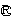Patents
Literature
48results about How to "Guaranteed content uniformity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Partial pressure sintering method for sintering neodymium-iron-boron magnet
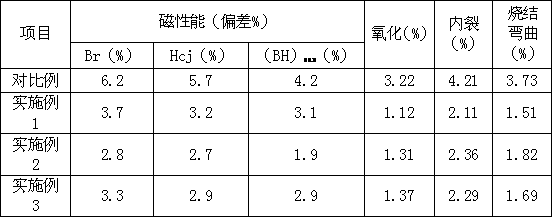
ActiveCN103000363AEnsure consistencyGuaranteed uniformityInductances/transformers/magnets manufactureMagnetic materialsMetallurgyVacuum pump
The invention relates to a partial pressure sintering method for sintering a neodymium-iron-boron magnet. Certain inert gases Ar are filled into various deflation sections in the sintering process, the pressure of the Ar gases inside a furnace is regulated according to blank deflation velocity and vacuum pump system exhaust velocity through vacuum degree control, so that heating sintering is carried out by uniformly deflating under different partial pressures of the Ar gases, and negative pressure is still kept. The partial pressure sintering method disclosed by the invention ensures the consistency and uniformity of magnetic property and saves the sintering time.
Owner:宁波永久磁业有限公司
Metformin compound pharmaceutical composition and preparation method thereof
InactiveCN103239719AUniform contentSimple preparation processOrganic active ingredientsMetabolism disorderLarge doseDiabrezide
The invention provides a metformin compound pharmaceutical composition for treating type II diabetes and a preparation method thereof. The pharmaceutical composition contains a small dose of anti-diabetic medicine and a relatively large dose of metformin hydrochloride as active ingredients. The preparation method has a simple process, does not need special auxiliary materials, and is suitable for industrial production. Two active ingredients in the prepared pharmaceutical composition have the advantages of uniform content and good dissolubility.
Owner:2Y CHEM
Tadalafil oral soluble film agent and preparation method
InactiveCN106389392AImprove dispersion uniformityDissolution rate is fastOrganic active ingredientsPharmaceutical non-active ingredientsOrganic acidCellulose
The invention provides a Tadalafil oral soluble film and a preparation method. The Tadalafil oral soluble film is prepared from a Tadalafil oral soluble film containing specific components through a conventional technology and particularly prepared from Tadalafil, a film-forming material, a plasticizer, an optionally existing corrective, an optionally existing coloring agent and an optionally existing aromatic agent. The Tadalafil oral soluble film is characterized in that the film-forming material is prepared from one or more than two components in polysaccharide, one or more than two components in organic acid and one or more than two components in hydroxyalkyl cellulose. Compared with the prior art, the Tadalafil oral soluble film has the advantages that the preparation technology is simple, no special requirement is needed on production equipment, the preparation stability is high, reproduction is easy, the requirements on storing conditions are low, and packaging, transporting and storing are easy.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +2
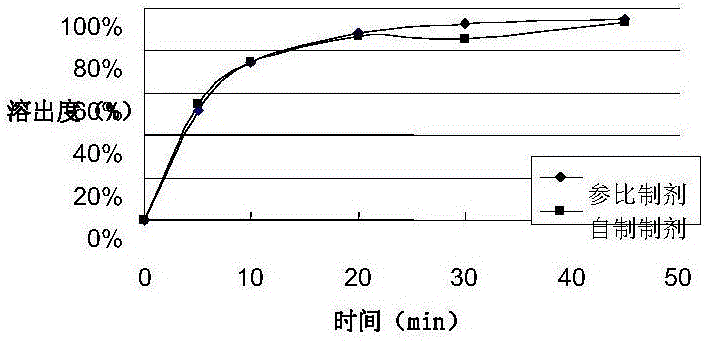
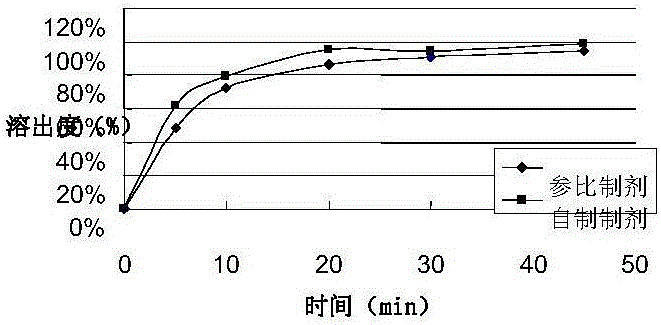
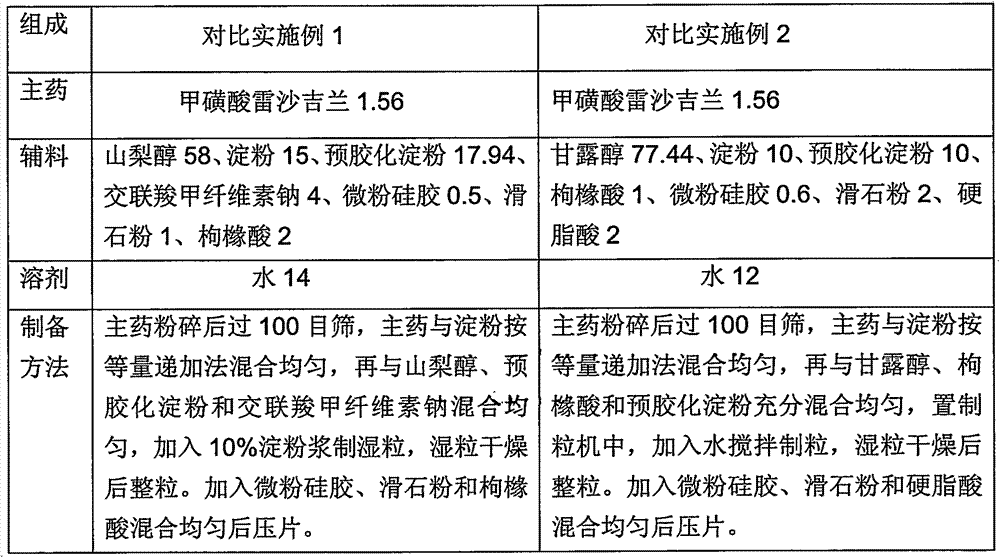
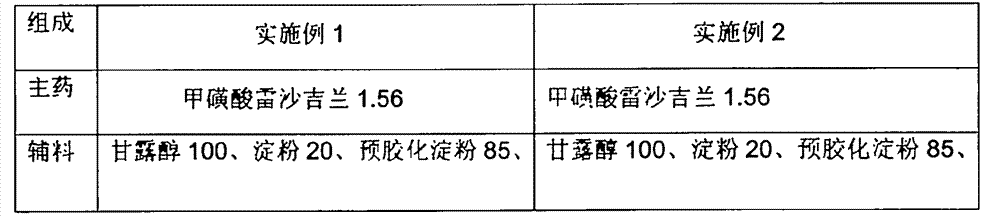
Rasagiline preparation and preparation method thereof
ActiveCN103315983AHigh dissolution rateImprove stabilityOrganic active ingredientsNervous disorderDissolutionPharmacology
The invention provides a rasagiline preparation and a preparation method thereof. The rasagiline preparation provided by the invention comprises rasagiline pharmaceutically acceptable salt and pharmaceutically acceptable auxiliary materials comprising a stabilizer and a filling agent. With the method provided by the invention, dissolution rate and stability of the rasagiline preparation are improved, preparation content uniformity is ensured, treatments of crushing and sieving are avoided, and loss and pollution are reduced. The method also has the advantages of simple operation, low cost, and no requirement on special equipment. The method is suitable to be applied on industrial productions.
Owner:SHANGHAI ZHONGXI PHARMA
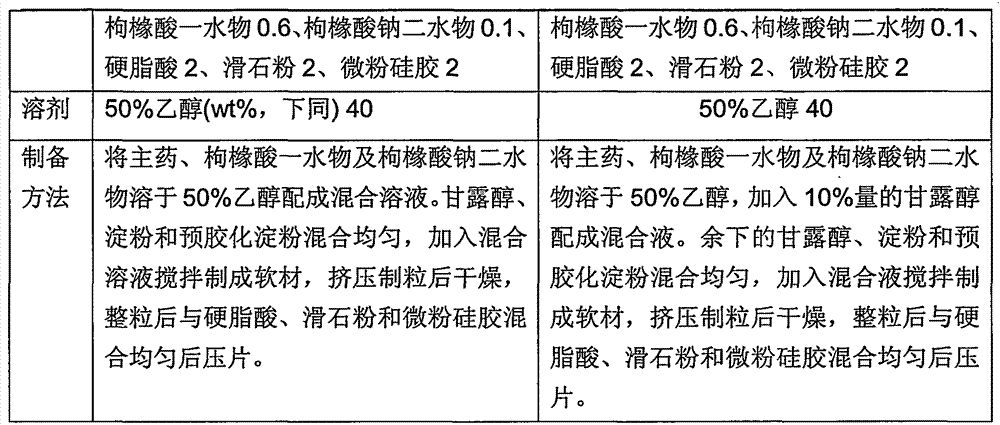
Calcium carbonate D3 chewable tablets for children calcium supplement, and preparation method of chewable tablets
ActiveCN106619706AStability impactGuaranteed content uniformityOrganic active ingredientsMetabolism disorderHardnessMannitol
The invention discloses calcium carbonate D3 chewable tablets for children calcium supplement, and a preparation method of the chewable tablets. The chewable tablets are prepared from the following components: calcium carbonate, vitamin D3 powder, maltodextrin, mannitol, magnesium stearate and essence. The preparation method comprises the following steps: (1) checking the needed raw materials and auxiliary materials, and respectively carrying out pretreatment on the qualified materials for later use; (2) preparing the calcium carbonate into suspension liquid, preparing the maltodextrin into a water solution, and evenly mixing the suspension liquid and the water solution; (3) carrying out spray drying to prepare maltodextrin-embedded calcium carbonate granules; (4) finishing the granules; (4) blending all the materials; (6) measuring the content of an intermediate, tabletting and bottling. After a spray drying technology is adopted, the gritty texture of the calcium carbonate can be effectively improved by embedding the calcium carbonate by using the maltodextrin; by adopting a direct tabletting technology, the production technology is greatly simplified; the calcium carbonate D3 chewable tablets are moderate in hardness, thus being suitable for children to chew.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Capsule type tiotropium bromide inhalation powder
ActiveCN101032484AGuaranteed content uniformitySimple processPowder deliveryAerosol deliveryActive componentTIOTROPIUM BROMIDE MONOHYDRATE
The present invention discloses one kind of tiotropium bromide capsule atomized powder preparation, which includes tiotropium bromide or tiotropium bromide monohydrate in 0.04-1.5 wt% and fine lactose powder of size smaller than 15 microns for adsorbing tiotropium bromide or tiotropium bromide monohydrate. The present invention also provides the preparation process of the atomized powder preparation. The tiotropium bromide capsule atomized powder preparation has simple preparation process, excellent flowability and homogeneously distributed active component.
