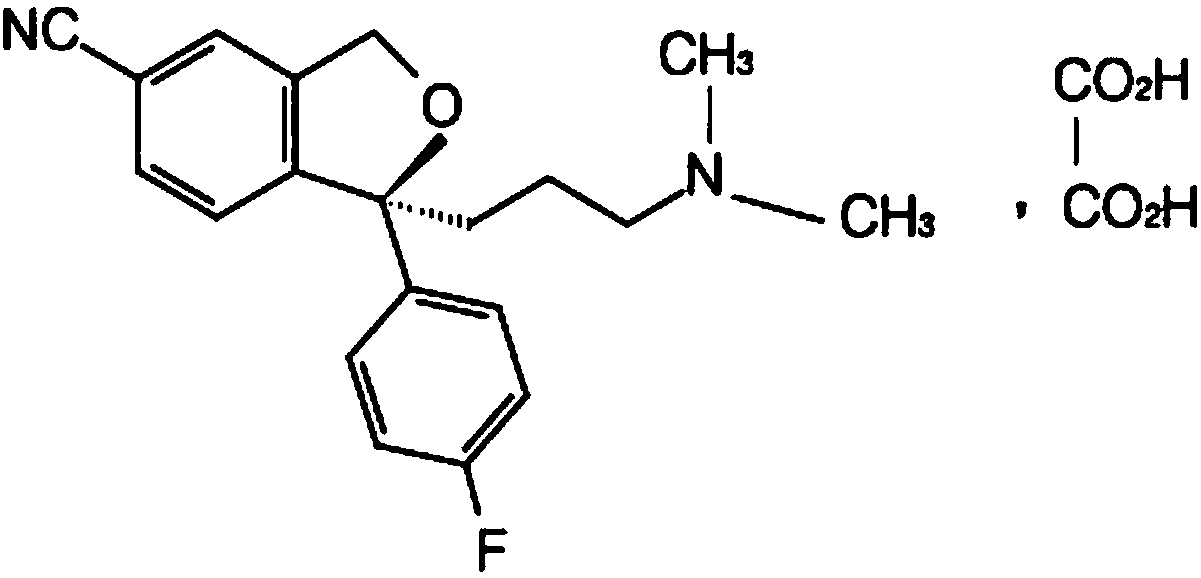
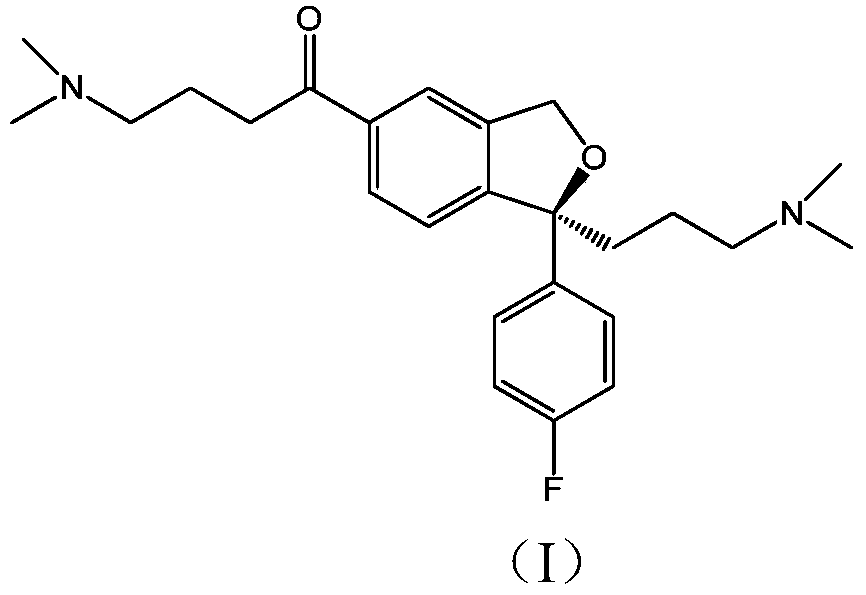
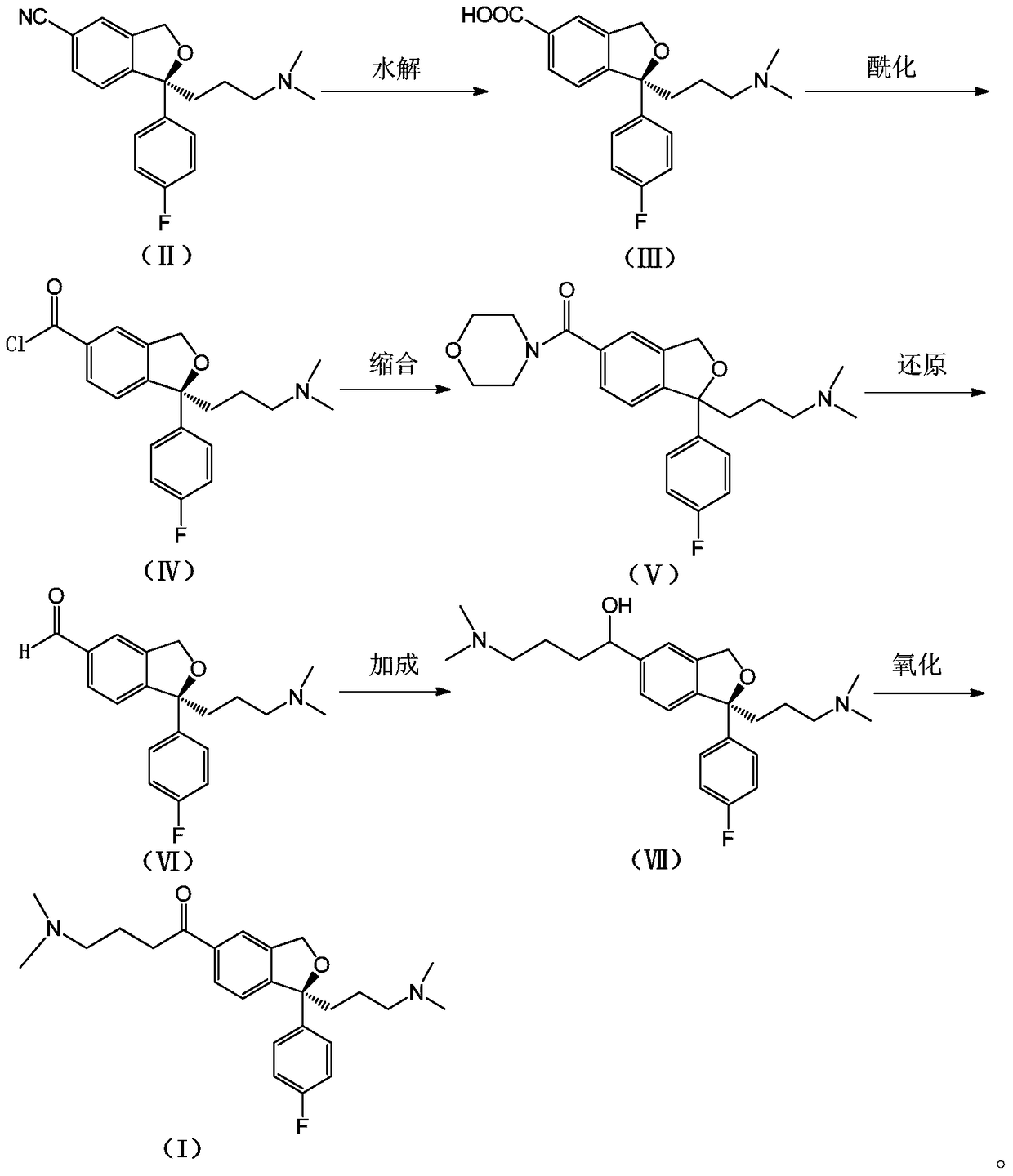
The invention relates to an escitalopram oxalate related substance and a preparation method thereof, and particularly, relates to the related substance of an antidepressant drug, the escitalopram oxalate (namely, (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-formonitrile oxalate), represented as the formula (I) and a preparation method thereof. The escitalopram oxalate, as the target compound, is synthesized through reactions comprising hydrolysis, acylation, condensation, reduction, addition and oxidization to the compound (II) (namely, (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-formonitrile). According to the method, the compound (I) is chemically synthesized for the first time. By means of the method, the target compound can be obtained through high-efficient and quick separation.
