Medicinal composition containing candesartan cilexetil and hydrochlorothiazide and preparation method thereof
A technology for candesartan medoxomil and hydrochlorothiazide is applied in the field of pharmaceutical compositions containing candesartan medoxomil and hydrochlorothiazide and the field of preparation thereof, which can solve the problems of poor formulation dissolution rate, large numerical value, etc. Production cost, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
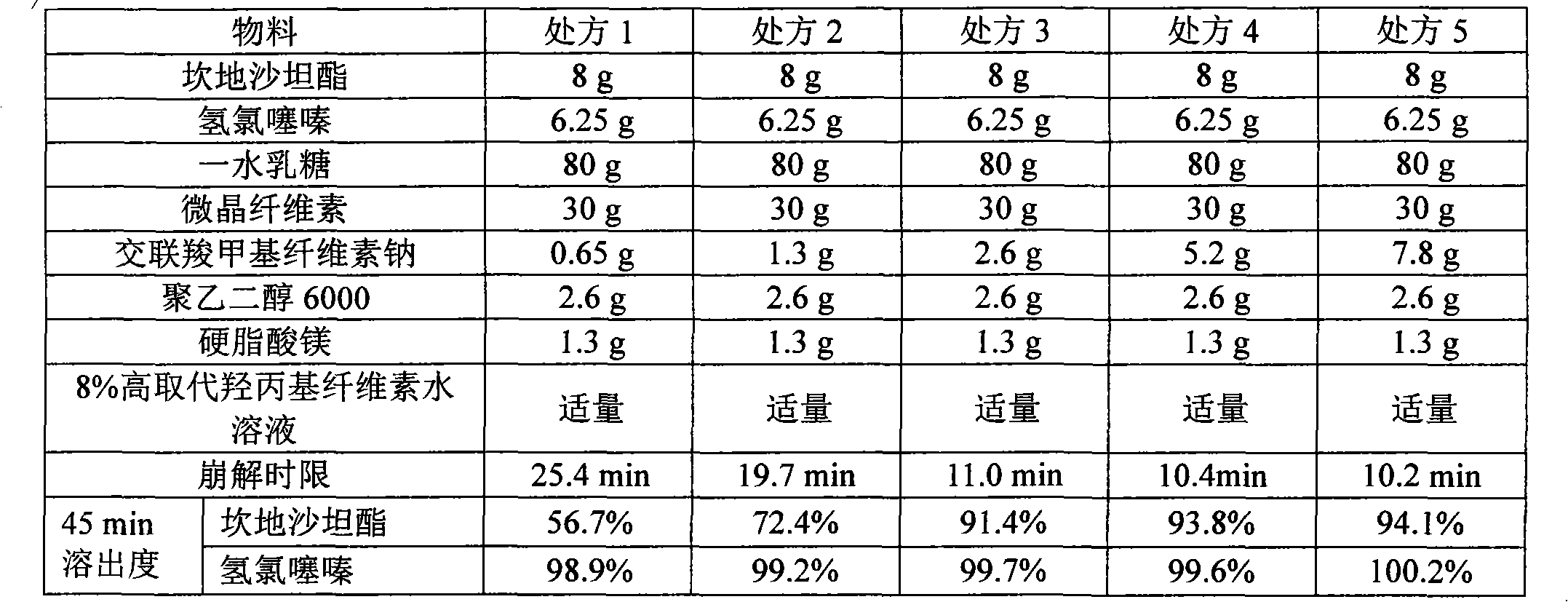
[0053] 1. Prescription
[0054] candesartan cilexetil
8g
6.25g
lactose monohydrate
80g
30g
2.6g
polyethylene glycol 6000
2.6g
1.3g
8% high substituted hydroxypropyl cellulose aqueous solution
Appropriate amount
production
1000 pieces
[0055] 2. Preparation process
[0056] 1) Pass candesartan cilexetil and hydrochlorothiazide through an 80-mesh sieve respectively, and set aside;
[0057] 2) Pass lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, polyethylene glycol 6000, magnesium stearate, and high-substituted hydroxypropyl cellulose through a 60-mesh sieve respectively, and set aside;
[0058] 3) Weigh the candesartan cilexetil, hydrochlorothiazide, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium and polyethylene glycol 6000 in th...
Embodiment 2
[0063] 1. Prescription
[0064] candesartan cilexetil
8g
6.25g
70g
40g
2.6g
polyethylene glycol 6000
2.6g
Magnesium stearate
1.3g
8% high substituted hydroxypropyl cellulose aqueous solution
Appropriate amount
production
1000 pieces
[0065] 2. Preparation process
[0066] 1) Pass candesartan cilexetil and hydrochlorothiazide through an 80-mesh sieve respectively, and set aside;
[0067] 2) Pass lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, polyethylene glycol 6000, magnesium stearate, and high-substituted hydroxypropyl cellulose through a 60-mesh sieve respectively, and set aside;
[0068] 3) Weigh the candesartan cilexetil, hydrochlorothiazide, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium and polyethylene glycol 6000 in th...
Embodiment 3
[0073] 1. Prescription
[0074] candesartan cilexetil
8g
6.25g
lactose monohydrate
80g
microcrystalline cellulose
30g
5.2g
polyethylene glycol 6000
2.6g
Magnesium stearate
1.3g
8% high substituted hydroxypropyl cellulose aqueous solution
Appropriate amount
production
1000 pieces
[0075] 2. Preparation process
[0076] 1) Pass candesartan cilexetil and hydrochlorothiazide through an 80-mesh sieve respectively, and set aside;
[0077] 2) Pass lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, polyethylene glycol 6000, magnesium stearate, and high-substituted hydroxypropyl cellulose through a 60-mesh sieve respectively, and set aside;
[0078] 3) Weigh the candesartan cilexetil, hydrochlorothiazide, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium and polyethylene glycol 6000 in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com