Owner:NANJING CAVENDISH BIO ENG TECH +1
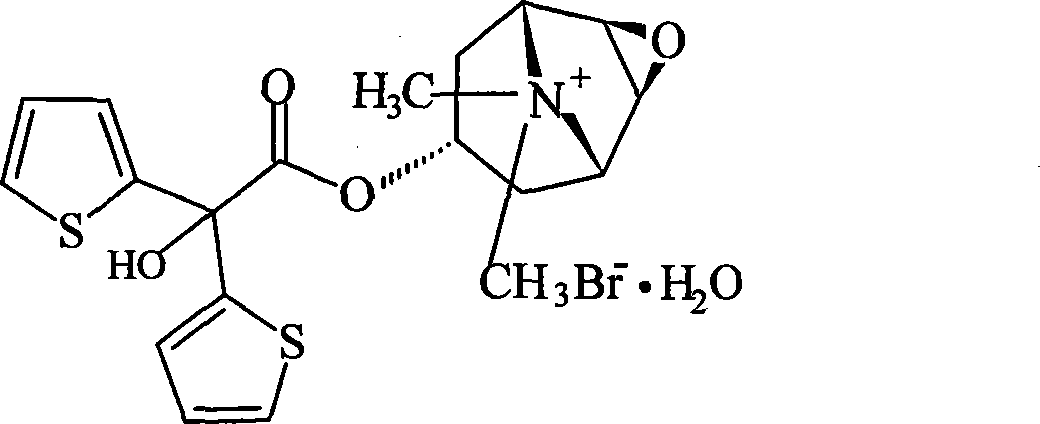
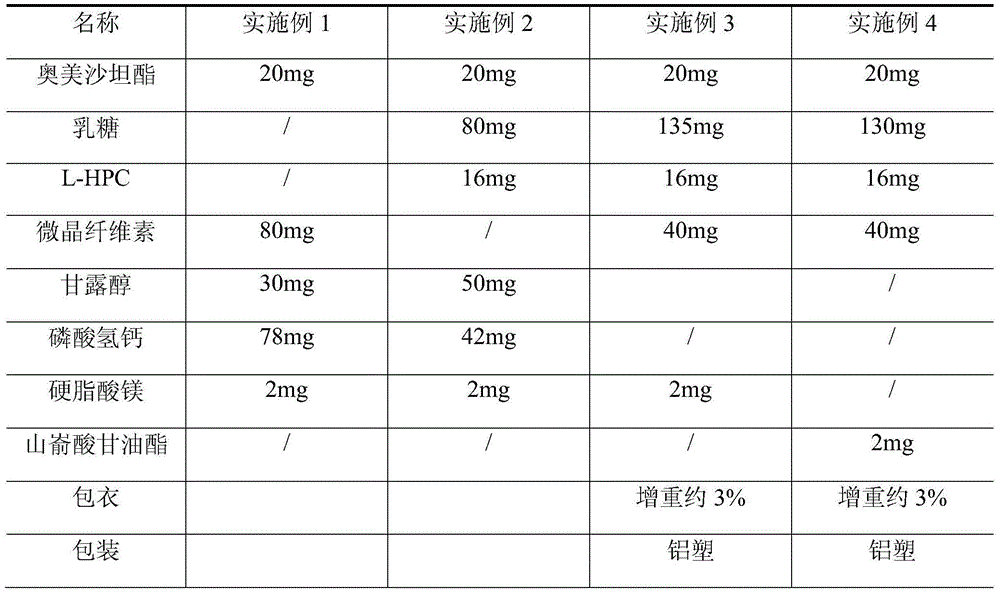
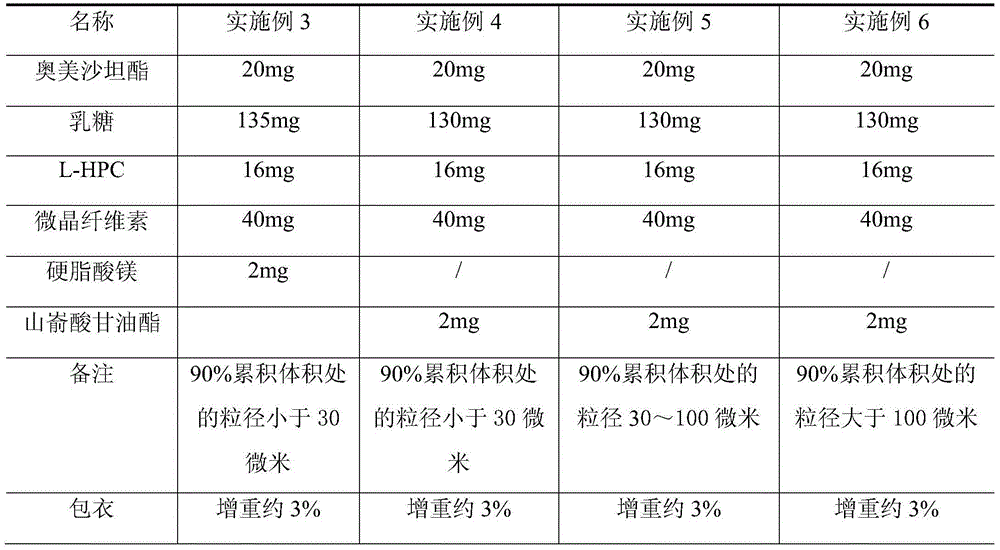
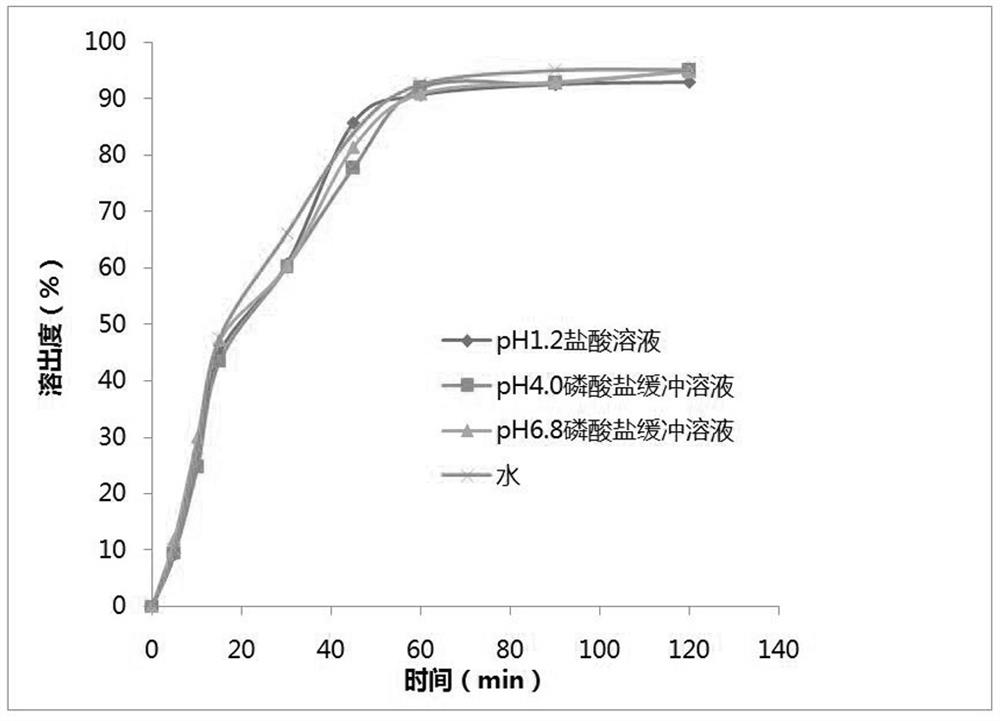
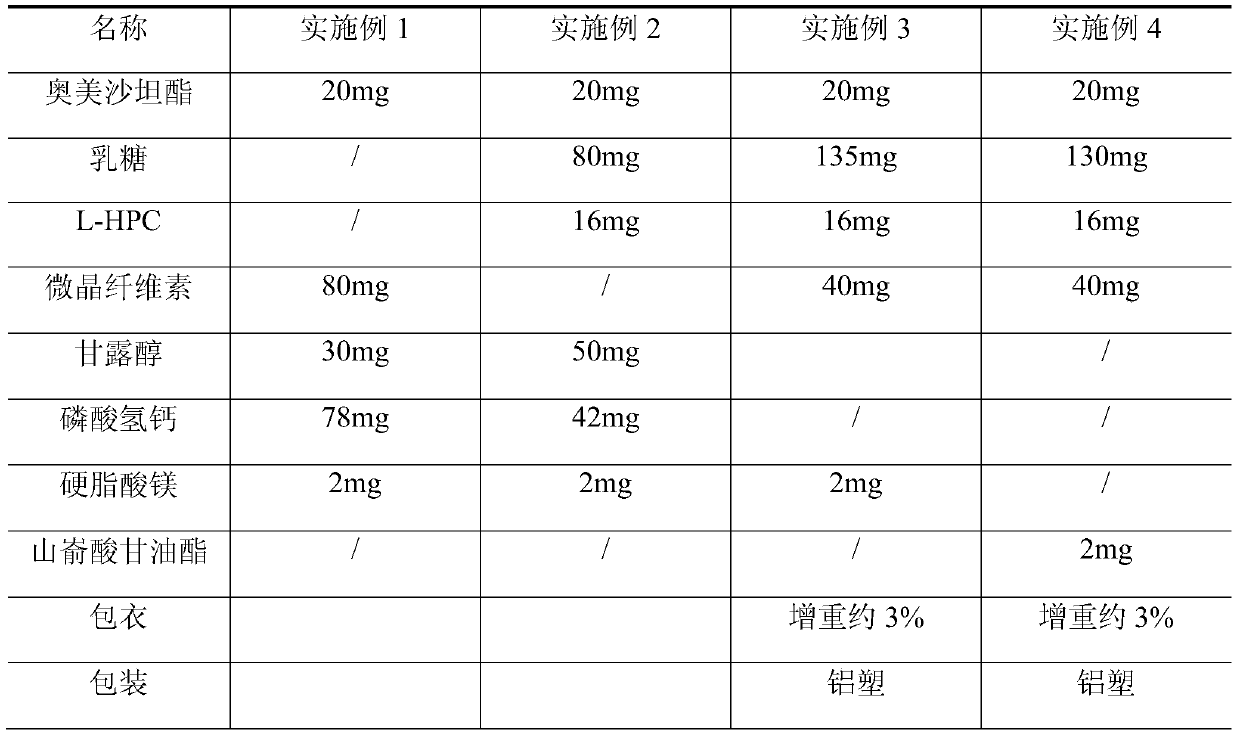
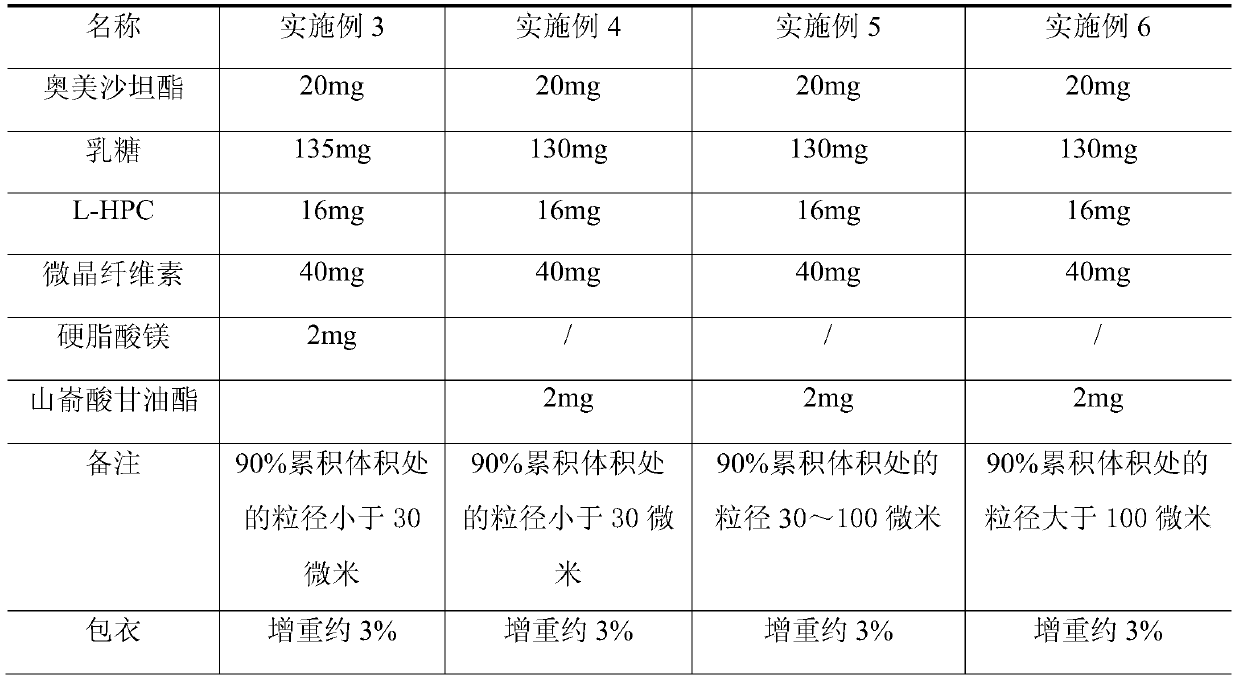
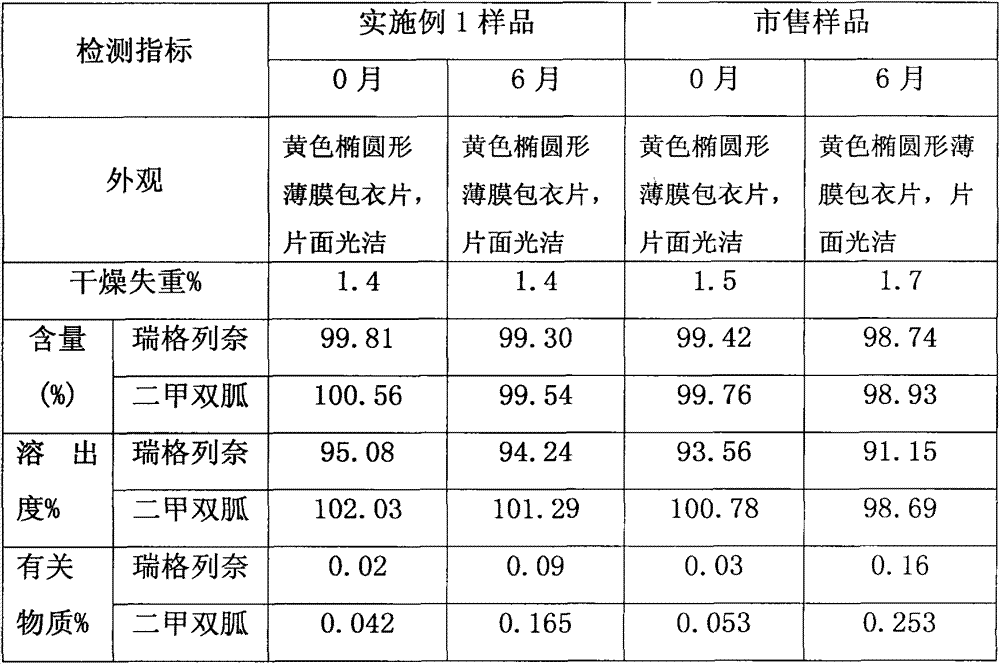
Olmesartan medoxomil tablet and preparation technology thereof
ActiveCN104398483AImprove stabilityGood particle size dissolutionOrganic active ingredientsOil/fats/waxes non-active ingredientsOlmesartanDissolution
The invention relates to an Olmesartan medoxomil tablet and preparation technology thereof. The core of the Olmesartan medoxomil tablet is prepared by directly pressing Olmesartan medoxomil and medicinal assistants, and the particle size of a position accumulating 90% of Olmesartan medoxomil is 1-100[mu]m; and the medicinal assistants comprise a filler, a disintegrating agent and a lubricant, wherein the lubricant is one or more of neutral or inert lubricants. Raw materials of the core of the tablet comprise, by weight, 20 parts of Olmesartan medoxomil, 80-180 parts of the filler, 10-20 parts of the disintegrating agent and 2-10 parts of the lubricant. The Olmesartan medoxomil is crushed in an airflow crushing mode, the particle size is controlled, and the preparation technology adopts a direct powder pressing method, so the introduction of water in a wet granulation process and the degradation of active substances in a wet particle drying process are avoided, and the incidence of a hydrolysis reaction is prevented. The Olmesartan medoxomil tablet has the advantages of stability, good dissolution and simple preparation.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Method for preparing 'youkadan' granule