Owner:连云港恒运药业有限公司
Crystalline composition containing escitalopram
InactiveUS20050147674A1Granulation avoidedReduce stepsBiocidePowder deliveryCrystallographyCrystalline particle
Crystalline particles of escitalopram oxalate with a particle size of at least 40 μm is disclosed. Method for the manufacture of said crystalline particles and pharmaceutical compositions comprising said crystalline particles are also disclosed.
Owner:H LUNDBECK AS
Pharmaceutical composition for treating depression and preparation method thereof
InactiveCN103565788AGuaranteed content uniformityPreparation method is stableOrganic active ingredientsNervous disorderIsobenzofuranPharmaceutical drug
The invention belongs to the field of medicine, and particularly relates to a pharmaceutical composition for treating depression and a preparation method thereof. The preparation is an oral solid preparation and contains an active component with general formula of (S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofuran carbonitrile oxalate (Escitalopram oxalate), microcrystalline cellulose and a compressible excipient; and the preparation has the characteristic of quick dissolution and is of great significance to the treatment of depression.
Owner:BEIJING D VENTUREPHARM TECH DEV
Crystalline composition containing escitalopram
InactiveUS20030212128A1Granulation avoidedReduce stepsPowder deliveryBiocideCrystallographyCrystalline particle
Crystalline particles of escitalopram oxalate with a particle size of at least 40 mum is disclosed. Method for the manufacture of said crystalline particles and pharmaceutical compositions comprising said crystalline particles are also disclosed.
Owner:H LUNDBECK AS
Formula and preparation process of escitalopram oxalate effervescent tablet
ActiveCN105343026AThe phenomenon of bubble disintegration is intuitive and interestingGreat tasteOrganic active ingredientsNervous disorderEffervescent tabletPanic disorder
Owner:YANGTAI PHARMA SHANDONG
Crystalline composition containing escitalopram oxalate
