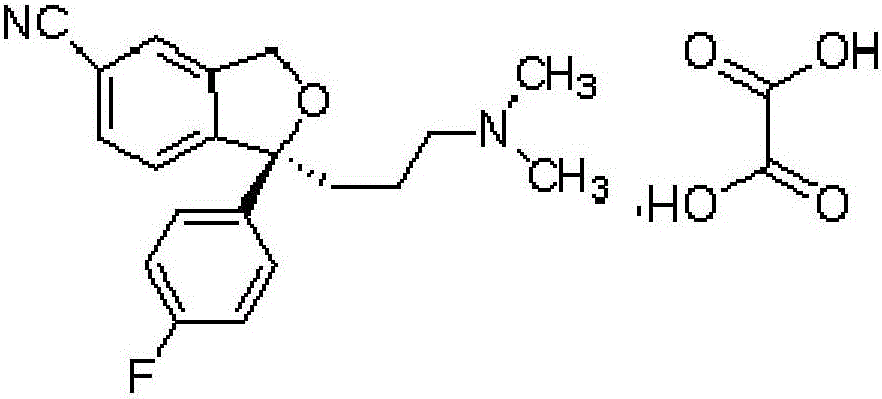
Escitalopram oxalate oral dissolution film agent and preparation method thereof
A film-dissolving and oxalic acid technology, applied in pharmaceutical formulations, sheet delivery, nervous system diseases, etc., can solve problems such as unpublished product stability data, unfavorable large-scale production, etc., achieving good environmental and economic benefits, and good taste , the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
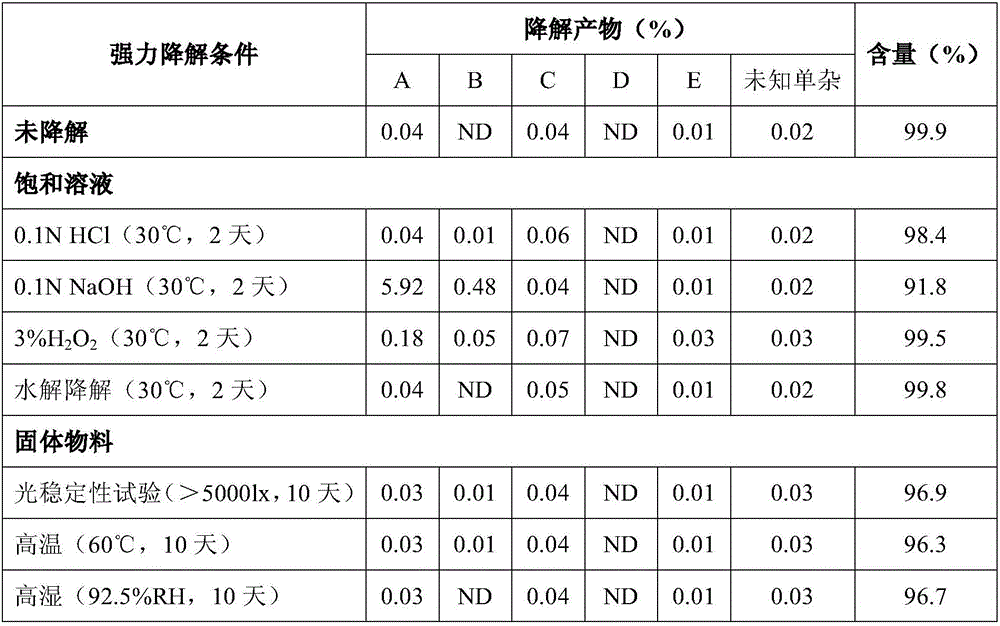
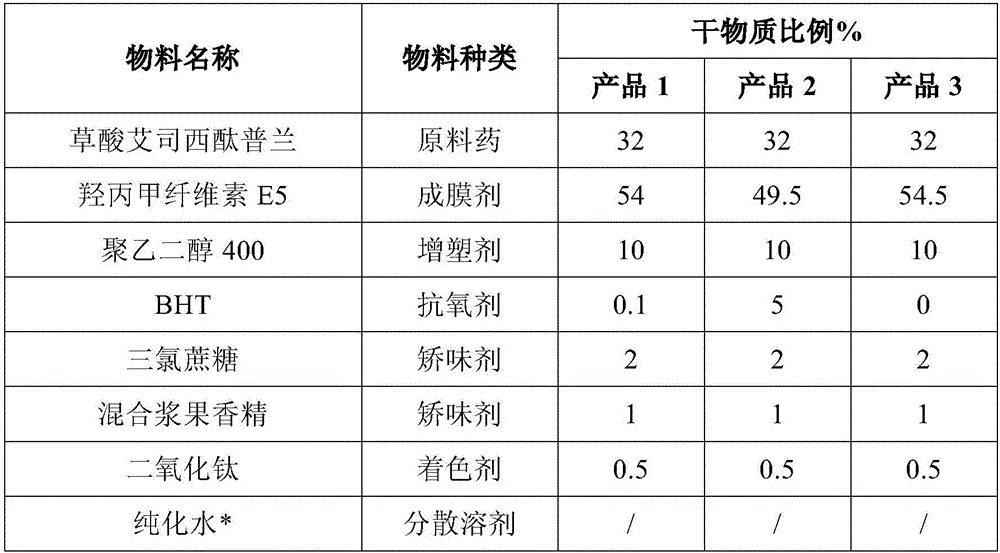
[0036] Embodiment 1: Antioxidant influences on product stability
[0037]
[0038] *Used in prescription but removed in final product.
[0039] Preparation:
[0040] [1] Add plasticizers, flavoring agents, colorants, antioxidants and raw materials into water and stir evenly at room temperature to obtain a dispersion system;
[0041] [2] Add a polymer film-forming material to the dispersion system in step 1, stir fully at room temperature to obtain a drug-containing glue, and perform degassing treatment if necessary;
[0042] [3] Coating the drug-containing glue in step 2, drying at 30°C, and cutting after drying to obtain the mouth-melting film.
[0043] The prepared film is white in color, has good smoothness and plasticity, and meets the requirements of cutting, packaging, transportation and clinical use.
[0044] Product 1, product 2 and product 3 were placed under the conditions of 40°C and 75% relative humidity for 30 days respectively, and samples were taken to det...
Embodiment 2
[0049] Embodiment 2: drying temperature influences on product stability
[0050]
[0051] *Used in prescription but removed in final product.
[0052] Preparation:
[0053] [1] Add plasticizers, flavoring agents, colorants, antioxidants and raw materials into water and stir evenly at room temperature to obtain a dispersion system;
[0054] [2] Add a polymer film-forming material to the dispersion system in step 1, stir fully at room temperature to obtain a drug-containing glue, and perform degassing treatment if necessary;
[0055] [3] Apply the drug-containing glue in step 2, dry under predetermined temperature conditions (35°C for both products 4 and 5; 40°C for product 6), and cut after drying to obtain the mouth-melting film.
[0056] The prepared film is white in color, has good smoothness and plasticity, and meets the requirements of cutting, packaging, transportation and clinical use.
[0057] Product 4, product 5 and product 6 were placed under the conditions of ...
Embodiment 3
[0069] Example 3: BHA as an antioxidant
[0070]
[0071]
[0072] *Used in prescription but removed in final product.
[0073] Preparation:
[0074] [1] Add plasticizers, flavoring agents, colorants, antioxidants and raw materials into water and stir evenly at room temperature to obtain a dispersion system;
[0075] [2] Add a polymer film-forming material to the dispersion system in step 1, stir fully at room temperature to obtain a drug-containing glue, and perform degassing treatment if necessary;
[0076] [3] Coating the drug-containing glue in step 2, drying at a predetermined temperature, and cutting after drying to obtain the mouth-melting film.
[0077] The prepared film is dark yellow in color, has good smoothness and plasticity, and meets the requirements of cutting, packaging, transportation and clinical use.
[0078] Product 7 was placed at 40°C and 75% relative humidity for 30 days, and samples were taken to detect related substances on days 0 and 30. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com