InactiveCN101057865AGuaranteed content uniformityAvoid adverse reactionsAntipyreticAnalgesicsCommon coldGallstones
The invention discloses a process for preparing pediatric paracetamol and amantadine hydrochloride granules for treating children's common cold, wherein each unit of the preparation comprises Paracetamol 100mg, amantadine hydrochloride 40mg, artificial ox gallstone 4mg, caffeine 6mg and chlorpheniramine maleate 0. 8mg.
Owner:杨文龙
Methyldigoxin preparation capable of being rapidly absorbed in oral cavity and preparation method of methyldigoxin preparation
ActiveCN106309392AFast blood flowQuick effectOrganic active ingredientsPharmaceutical non-active ingredientsDrugPartition coefficient
The invention relates to a methyldigoxin preparation capable of being rapidly absorbed in an oral cavity and a preparation method of the methyldigoxin preparation. The methyldigoxin preparation capable of being rapidly absorbed in the oral cavity comprises an active center, a compound absorption accelerating system and other pharmaceutically acceptable auxiliary materials. By utilizing characteristics that a sublingual mucosa has abundant blood vessels and a rapid blood flow, the methyldigoxin is prepared into the preparation capable of being rapidly absorbed in the oral cavity and is absorbed through the sublingual mucosa, and absorbed medicine directly enters systemic circulation. The technical difficulty that the methyldigoxin has a relatively small oil-water partition coefficient and low membrane permeability, is easy to degrade under an acidic condition and cannot be easily prepared into the preparation capable of being rapidly absorbed in the oral cavity in theory is overcome; the methyldigoxin is developed into the preparation which is high in membrane permeability, can rapidly have effect through the sublingual mucosa and has good compliance.