InactiveCN101492436ABroad particle size distributionLess quantityOrganic active ingredientsPowder deliveryPhenyl groupCitalopram
The present invention discloses crystalline particles of escitalopram oxalate which either have a broad particle size distribution or comprise at least 0.01 % (w / w) of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, said particles being suitable for use in direct compression. Furthermore, the invention discloses a novel pharmaceutical unit dosage form containing such crystalline particles of escitalopram oxalate as well as methods for manufacture of such crystalline particles of escitalopram oxalate. Finally, the invention provides a method for reduction of the amount of hydroxyl containing impurities in a solution of citalopram or escitalopram.
Owner:H LUNDBECK AS
Escitalopram oxalate tablet composition and quality control method
Owner:HUNAN DONGTING PHARMA
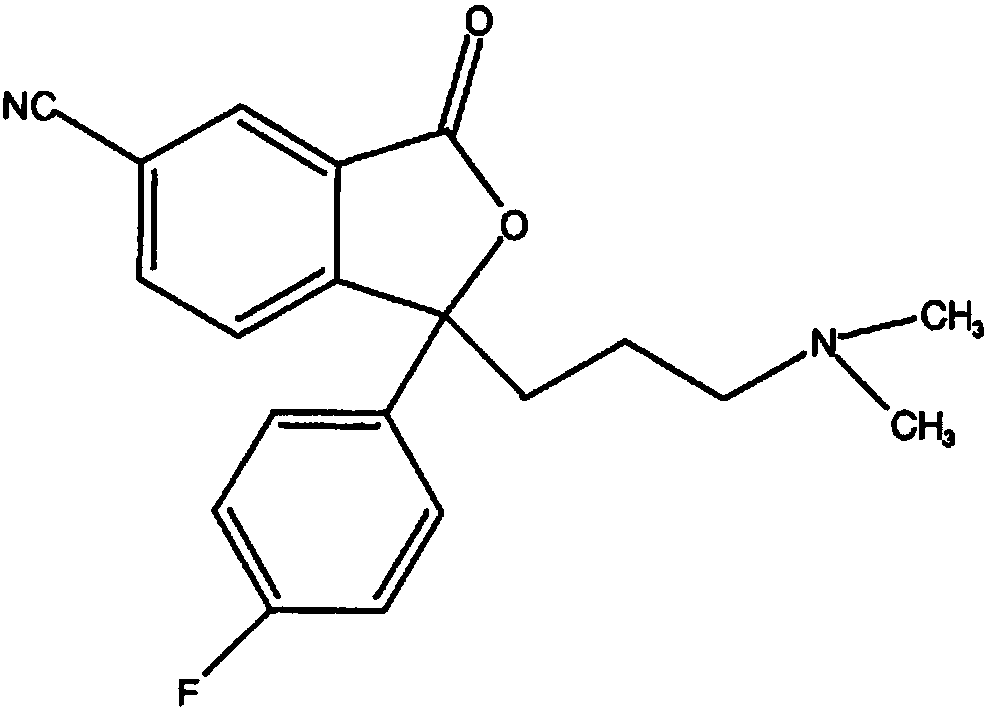
Preparation method of escitalopram oxalate impurity C
InactiveCN102952106AReduce processing costsGood stability and reproducibilityOrganic chemistryIsobenzofuranPhenyl group
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation method for escitalopram oxalate
ActiveCN102336729AMild conditionsEasy to separate and purifyCarboxylic acid salt preparationSynthesis methodsSolvent
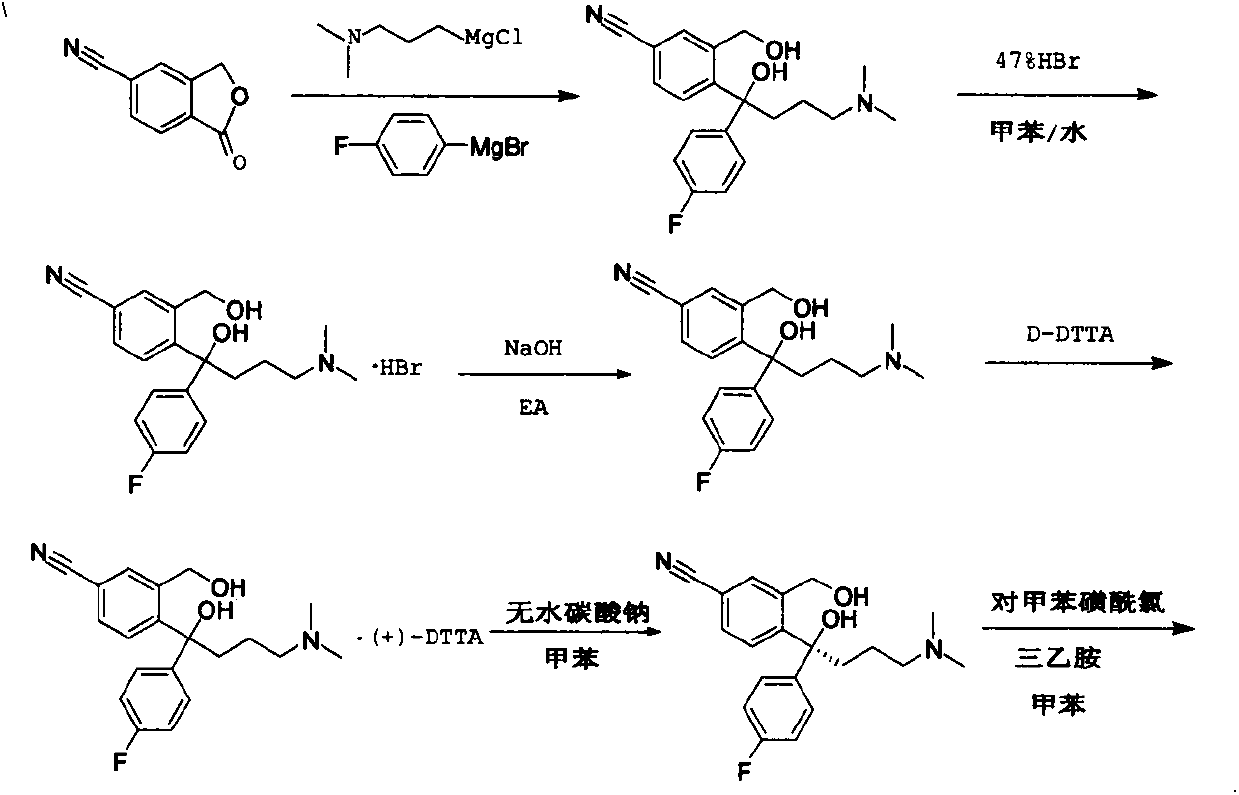
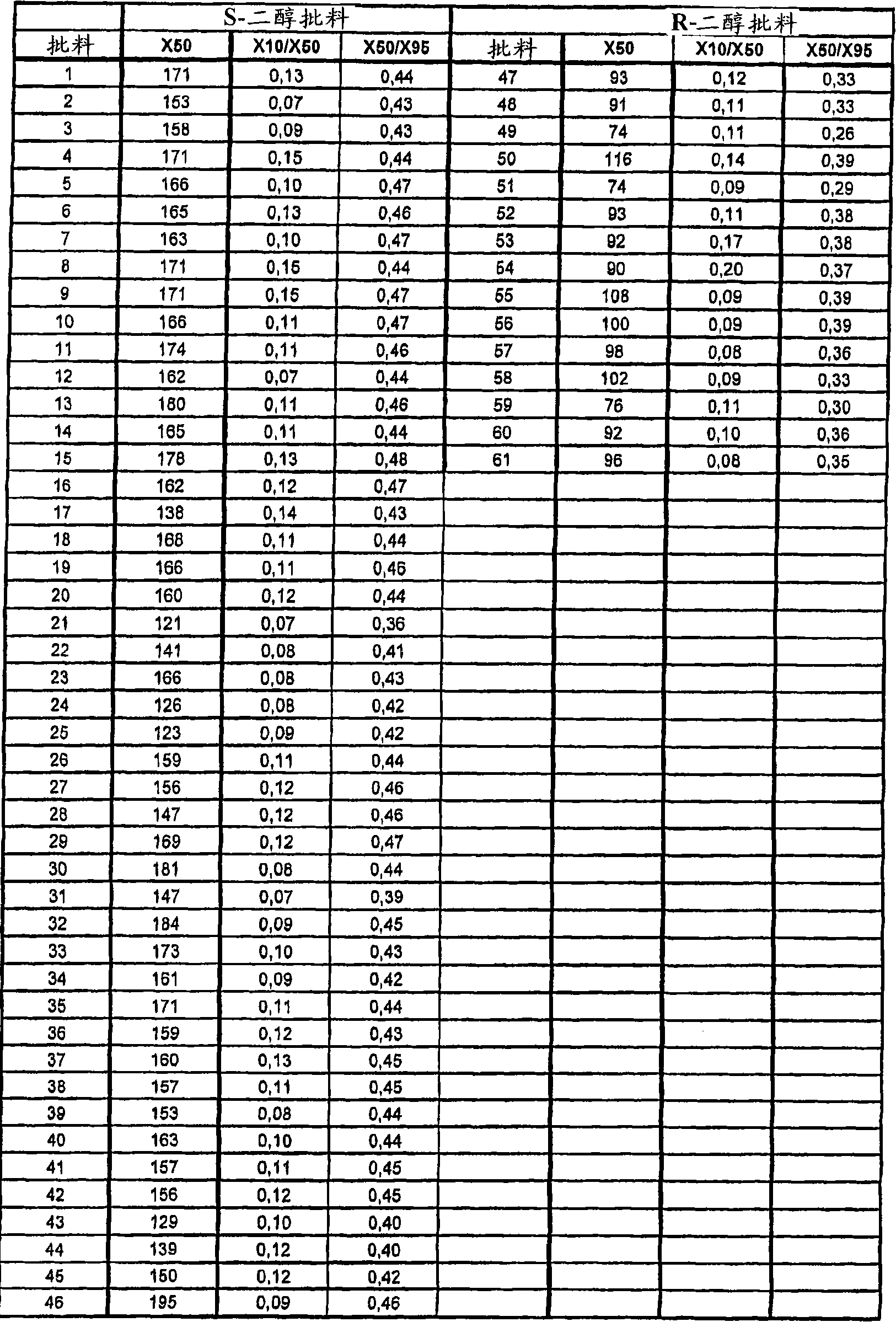
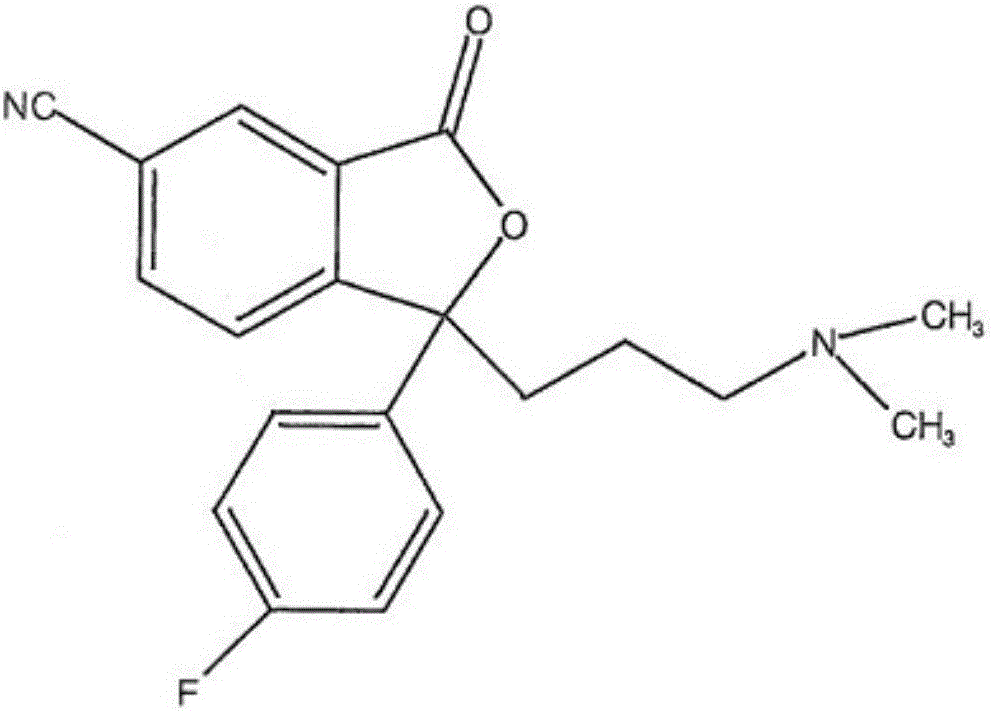
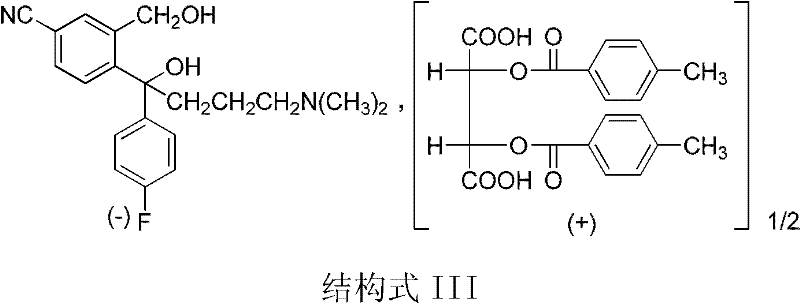
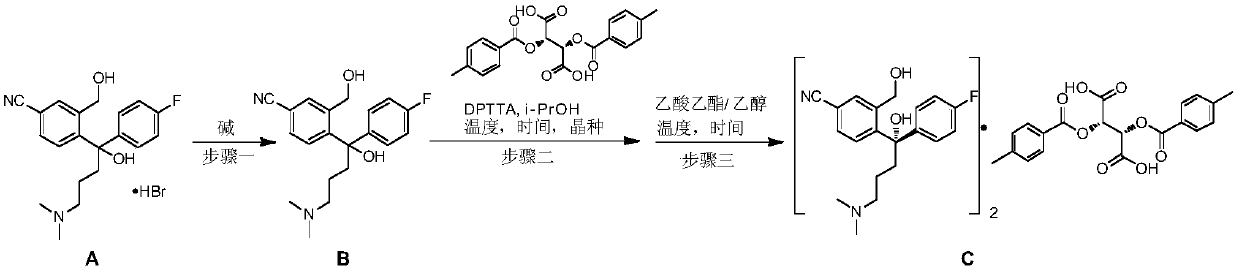
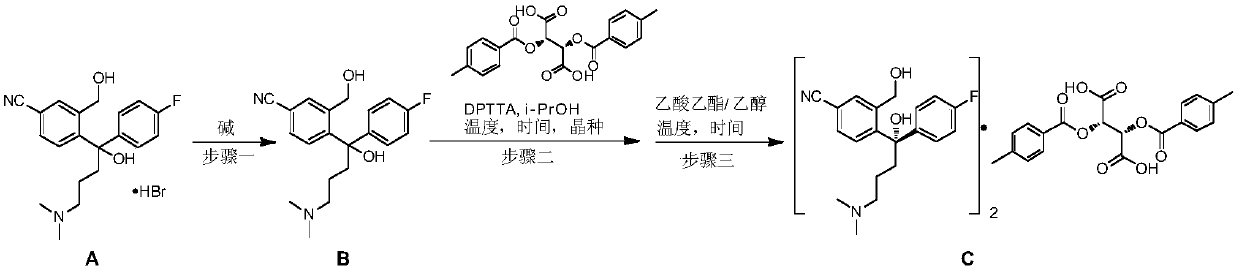
The invention discloses a preparation method for escitalopram oxalate, which is characterized in that D-toluoyl tartrate of S-diol is dissolved into water, and sodium hydroxide is used for neutralization to obtain free S-diol; then, the temperature is controlled to be 0 to 10 DEG C, paratoluensulfonyl chloride is added, then, inorganic alkaline solution is dripped, and the ring closure reaction is carried out after the reaction for 2 to 4 hours; after the reaction is completed, the pH value is regulated to 12 to 13, and then, the extraction, the water washing and the liquid separation are carried out; and after oxalic acid is added for salt forming, the temperature reduction, the filtering and the drying are carried out, and products are obtained. In the ring closure reaction, water is adopted as solvents, the condition is mild, the environment is friendly, byproducts of each step of reaction in the synthesis method are few, the yield is high, in addition, products in each step of reaction are easy to separate and purity, raw materials used in the synthesis method are easy to obtain, the operation is simple, and the industrial production is easy.
Owner:山东安信制药有限公司
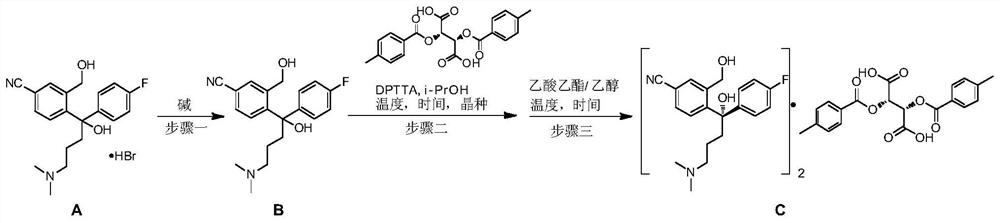
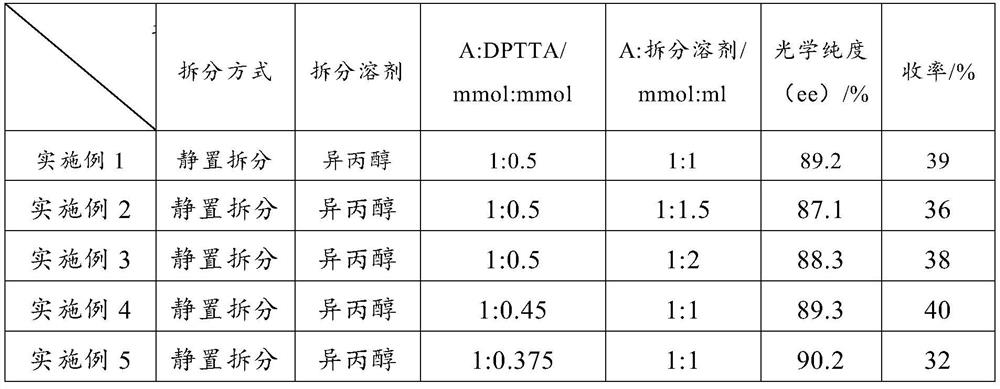
Preparation method of high-optical-purity escitalopram oxalate intermediate S-configuration diol
ActiveCN109988083AHigh yieldHigh optical purityOrganic compound preparationOrganic chemistry methodsHydrobromideOrganic solvent
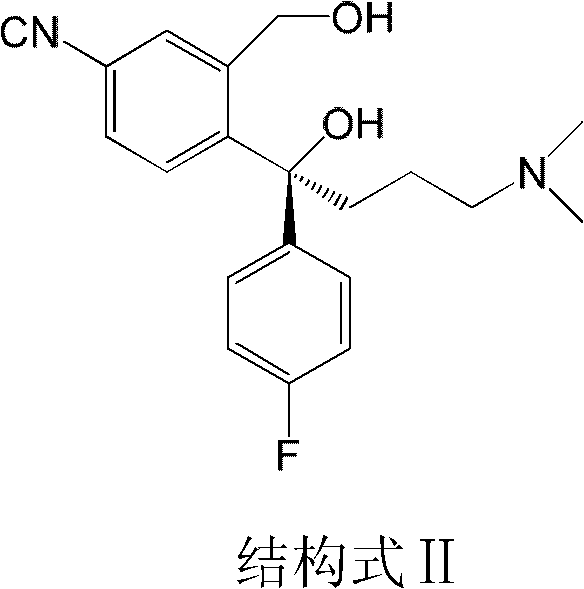
The invention discloses a preparation method of an escitalopram oxalate intermediate S-configuration diol. The preparation method comprises the following steps: (1) carrying out hydrobromic acid salinization on racemic diol (4-(4-dimethylamino-1-p-fluorophenyl-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile) to obtain free racemic diol; and (2) carrying out chiral resolution on the free racemic diolby using (+)-di-p-toluoyl-D-tartaric acid in isopropanol by adopting a standing resolution mode, and conducting crystallization to obtain an escitalopram oxalate intermediate S-configuration diol crude product, and carrying out recrystallization refining on the crude product in an organic solvent to obtain the escitalopram oxalate intermediate with high optical purity. The method disclosed by theinvention is high in resolution efficiency and high in product yield, and the prepared diol intermediate is high in optical purity and can meet the requirements of industrial production.