Owner:南京斯泰尔医药科技有限公司
Indissolvable drug oral sustained-release composition and preparation method thereof
ActiveCN111728949AGrow fastAchieve dissolutionOrganic active ingredientsPill deliveryPharmaceutical drugOrganic chemistry
The invention discloses an indissolvable drug oral sustained-release composition, which is especially suitable for low-dose indissolvable drugs. The indissolvable drug oral sustained-release composition comprises sustained-release particles and a gel skeleton, wherein the sustained-release particles comprise an indissolvable drug, an enteric material and a liquid strong adsorption carrier; the gelskeleton comprises a hydrophilic gel skeleton material; and the sustained-release particles are obtained by preparing a suspension from the indissolvable drug and the enteric material and then spraying the suspension onto the liquid strong adsorption carrier. The sustained-release particles are partially wrapped by the gel skeleton to form a multi-sustained-release system technology, the releasetime is prolonged, the preparation process is simple, the efficiency is high, the drug mixing is uniform, and the content loss is less.
Owner:AC PHARMA CO LTD
Method for preparing 'kelike' capsule
InactiveCN101057866AGuaranteed content uniformityAvoid adverse reactionsAntipyreticAnalgesicsGallstonesCaffeine
The invention discloses a process for preparing compound paracetamot and amantadine lydrochloide capsules for treating common cold, wherein each tablet of the compound paracetamot and amantadine lydrochloide capsules comprises acetaminopher 250mg, amantadine hydrochloride 100mg, artificial ox gallstone 10mg, caffeine 15mg, chlorpheniramine maleate 2mg.
Owner:杨文龙
Medicinal composition containing candesartan cilexetil and hydrochlorothiazide and preparation method thereof
InactiveCN102138920APromote dissolutionImprove stabilityOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATEHydrochlorothiazide
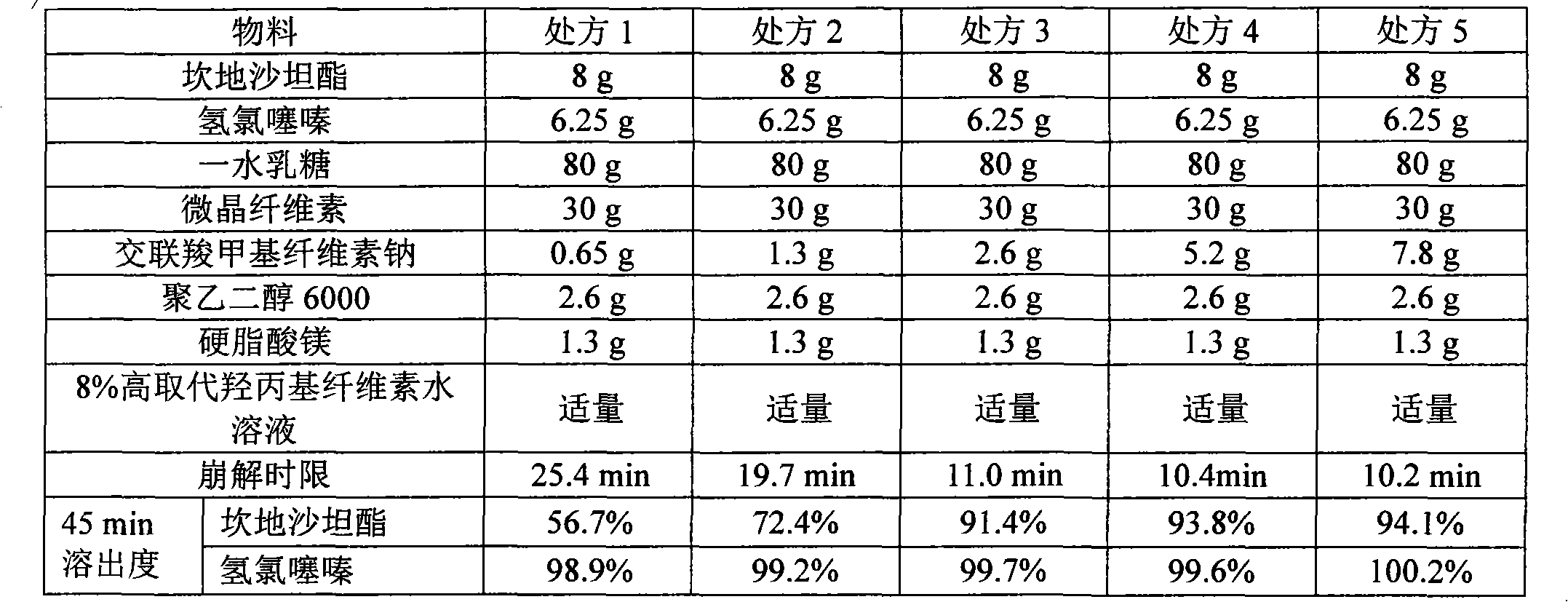
The invention relates to a medicinal composition containing candesartan cilexetil and hydrochlorothiazide and a preparation method thereof. The medicinal composition comprises the following components in parts by weight: 4 or 8 parts of candesartan cilexetil, 6.25 parts of hydrochlorothiazide, 60 to 100 parts of lactose monohydrate, 15 to 40 parts of microcrystalline cellulose, 2.6 parts of croscarmellose sodium, 2.6 parts of polyglycol 6000, and 1.3 parts of magnesium stearate. After the medicinal composition is uniformly mixed, an 8 percent high-substitution hydroxypropyl cellulose aqueous solution is used as a binder to granulate by a wet method and to tablet. In the method, by adopting a certain amount of the polyglycol 6000 as a stabilizer, the preparation stability is remarkably improved; and over 90 percent of dissolution rate