Owner:BEIJING MEDISAN TECH +1
Escitalopram oxalate oral disintegrating tablet and preparation method thereof
The invention discloses an oral disintegrating tablet containing escitalopram oxalate. The preparation is composed of a filling agent, especially referring to mannitol with good taste, microcrystalline cellulose having a certain disintegration function, an adhesive, a disintegrating agent, a flavoring agent and a lubricating agent. Silicon dioxide is added, so that the taste is effectively improved. When a patient takes the escitalopram oxalate oral disintegrating tablet disclosed by the invention, no water or less water is required and no chewing is required; when the drug is placed into the oral cavity, the drug is quickly dissolved or disintegrated after meeting saliva, and then the drug enters the digestive system following the autonomous or heteronomous swallowing action of the patient and then is absorbed and becomes effective. The oral disintegrating tablet is suitable for the acute and maintenance treatment for major depressive disorder of an adult and the acute treatment for generalized anxiety disorder of the adult. The invention also provides a preparation method which is simple in technique, is low in cost and belongs to the field of pharmaceutical technology.
Owner:BEIJING VENTUREPHARM BIOTECH
Tablets containing escitalopram oxalate and preparation method thereof
InactiveCN107441052AUniform contentQuality improvementOrganic active ingredientsPill deliveryMedicineBULK ACTIVE INGREDIENT
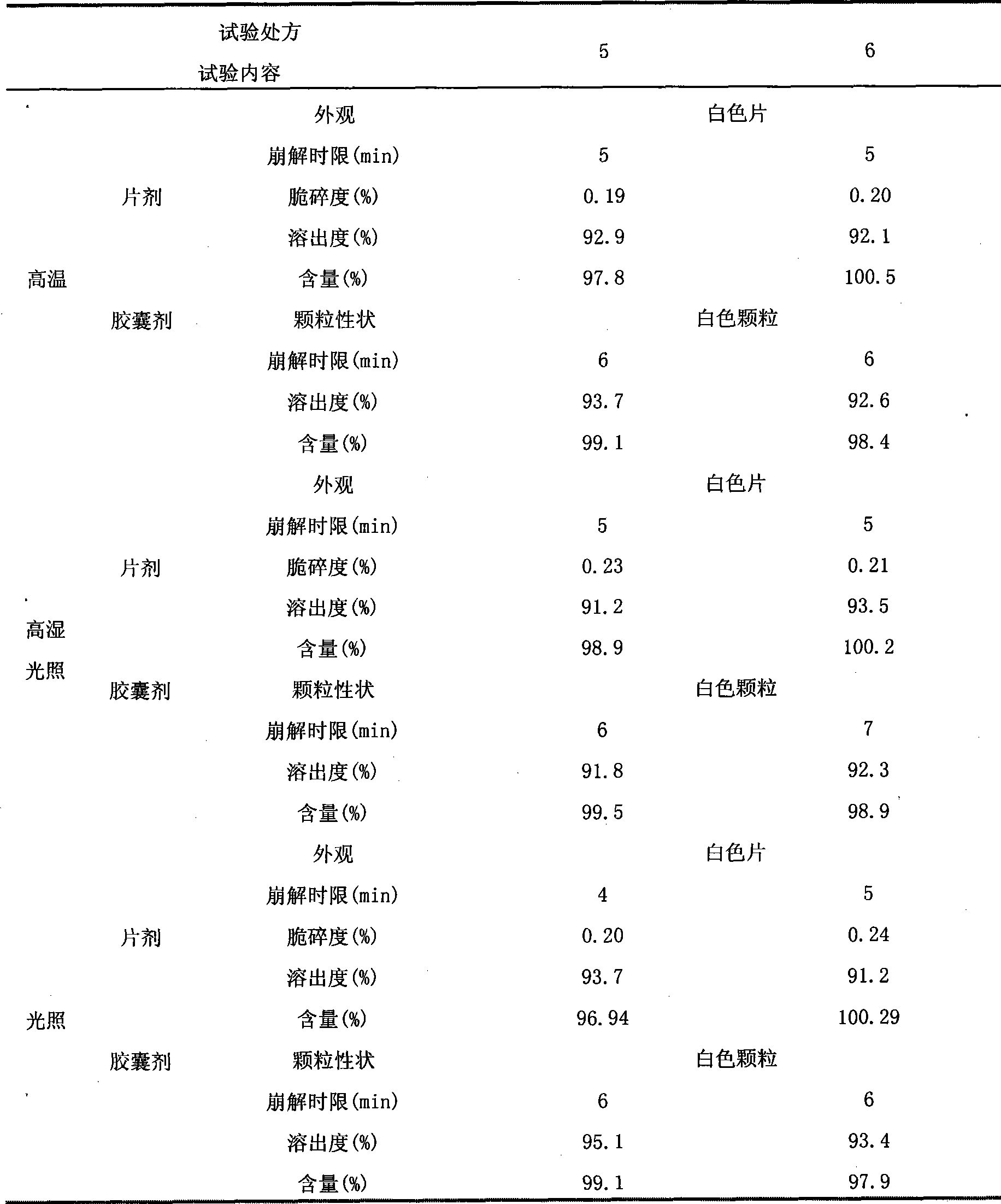
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to escitalopram oxalate tablets and a preparation method thereof capable of ensuring stable quality. According to the preparation method, an escitalopram oxalate raw material with the median diameter of not less than 300 microns is used, and auxiliary materials include magnesium stearate and other components, wherein the weight percentage of magnesium stearate in the tablets is less than 0.1%. The preparation method effectively solves the problems of poor raw material fluidity, high sticking tendency and non-uniform mixing of materials, and also solves the problem that compatibility between magnesium stearate and active ingredients is poor, causing increase of related substances. The method has the advantages of easy operation and easy industrialization, and the obtained tablets are high in content uniformity and stable in quality within the valid period.
Owner:YANGTAI PHARMA SHANDONG
Pharmaceutical composition containing escitalopram oxalate and preparation method of pharmaceutical composition
InactiveCN111514110ASolve liquidity problemsSolve for uniformityOrganic active ingredientsNervous disorderPolyethylene glycolPharmaceutical Substances
The invention discloses pharmaceutical composition containing escitalopram oxalate and a preparation method of the pharmaceutical composition. The pharmaceutical composition is tablets prepared from polyethylene glycol 6000 as a lubricant through fluidized bed granulation. The method well solves the problems of poor material flowability and non-uniform tablet content.
Owner:FUJIAN HAIXI PHARMA
A high performance liquid phase detection method for related substances of escitalopram oxalate
ActiveCN106324141BGood linear relationshipStrong specificityComponent separationFluid phaseGradient elution
The invention belongs to the field of pharmaceutical analysis and particularly relates to a HPLC (high-performance liquid chromatography) detection method for escitalopram oxalate related substances. The escitalopram oxalate related substances are determined with HPLC, a C18 column is taken as a chromatographic column, a phosphate buffer solution is taken as a mobile phase A, the mobile phase A and an acetonitrile solution are mixed in a certain ratio to form a mobile phase B, the mobile phase A and the acetonitrile solution are mixed in a certain ratio to form a mobile phase C, and the escitalopram oxalate related substances are detected with a gradient elution method. The method can separate impurities, is high in specificity and sensitivity and good in repeatability and durability, can well control known impurities and unknown impurities of escitalopram oxalate and guarantees safety of escitalopram oxalate.
Owner:山东锐顺药业有限公司
Preparation method of escitalopram oxalate inclusion compound oral liquid
InactiveCN110787304AGreat tasteImprove medication complianceOrganic active ingredientsNervous disorderBitter tasteOrganic chemistry
The invention discloses escitalopram oxalate inclusion compound oral liquid and a preparation method of the escitalopram oxalate inclusion compound oral liquid. In order to overcome the defect of a conventional flavor correction process of escitalopram oxalate, the preparation method disclosed by the invention has the advantages that the flavor is corrected commonly by adopting an inclusion technology and a flavor correction agent, so that a bitter taste of the escitalopram oxalate is covered, and an oral liquid preparation having an appropriate mouth feel is developed, and thus, the medicineapplication compliance of patients is improved.
Owner:BEIJING VENTUREPHARM BIOTECH
Escitalopram oxalate tablet composition and quality control method
ActiveCN106018618BEffective quality controlOrganic active ingredientsComponent separationQuality controlMagnesium stearate
The invention relates to an escitalopram oxalate tablet composition and a quality control method and in particular relates to a method for controlling quality of an escitalopram oxalate tablet composition. The method comprises the following steps: carrying out limitation test on escitalopram R-enantiomeric impurities in the composition, measuring the content of the escitalopram R-enantiomeric impurities in the composition and testing related substances in the composition. Furthermore, the invention also relates to an escitalopram oxalate tablet composition. The escitalopram oxalate tablet composition comprises an effective dose of escitalopram oxalate, magnesium stearate and optional pharmaceutically acceptable carriers. The method can implement the excellent technical effect as described in the description.
Owner:HUNAN DONGTING PHARMA
Pharmaceutical composition
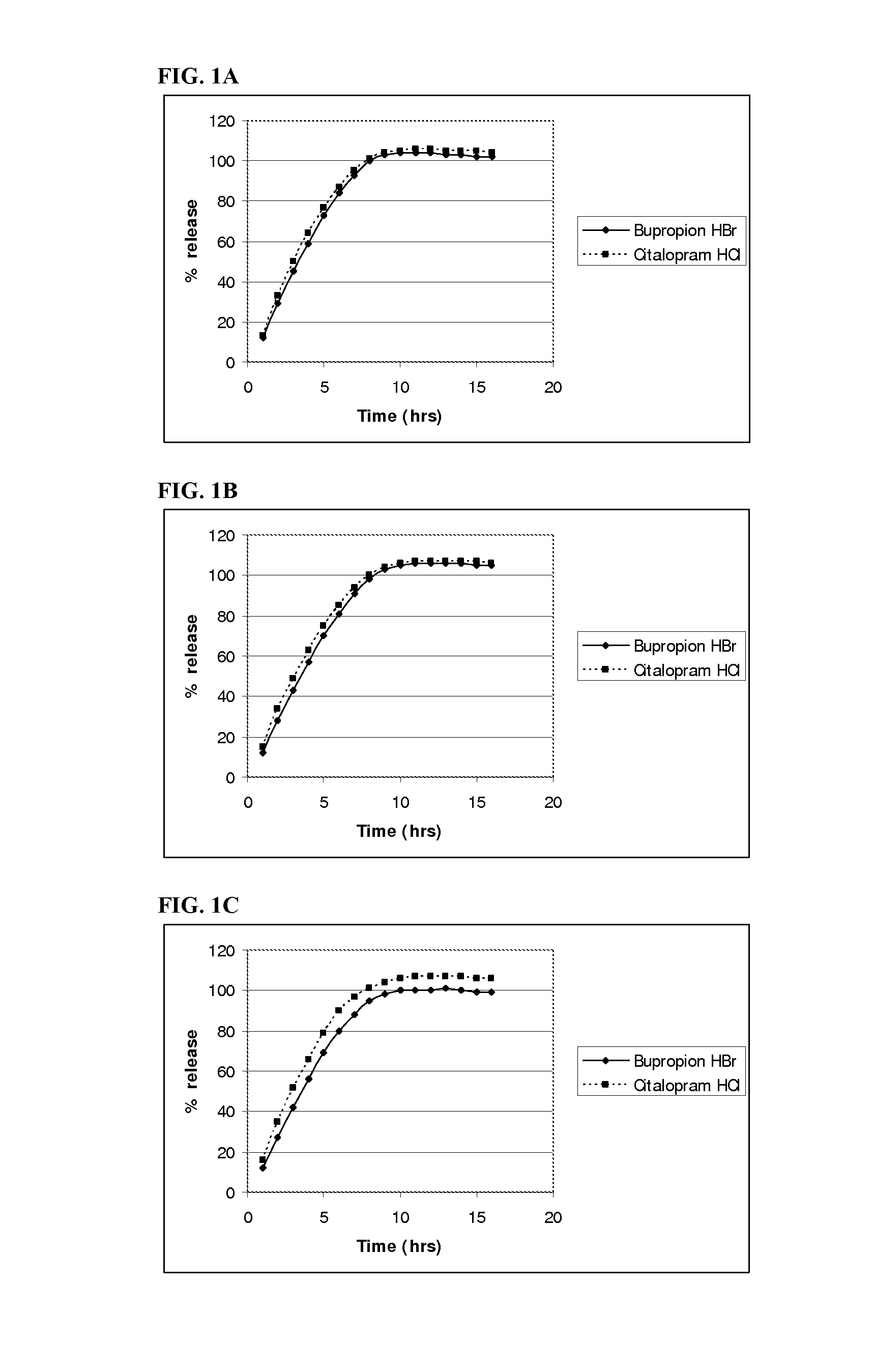
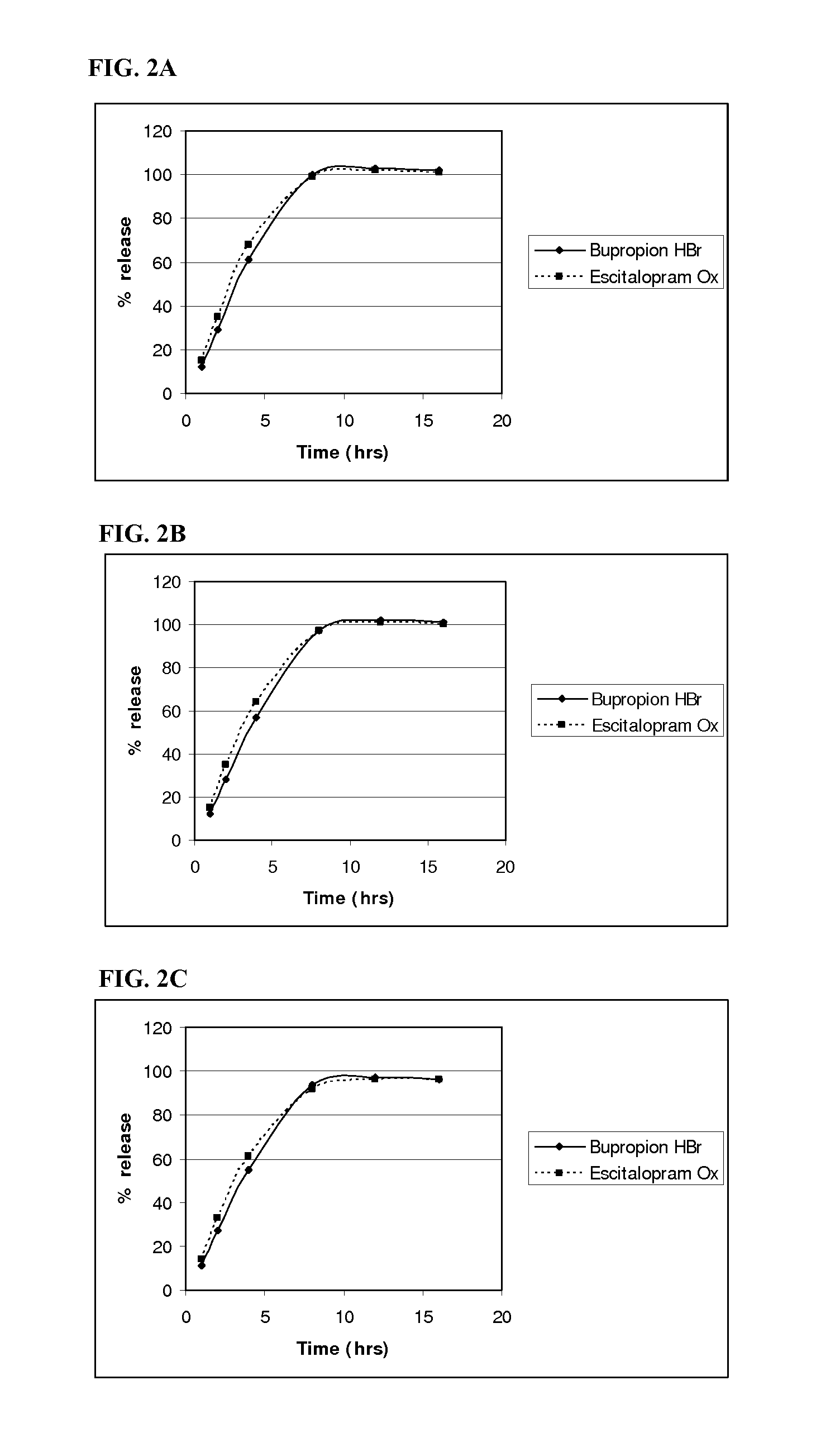