can be achieved in 45 minutes by adopting less disintegrant and binder; the preparation method has a simple production process without adding extra equipment; the tablets produced according to the method has good stability and high disintegration speed, so that the dissolution of the medicament is remarkably improved, and measurement proves that: the dissolution of the tablets prepared by adopting the method reaches over 90 percent in 45 minutes.
Owner:HAINAN ZHONGJI MEDICAL TECH
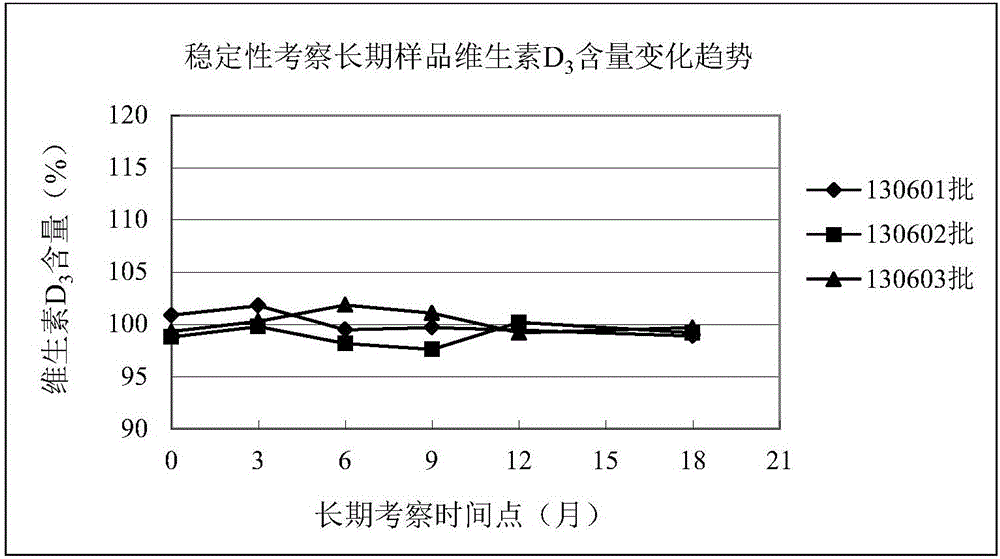
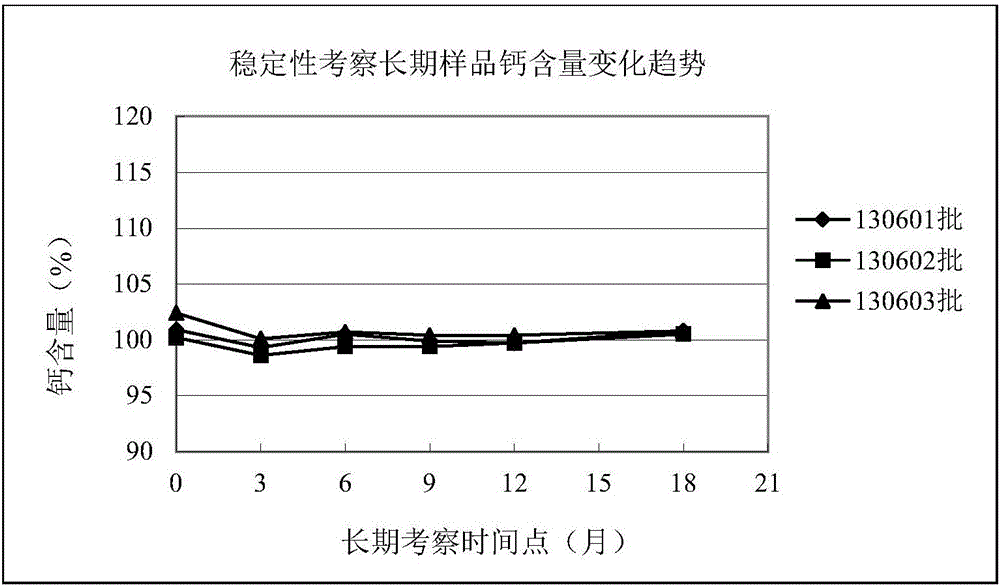
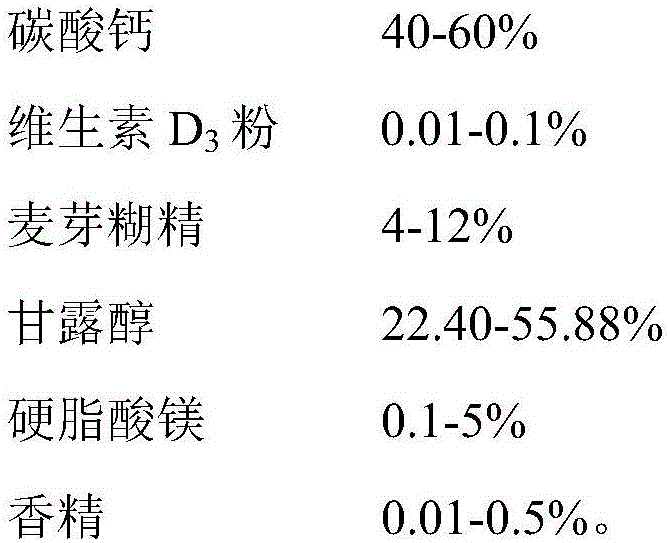
CaCO3 vitamin D3 preparation and preparation method thereof
ActiveCN109010361AGuaranteed molding effectGuaranteed stabilityOrganic active ingredientsMetabolism disorderFiller ExcipientMedicine
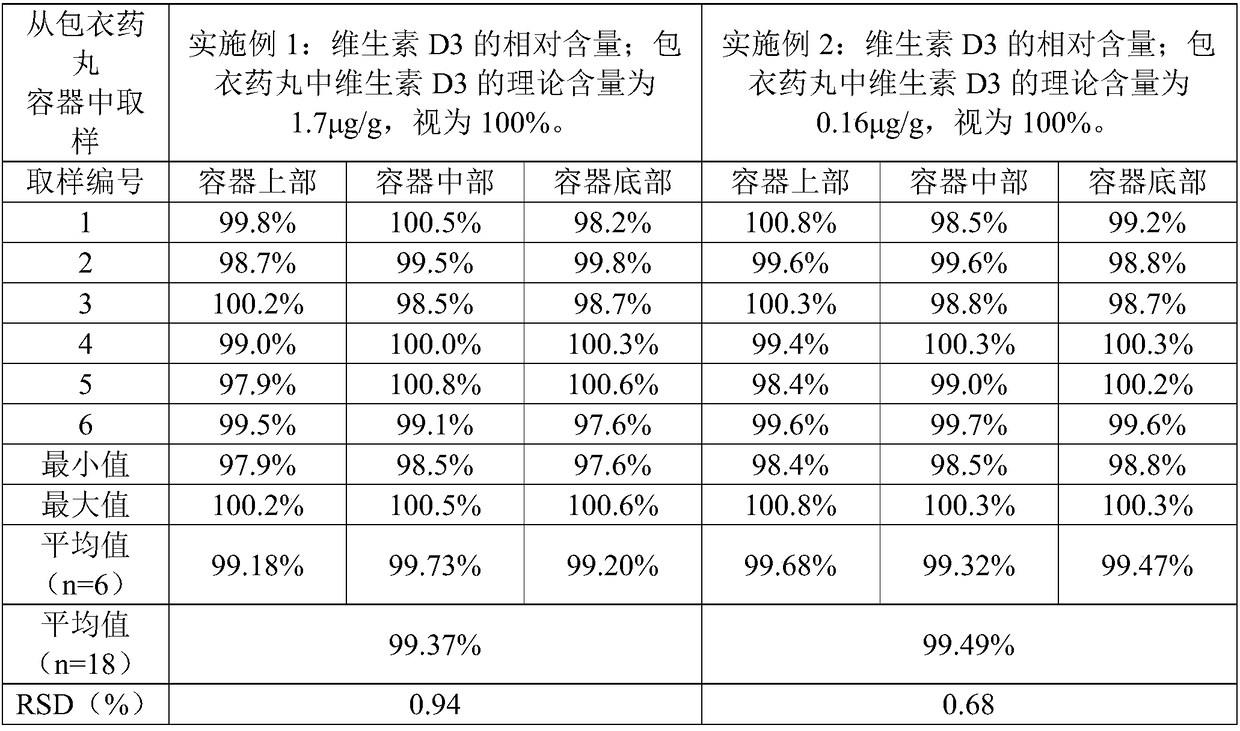
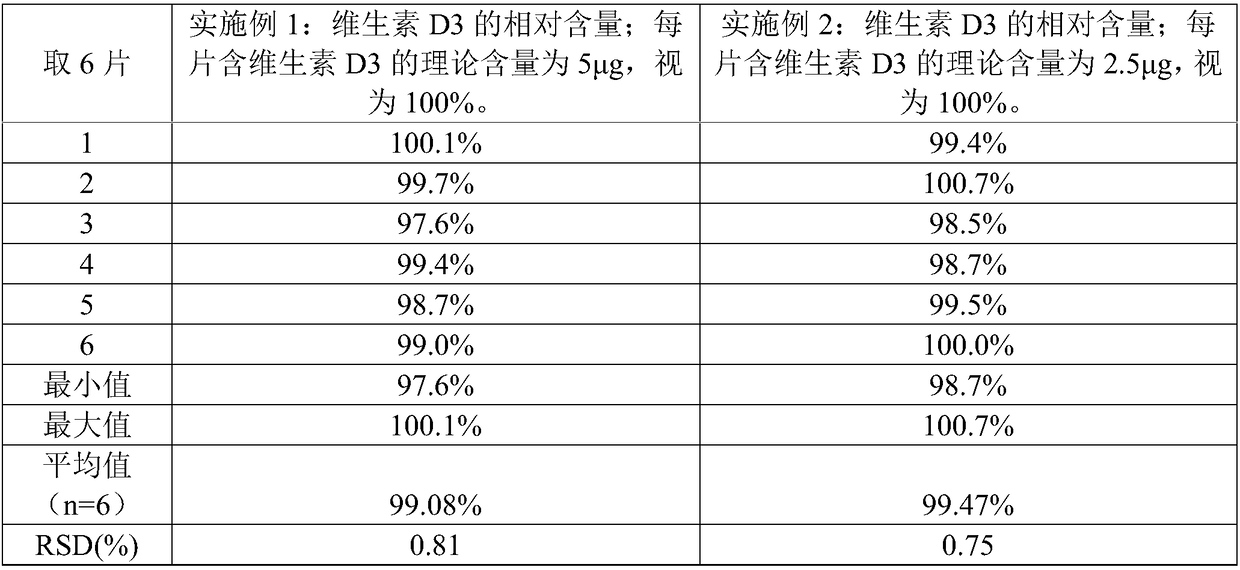
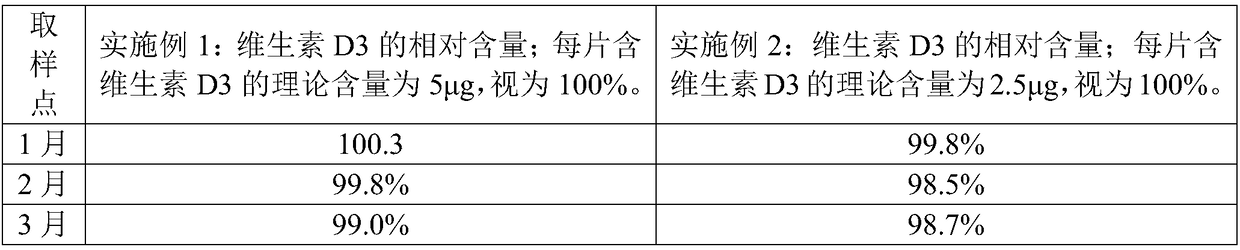
The invention relates to a CaCO3 vitamin D3 preparation and a preparation method thereof. The CaCO3 vitamin D3 preparation is mainly prepared from coated pills, wherein the coated pill includes a pillcore, a medicine coating layer and a protective coating layer; the medicine coating layer and the protective coating layer successively coat the pill core. The pill core includes: CaCO3, a disintegrating agent, filler and a binder; the medicine coating layer includes: vitamin D3 and a binder; the protective coating layer is mainly prepared from coating powder. By dispersing the CaCO3 in the pillcore, containing the disintegrating agent and filler, of the coated pill and dispersing the vitamin D3 in the medicine coating layer coating the pill core, and binding the pill core with the medicinecoating layer through the binder and coating the pill with the protective coating layer, a problem of degradability of vitamin D3 is avoided, and uniformity of the CaCO3 and vitamin D3 is increased and stability of the vitamin D3 is improved.
Owner:CHINESE MEDICINES GUANGZHOU
Pharmaceutical composition for treating depression and preparation method thereof
InactiveCN103565788AGuaranteed content uniformityPreparation method is stableOrganic active ingredientsNervous disorderIsobenzofuranPharmaceutical drug
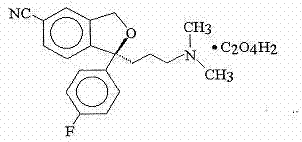
The invention belongs to the field of medicine, and particularly relates to a pharmaceutical composition for treating depression and a preparation method thereof. The preparation is an oral solid preparation and contains an active component with general formula of (S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofuran carbonitrile oxalate (Escitalopram oxalate), microcrystalline cellulose and a compressible excipient; and the preparation has the characteristic of quick dissolution and is of great significance to the treatment of depression.