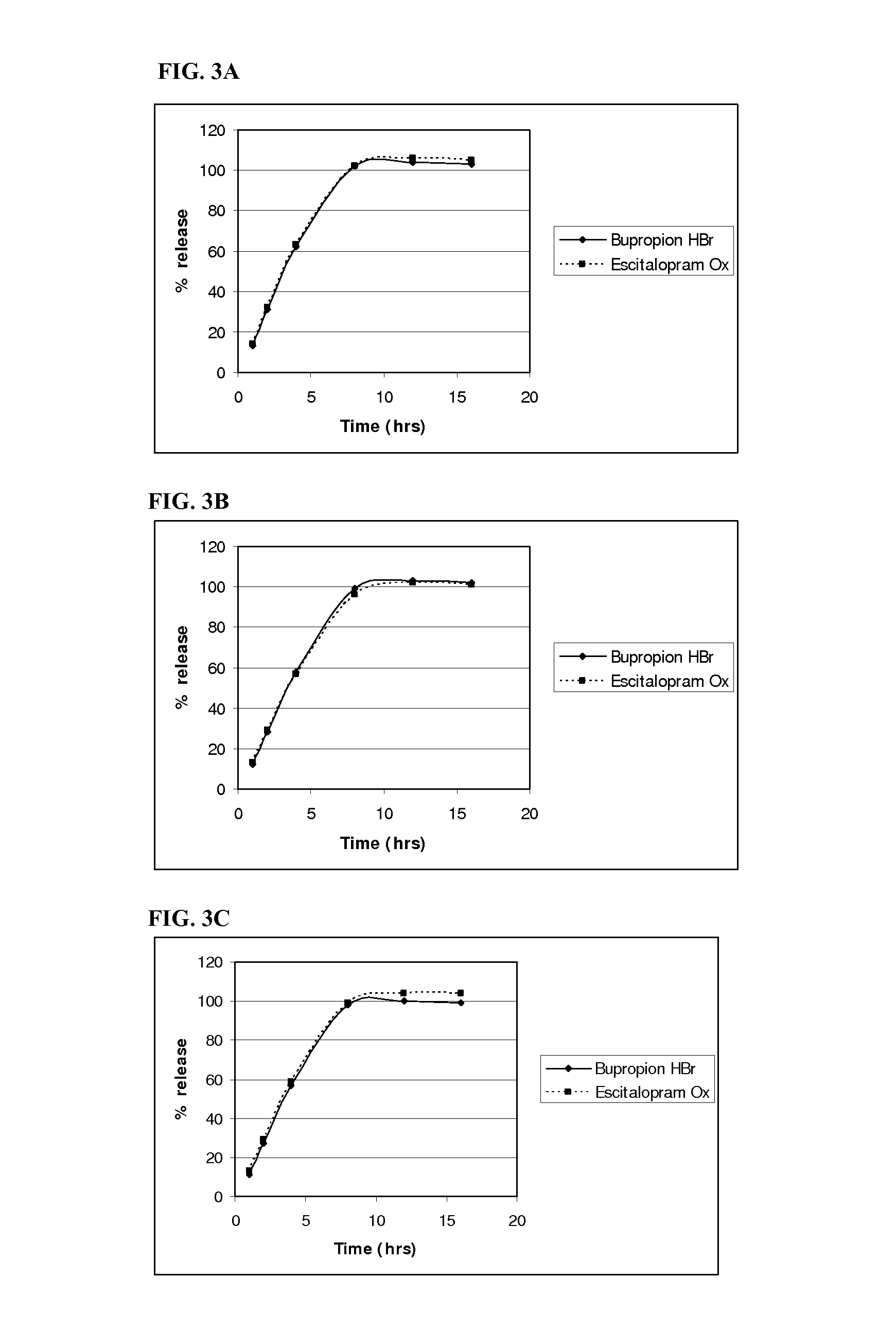
InactiveUS20110052684A1Promote absorptionImprove bioavailabilityBiocidePowder deliveryControlled releaseBupropion hydrochloride
The present invention relates to a pharmaceutical composition comprising a tablet core comprising a combination of actives selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, bupropion hydrobromide and escitalopram oxalate, and bupropion hydrobromide and quetiapine fu-marate, and at least one pharmaceutically acceptable excipient, and a control-releasing coat surrounding the tablet core, wherein said composition surprisingly provides for a synchronous release of the combination of active agents in-vitro. The once-daily pharmaceutical composition surprisingly also provides for enhanced absorption of bupropion hydrobromide when administered to a subject in need of such administration.
Owner:BIOVAIL LAB INT SRL
Preparation method of escitalopram oxalate drop
The invention relates to the technical field of medicine, in particular to a preparation method of an escitalopram oxalate drop. The escitalopram oxalate drop is composed of escitalopram oxalate, a pH buffer agent, a flavoring agent, a solvent and a preservative. The escitalopram oxalate contained in the drop prepared by the invention is racemic citalopram-S-enantiomer, and the action mechanism of the escitalopram oxalate may be related to the behavior of enhancing serotonin activation by inhibiting the 5-hydroxytryptamine reabsorption of neurons of a central nervous system. The escitalopram oxalate drop is clinically used for treating severe depression and general anxiety; compared with other dosage forms, the escitalopram oxalate drop has the advantages of being convenient to take, accurate in dosage, convenient to carry and transport and the like; and when the escitalopram oxalate drop is taken, a matched dropper is used for sucking liquid to a specified scale line, and the escitalopram oxalate drop is directly dropped into a mouth or added into other food to be taken.