Owner:BEIJING D VENTUREPHARM TECH DEV
Antiallergic pharmaceutical composition and preparation method thereof
InactiveCN103768031AGuaranteed content uniformityPreparation method is stableOrganic active ingredientsPharmaceutical non-active ingredientsAcetic acidChlorobenzene
The invention belongs to the field of pharmaceutical preparations, and relates to an antiallergic pharmaceutical composition and a preparation method thereof. The preparation is orally disintegrating tablets, and contains an active ingredient with a general formula of (R)-(-)-2-[3-[4-[(4-chlorphenyl)benzyl]-1-piperazinyl]ethoxy]acetic acid dihydrochloride. The preparation of the invention can be rapidly disintegrated in oral cavity, and has the characteristics of no gritty feel, good mouth feel and rapid dissolution.
Owner:BEIJING VENTUREPHARM BIOTECH
Crystal form A of Bictegravir sodium salt, and preparation method and use of crystal form A of Bictegravir sodium salt
ActiveCN111978333AImprove liquiditySmall weight differenceOrganic active ingredientsOrganic chemistry methodsPhysical chemistryBiomedicine
The invention relates to the technical field of biomedicine, in particular to a crystal form A of Bictegravir sodium salt, and a preparation method and use of the crystal form A of Bictegravir sodiumsalt. Compared with existing crystal forms, the crystal form A of Bictegravir sodium salt provided by the invention has lower hygroscopicity; has good solubility in simulated biological media and purewater; has better fluidity; has better compressibility; and has less adhesion.
Owner:CHANGZHOU PHARMA FACTORY
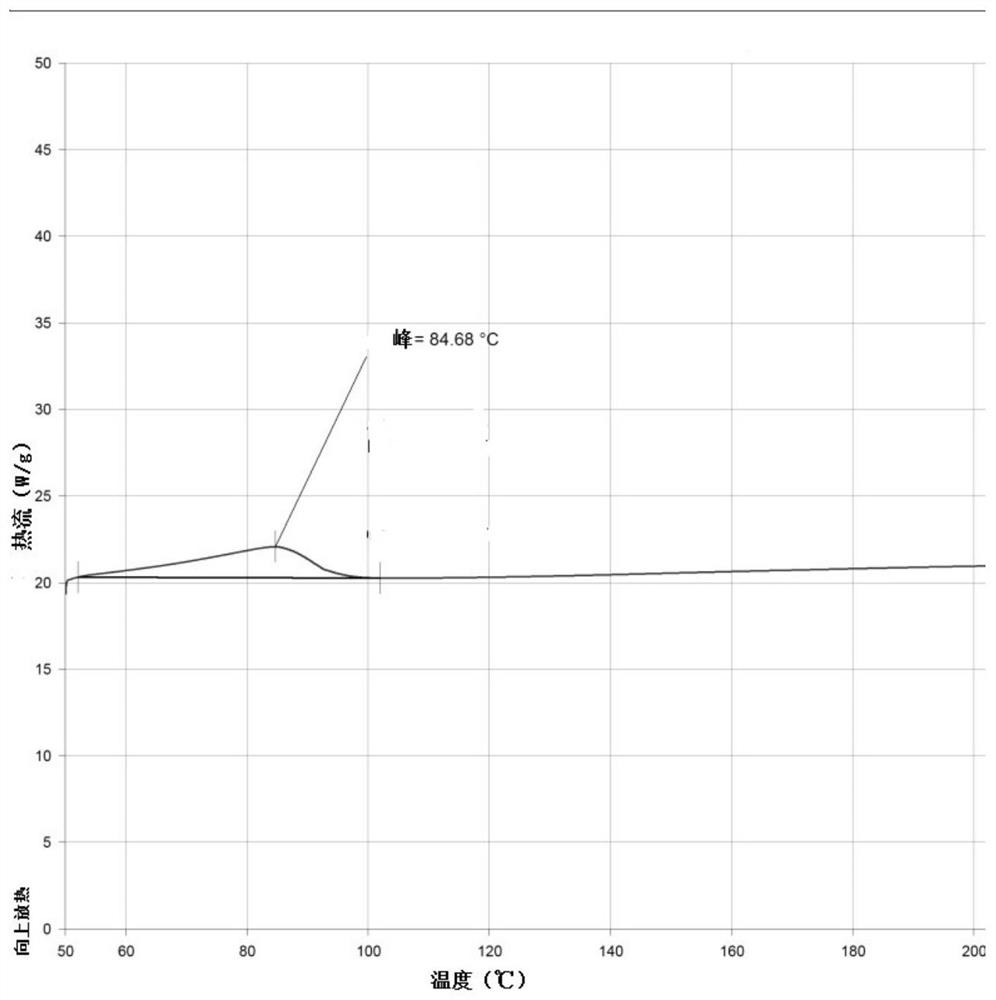
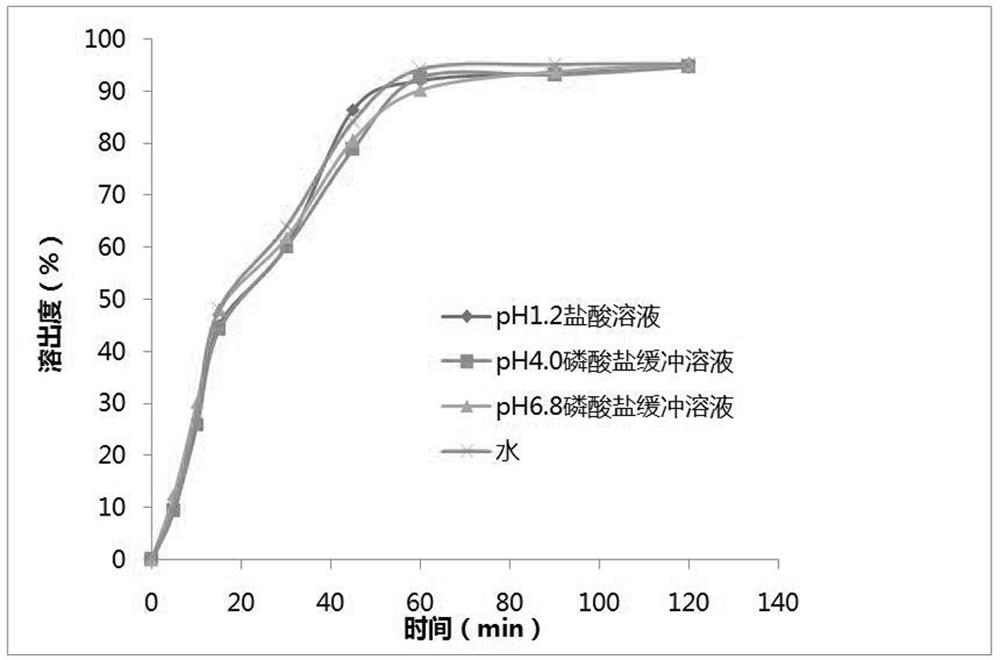
Naproxen and esomeprazole magnesium compound enteric coated tablets and preparation method thereof
InactiveCN106606496AGood effectGuaranteed content uniformityOrganic active ingredientsAntipyreticActive componentPharmaceutical Adjuvants
The invention provides naproxen and esomeprazole magnesium compound enteric coated tablets, which are composed of an active component and a pharmaceutical adjuvant. The active component comprises (1) naproxen enteric pellets and (2) esomeprazole magnesium quick-release particles. Each tablet comprises 375-500 mg of naproxen and 20 mg of esomeprazole magnesium based on esomeprazole per single dosage. The releasing degree of the compound enteric coated tablets is measured, and the result shows that the releasing degree is more than 80%, and reaches 85-90%. In an acceleration condition, compared with enteric coated tablets placed for zero month, enteric coated tablets placed for three months have no obvious change in property, releasing degree, content, and related substances and are stable in quality. According to a preparation method of the naproxen and esomeprazole magnesium compound enteric coated tablets, the content of esomeprazole magnesium is easy to control, and the size of prepared naproxen enteric pellets is close to that of the esomeprazole magnesium quick-release particles, so that the naproxen enteric pellets and esomeprazole magnesium quick-release particles are easy to be uniformly mixed. The content uniformity of pressed tablets meets requirements, and the preparation method is suitable for massive industrial production.
Owner:SHANGHAI SUNTECH PHARMA
Indapamide tablet and preparation method thereof
PendingCN112245402AAvoid damageUniform and stable particle size distributionOrganic active ingredientsDrageesOrganic solventIndapamida
The invention provides an indapamide tablet. The tablet is composed of a tablet core and a film coating layer, wherein the tablet core is prepared from the following raw materials in parts by weight of 20-30 parts of indapamide, 600-800 parts of a filler, 100-300 parts of an adhesive, 8-50 parts of a lubricant and 2-25 parts of a glidant; and the mass of the film coating layer is 2-5% of the massof the tablet core. A preparation method comprises the following steps of pretreatment of raw materials and auxiliary materials; weighing and proportioning; dry powder mixing; dry granulation; total mixing; tabletting; and coating. According to the invention, dry granulation is adopted, the operation process is simple, the intra-batch and inter-batch difference is small, the particle size distribution of powder is uniform and stable, and the tablet weight difference and the tablet hardness in the tabletting process are easy to control; water or an organic solvent does not need to be added, andhigh-temperature drying is not needed, so that damage to main active ingredients is small; the dissolution of the indapamide tablet is consistent with that of an original drug; and according to the indapamide tablet prepared by the invention, API distribution is more uniform, and a burst release phenomenon does not easily occur during release.
Owner:濮阳市汇元药业有限公司
A kind of olmesartan medoxomil tablet and preparation technology thereof
ActiveCN104398483BImprove stabilityGood particle size dissolutionOrganic active ingredientsOil/fats/waxes non-active ingredientsMedicineOlmesartan
The invention relates to an olmesartan medoxomil tablet and a preparation process thereof. The olmesartan medoxomil tablet core is made by direct compression of olmesartan medoxomil and pharmaceutical excipients, and the particle size at 90% cumulative volume of olmesartan medoxomil is 1 ‑100 microns; the pharmaceutical excipients include fillers, disintegrants and lubricants, wherein the lubricants are selected from one or more of neutral or inert lubricants. The weight ratio of raw materials in the tablet core is 20 parts of olmesartan medoxomil, 80-180 parts of filler, 10-20 parts of disintegrant, and 2-10 parts of lubricant. Olmesartan medoxomil of the present invention is pulverized by means of airflow pulverization to control the particle size. The preparation process adopts the powder direct tableting method, which avoids the introduction of moisture in the wet granulation process, the degradation of active substances in the wet granule drying process, and prevents hydrolysis. The reaction takes place, the medicine is stable, the dissolution property is good, and the preparation is simple.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Pharmaceutical composition for treating diabetes mellitus
ActiveCN103494816AGuaranteed DissolutionGuaranteed content uniformityOrganic active ingredientsMetabolism disorderDrug productRepaglinide
The invention relates to the technical field of a pharmaceutical composition for treating diabetes mellitus, particularly relates to a compound composition of repaglinide and metformin, and belongs to the technical field of medicine. According to the technical scheme, the pharmaceutical composition is a composite tablet of the repaglinide and the metformin and is characterized in that the repaglinide is micronized into 3-10 microns; the micronized repaglinide compound is dissolved into an ethanol solution of opadry and cladded at the periphery of an uncoated metformin tablet. The pharmaceutical composition has the beneficial effects that the preparation method is simplified; the cost is reduced; the stability of the medicine is improved.
Owner:DISHA PHARMA GRP
Telmisartan oral solid preparation with stable product performance and preparation method of telmisartan oral solid preparation
ActiveCN114344294AApply medicine evenlyGuaranteed content uniformityHydroxy compound active ingredientsPharmaceutical non-active ingredientsCelluloseMagnesium stearate
The invention relates to a telmisartan preparation and a preparation method thereof, in particular to a telmisartan oral solid preparation with stable product performance and a preparation method thereof. The telmisartan tablet comprises the following raw material components: an active component telmisartan, a basifier, an adhesive, a filler and a lubricant, the filler is a mannitol pellet core, and the telmisartan tablet is prepared by the following method: (1) sequentially adding telmisartan, sodium hydroxide, meglumine and povidone to prepare slurry at 40 + / -5 DEG C; (2) adding the mannitol pellet cores into a fluidized bed, and spraying slurry and granulating; (3) granulating; (4) adding the external auxiliary materials including hydroxypropyl methylcellulose, silicon dioxide, magnesium stearate and talcum powder, and mixing; and (5) tabletting or filling. The mannitol pellet core is adopted, so that the pellet core is more uniform in medicine application, and the content uniformity of the medicine is ensured. And meanwhile, the process parameters are optimized, so that the smooth proceeding of the process is ensured.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
High-dose clinorhomboidal adefovir dipivoxil preparation, and preparation method and application thereof
InactiveCN102716136AGuaranteed content uniformityEnsure mixing uniformityOrganic active ingredientsGroup 5/15 element organic compoundsCurative effectHigh doses
The invention relates to a clinorhomboidal adefovir dipivoxil preparation. The clinorhomboidal adefovir dipivoxil preparation is characterized in that each unit of the clinorhomboidal adefovir dipivoxil preparation comprises 15 to 25mg of clinorhomboidal adefovir dipivoxil and a pharmaceutically acceptable carrier. The invention also relates to a preparation method for the preparation. The invention further relates to the application of the clinorhomboidal adefovir dipivoxil preparation to preparation of a medicine for relieving hepatitis symptoms which are caused by hepatitis B virus. The high-dose clinorhomboidal adefovir dipivoxil preparation has the advantages of high safety, quick hepatitis B virus resistance response, high curative effect, ideal comprehensive treatment effect, and the like.
Owner:CHF SHANGHAI PHARMA
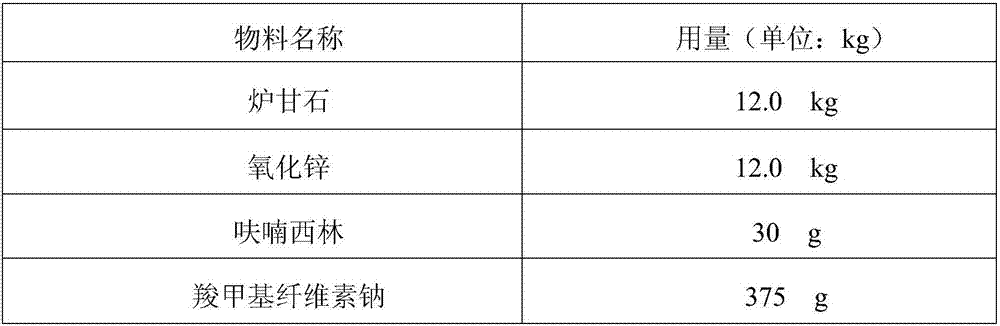
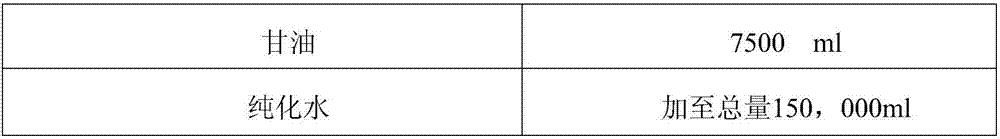
Novel method for preparing calamine and nitrofurazone pharmaceutical composition
InactiveCN107007548ASedimentation volume ratio is smallGood uniformityOrganic active ingredientsAntipyreticChemistrySodium carboxymethylcellulose
Provide a new preparation method of calamine nitrofurazone pharmaceutical composition, especially the new preparation method of calamine nitrofurazone lotion, the raw materials of described calamine nitrofurazone lotion include calamine, zinc oxide, nitrofurazone, glycerin , sodium carboxymethylcellulose, purified water, characterized in that the method comprises first mixing nitrofurazone, at least a part of sodium carboxymethylcellulose and at least a part of water, and then mixing with the remaining components in the composition A step of. The calamine nitrofurazone lotion prepared by the new preparation method of the invention has the advantages of small sedimentation volume ratio, good uniformity, stable quality, and favorable transdermal absorption.
Owner:BEIJING SHOUER PHARMA FACTORY
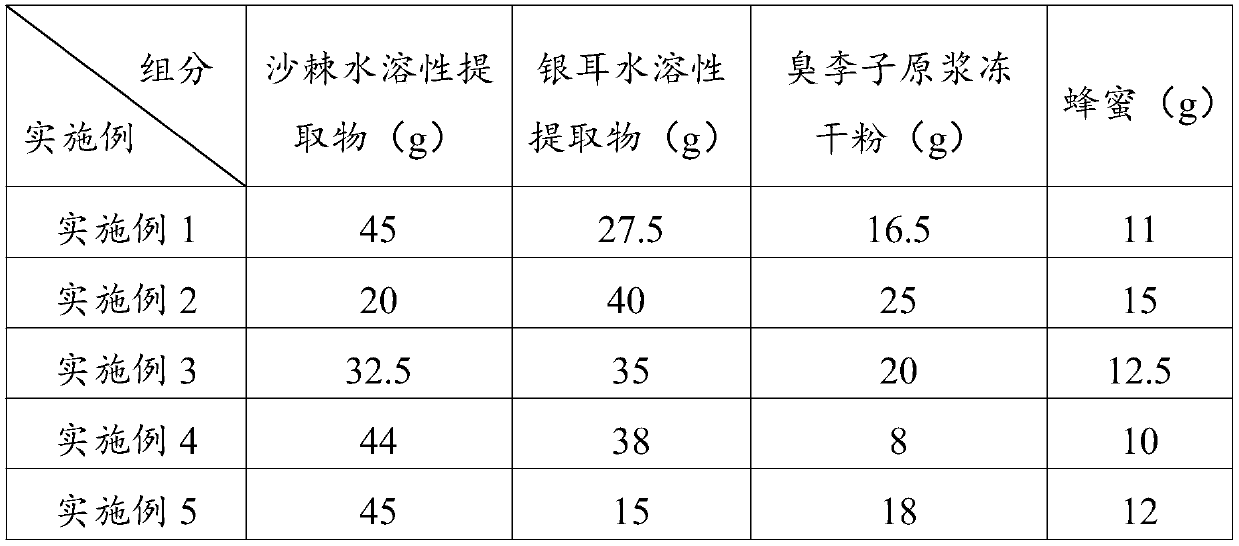
Seabuckthorn-tremella fuciformis lung-clearing solid beverage and preparation method thereof
InactiveCN110122725AExtended shelf lifeEasy to prepareNatural extract food ingredientsFood ingredient functionsTremellaAdditive ingredient
The invention discloses a preparation method of an instant seabuckthorn-tremella fuciformis solid beverage with lung-clearing effect. The seabuckthorn-tremella fuciformis solid beverage is prepared byscientific and reasonable proportion cooperation of a seabuckthorn fruit water-soluble extract, a tremella fuciformis water-soluble extract and other component part groups. The seabuckthorn-tremellafuciformis solid beverage has the advantages of being convenient to carry and easy to eat, has good lung-clearing effect, has the inhibiting effect caused by antagonism of haze, has the effects of clearing lung and relieving cough, can alleviate cough, excessive phlegm, chest tightness and many other symptoms caused by haze and dust, and can protect eyes.