Owner:BEIJING VENTUREPHARM BIOTECH
Tablet containing escitalopram oxalate and preparation method thereof
ActiveCN104523638BSolve Content Uniformity ProblemsSolve the sticking problemOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMedicinal chemistry
The invention discloses a tablet containing escitalopram oxalate and a preparation method thereof. According to the invention, the tablet containing escitalopram oxalate uses hydroxy propyl cellulose as an adhesive and uses escitalopram oxalate with median particle size less than or equal to 20mum, and the prepared tablet containing escitalopram oxalate has the advantages of beautiful appearance and stable quality. The invention also provides the preparation method of oral tablet, and has the advantages of simple process and adaptability of commercialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Escitalopram oxalate related substances and preparation method thereof
The invention relates to an escitalopram oxalate related substance and a preparation method thereof, and particularly, relates to the related substance of an antidepressant drug, the escitalopram oxalate (namely, (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-formonitrile oxalate), represented as the formula (I) and a preparation method thereof. The escitalopram oxalate, as the target compound, is synthesized through reactions comprising hydrolysis, acylation, condensation, reduction, addition and oxidization to the compound (II) (namely, (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-formonitrile). According to the method, the compound (I) is chemically synthesized for the first time. By means of the method, the target compound can be obtained through high-efficient and quick separation.
Owner:连云港恒运药业有限公司
Synthetic method of escitalopram impurity J
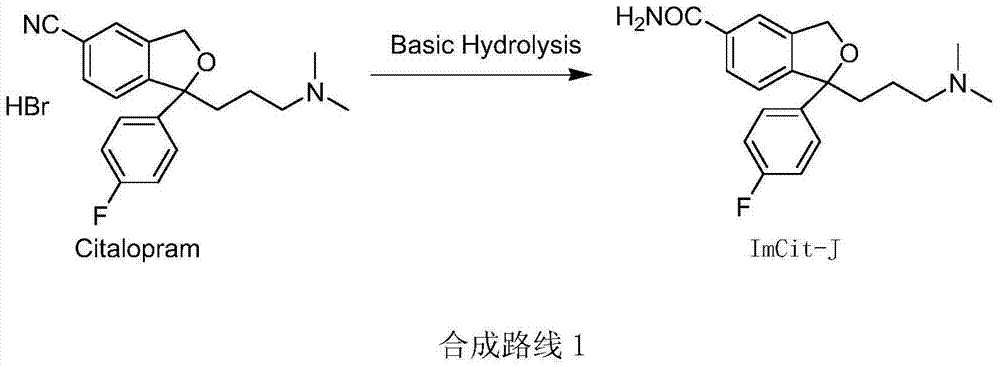
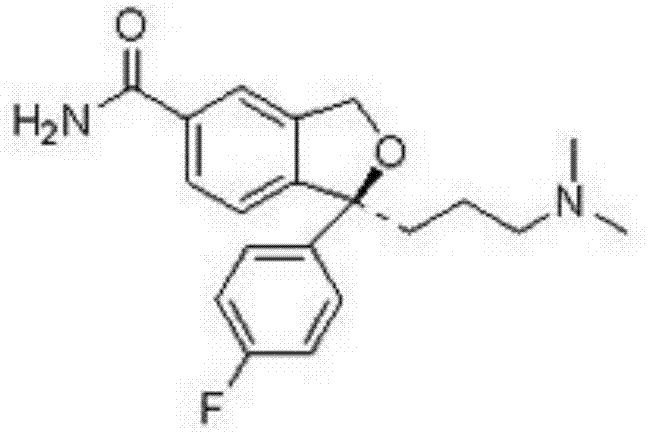
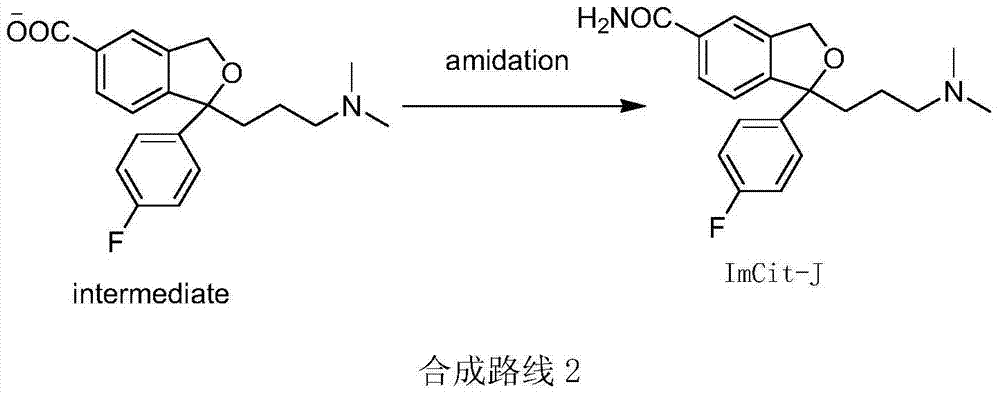
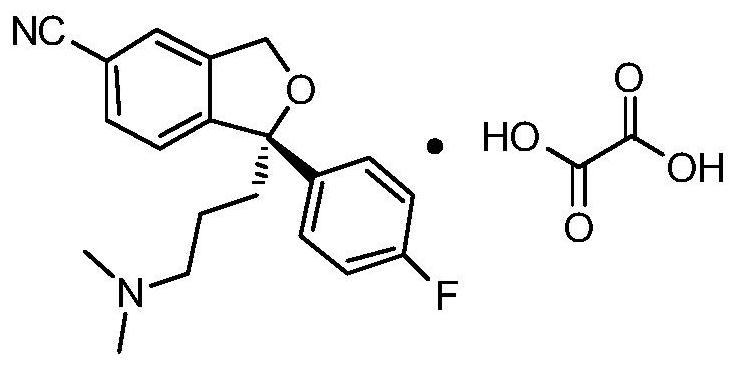
The invention discloses a synthetic method of an escitalopram impurity J, belonging to the technical field of medicaments. The synthetic route comprises the steps of using escitalopram oxalate as a raw material, adding an acid-binding agent into a solvent in the presence of hydrogen peroxide, and performing hydrolysis reaction to generate the escitalopram impurity J. The method disclosed by the invention uses escitalopram oxalate as a starting material; compared with a raw material required by a conventional method, escitalopram oxalate has the advantages of low cost and easy purchase; the synthetic route is simple, only needs a one-step reaction, is mild in reaction conditions and small in pollution caused by a reagent and the solvent adopted in the reaction, and has the characteristic of environmental friendliness; the escitalopram impurity J synthesized by the synthetic method is high in purity and yield.
Owner:成都诺维尔生物医药有限公司
Preparation method of s-configuration diol of high optical purity escitalopram oxalate intermediate
ActiveCN109988083BHigh yieldHigh optical purityOrganic compound preparationOrganic chemistry methodsHydrobromideOXALIC ACID DIHYDRATE
The invention discloses a preparation method of escitalopram oxalate intermediate S-configuration diol, comprising: (1) preparing racemic diol (4-(4-dimethylamino-1-p-fluorobenzene Base-1-hydroxybutyl)-3-(hydroxymethyl) benzyl cyanide) hydrobromide basification to obtain free racemic diol; (2) free racemic diol with D-(+)- Chiral resolution and crystallization of di-p-toluoyl tartaric acid in isopropanol by static resolution to obtain the crude product of the S-configuration diol of the escitalopram oxalate intermediate, and the crude product was carried out in an organic solvent The escitalopram oxalate intermediate with high optical purity was obtained through recrystallization and purification. The method of the invention has high resolution efficiency and high product yield, and the prepared diol intermediate has high optical purity and can meet the needs of industrial production.
Owner:BEIJING MEDISAN TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com