Owner:JIAMUSI UNIVERSITY
Paroxetine enteric sustained-release preparation and preparation method thereof
ActiveCN104173345ALow densityWell mixedOrganic active ingredientsNervous disorderLactoseEnteric coating
The invention discloses a paroxetine enteric sustained-release preparation and a preparation method thereof. The paroxetine enteric sustained-release preparation comprises a paroxetine tablet core and an enteric coating. The paroxetine tablet core comprises paroxetine or its salt, a hydrophilic gel material, a diluent and a binder. The diluent comprises a mixture of lactose and microcrystalline cellulose or a mixture of lactose and pregelatinized starch. The paroxetine enteric sustained-release preparation has good stability, active components are not influenced by extraneous factors easily, content is not changed basically, a release curve is stable, tablet surface appearance is good, and the preparation method is simple, can be operated easily and is suitable for industrial large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Medicine containing amlodipine besylate and lisinopril dehydrate and preparation method thereof
InactiveCN106333948AGuaranteed content uniformityReduce wasteOrganic active ingredientsDipeptide ingredientsState of artMedicine
The invention discloses a medicine containing amlodipine besylate and lisinopril dehydrate and a preparation method thereof. Per unit of the preparation is composed of, by weight, 2.5-10 parts of amlodipine besylate, 5-20 parts of lisinopril, 50-200 parts of a filler, 2-10 parts of a disintegrating agent and 0.5-2 parts of a lubricant. A preparation method of the medicine comprises the following steps: mixing: mixing the raw materials and auxiliary materials by an equivalent incremental mode; and tabletting: carrying out direct powder compression on the mixture obtained by the mixing. An amlodipine besylate-lisinopril tablet prepared from the above medicine by the above technology has the following advantages: use of auxiliary materials such as an adhesive, etc. is omitted in comparison with the prior art; the formula is simple; and safety is greatly raised; raw materials and auxiliary materials are mixed by the equivalent incremental mode in the preparation technology so as to ensure content uniformity of the medicine; and steps of granulation, drying and size stabilization, etc. are omitted, the technology is easy to operate, production efficiency is high, and the technology is very suitable for industrial production.
Owner:成都尚药科技有限公司
Pharmaceutical composition of bazedoxifene acetate tablets and preparation method thereof
ActiveCN112076163AConsistent qualityGuaranteed content uniformitySkeletal disorderPill deliveryVitamin CPharmacology
The invention relates to a pharmaceutical composition containing bazedoxifene acetate as an effective component and a preparation method thereof. The composition comprises bazedoxifene acetate; lactose; microcrystalline cellulose; pregelatinized starch; low-substituted hydroxypropyl cellulose; colloidal silica; sodium dodecyl sulfate; vitamin C; and glyceryl behenate. The invention also relates toa preparation method of the composition. The pharmaceutical composition of the present invention has the effects of improving the inter-batch difference on drugs, and ensuring the content uniformityof drugs and consistent dissolution of different batches of drugs, and has medicinal safety and effectiveness.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
A kind of crystal form of valbenazine di-p-toluenesulfonate and its preparation method and use
ActiveCN110621674BGood compressibilityHigh speedOrganic active ingredientsNervous disorderPharmaceutical drugToluene
A crystal form of Valbenazine di-p-toluenesulfonate and its preparation method, a pharmaceutical composition containing the crystal form, and the use of the crystal form in the preparation of vesicular monoamine transporter 2 inhibitors and pharmaceutical preparations for treating tardive dyskinesia use in .
Owner:CRYSTAL PHARMA CO LTD
A kind of paroxetine enteric-coated sustained-release preparation and preparation method thereof
ActiveCN104173345BLow densityWell mixedOrganic active ingredientsNervous disorderActive componentMedicine
The invention discloses a paroxetine enteric sustained-release preparation and a preparation method thereof. The paroxetine enteric sustained-release preparation comprises a paroxetine tablet core and an enteric coating. The paroxetine tablet core comprises paroxetine or its salt, a hydrophilic gel material, a diluent and a binder. The diluent comprises a mixture of lactose and microcrystalline cellulose or a mixture of lactose and pregelatinized starch. The paroxetine enteric sustained-release preparation has good stability, active components are not influenced by extraneous factors easily, content is not changed basically, a release curve is stable, tablet surface appearance is good, and the preparation method is simple, can be operated easily and is suitable for industrial large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Compound liquorice tablet capable of being prepared by dry powder direct compression process and preparation method of compound liquorice tablet
ActiveCN114010610AGuaranteed appearanceGood compressibilityOrganic active ingredientsInorganic non-active ingredientsPhysical chemistryPyrrolidinones
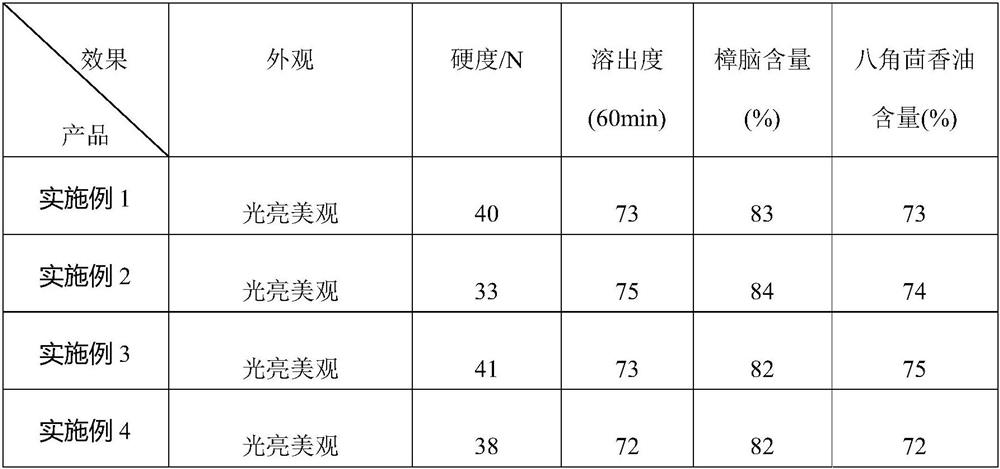
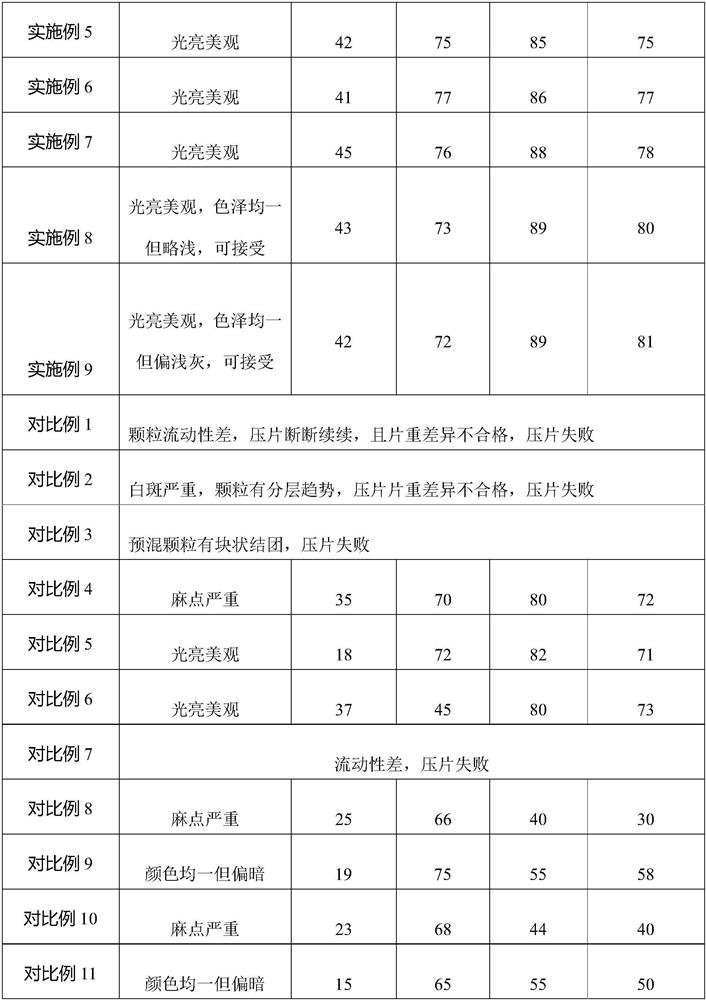
The invention discloses a compound liquorice tablet capable of being prepared by a dry powder direct compression process and a preparation method of the compound liquorice tablet. The preparation method comprises the following steps: weighing, primarily mixing, sieving, dissolving and adsorbing, premixing, totally mixing, tabletting and packaging. By controlling the average particle size of the primary mixture (the mixture of the licorice extract, the poppy fruit extract powder and the sodium benzoate), the appearance of the preparation is fundamentally ensured; by screening and selecting a novel auxiliary material polyvinylpyrrolidone, the problems of compressibility and dissolution rate are solved; by using silicon dioxide, the problems of adsorption and stability of volatile oil are solved, and the flowability and compressibility of particles are improved; by using a direct tabletting method, the process is simplified, the efficiency is improved, the content of volatile oil in a finished product is increased, the curative effect is improved, and the quality of the compound liquorice tablet is integrally improved.
Owner:宁波大红鹰药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com